International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 537
ISSN 2229-5518
Zeolite -Water Adsorption Refrigeration
System
Abstract--- This paper presents the utilization of Zeolite 4A from for application in adsorption refrigeration systems. The refrigeration system prototype used in the test consists of two steel cylinders one containing Zeolite and the other containing distilled water at a pressure corresponding to the room temperature boiling point of water, three transport pipes having one valve each. The refrigerator was constructed and tested at the Mechanical Engineering Department, India. The lowest evaporator temperature attained was 9.8 deg Celsius with a C.O.P of 0.4.
Index Terms--- Adsorption, Refrigeration, Coefficient of Performance, Zeolite, Water, Vacuum, Saturated, Desorption.
—————————— ——————————
1 INTRODUCTION
The present mechanical vapour compression systems are often used to satisfy the cooling demand. This system is popular and advantageous due to its high coefficient of performance, small size and low weight but the system comes with a disadvantage of contributing to global warming, ozone layer depletion coupled with high energy consumption. The main disadvantage is that the refrigerant used in such systems have chlorofluorocarbon (CFC), Hydro chlorofluorocarbon (CHFC) which in turn lead to global warming and Ozone Layer Depletion.
A lot of research and development to introduce adsorption refrigeration system using adsorbent-refrigerant pair is being carried out now-a-days. These systems makes use of the unique features of certain adsorbent refrigerant pairs to complete refrigeration cycles for cooling purposes which are well adapted to simple technology applications which operate without moving parts and with low grade heat from different sources such as residual heat or solar energy.
The use of adorption processes to produce refrigeration effect by using adsorbents like Zeolite, silica gel, activated carbon and alumina which have highly porous structures with surface–volume ratios in the order of several hundred that can selectively catch and hold refrigerants (Wang, et.al
2010).
best to be used in adsorption refrigeration because of the fact that they have extremely non-linear pressure dependence of its adsorption isotherms. The isotherms saturate at low partial pressure, after which the amount adsorbed becomes almost independent of pressure. At ambient temperature, zeolite can adsorb most of the vapour even at high partial pressure, corresponding to high condenser temperature. This unique property of the zeolite becomes a very important feature in high condenser temperature and thus only a moderate regeneration temperature might be employed. The zeolite-water pair is one of the most preferred adsorbent – adsorbate pairs because water has a high latent heat of vaporization and a convenient boiling point (N. O. Omisanya, et.al 2012).
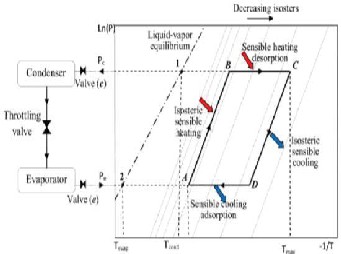
The adsorption cycle in this refrigeration system is similar to the vapor compression refrigeration cycle except the fact that the adsorbent and adsorbate takes the place of the compressor unit (Hassan et.al, 2011). The adsorption- desorption cycle consists of four stages and is presented by clapeyron diagram showing the pressure-temperature concentration (p-T-x) by thick lines ABCD in the figure.
The most preferred lowest temperature for the adsorption refrigerator is the room temperature, the boiling
point should be preferentially above 20 degC. Zeolite – water, Zeolite – methanol, Activated carbon- methanol etc might be mentioned among the adsorbent -adsorbate combinations that have been tested. Zeolite – water pair
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 538
ISSN 2229-5518
Figure 1
Clapeyron diagram for a conventional adsorption cycle. Source: Hassan et.al, 2011.
Step (A B): Isoteric heating
Step (B D): Desorption and condensation
Step (D F): Isoteric cooling
Step (F A): Adsorption and evaporation
Zeolite/water is good for cooling and overcoming environmental problems related to vapour compression refrigeration systems because they have zero ozone layer depletion potential and no global warming potential(El Fadar et.al, 2009). The most important feature of zeolite is that the zeolite crystals are eternal i.e. they can undergo infinite adsorption-desorption cycles.
2 REFRIGERATION APPARATUS
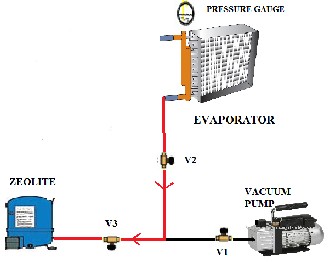
The system was fabricated and tested in Department of Mechanical Engineering, JSS Academy of Technical Education, Noida, India. The prototype system produced (as shown in the figure) consists of two cylinders, a refrigerating chamber, a vacuum pump, transport pipes with valves and Zeolite 4A.
0.3kg of the synthesized Zeolite 4A; 53μm particle size was loaded in one of the cylinder which is kept air tight (El I. Amber et.al, 2013). Since Zeolite-water pair has maximum adsorption capacity at ambient temperature (I. Amber et.al,
2013), 0.02m3 of distilled water is poured into the water cylinder.
Figure 2
One of the steel cylinders contains Zeolite 4A which is kept at complete vacuum and the other cylinder contains water which is kept at very low pressure such that the water boils at room temperature (at 23mbar). The valves V1 and V2 connects the vacuum pump and the evaporator whereas the valves V1 and V3 connects the vacuum pump and the Zeolite container.
3 TESTING PROCEDURE
The system operates in two loops, loop 1 is the desorption process and loop 2 is the adsorption process. The positioning of the Valves V1, V2 and V3 is identical in the apparatus as shown in the figure 3.
In the adsorption process, the valves V1 and V3 are opened, and the vacuum pump is used to extract all the air from the Zeolite cylinder and create complete vacuum inside it. The valve V3 is then closed and the cylinder containing water is then connected to the vacuum pump by opening valves V1 and V2. The pressure inside the water cylinder is lowered (to 23mbar in this case) such that water starts boiling at room temperature. The valve V1 is then closed and the two cylinders containing Zeolite and water are connected by opening the valves V2 and V3.
The absorbent (Zeolite 4A) act as a water vapour pump and starts adsorbing the water vapour from the water cylinder and hence the temperature inside it starts decreasing while the temperature of the Zeolite starts increasing and thus a cooling effect is produced. As soon as the temperature inside the water cylinder reaches 0 degC, the remaining water inside the cylinder freezes and hence ice is formed which provides the refrigeration effect till the Zeolite becomes saturated.
As soon as the Zeolite becomes saturated and is incapable of absorbing more water, the Zeolite container is heated such that the Zeolite loses water and is gets collected in the water container. This is the desorption cycle. As soon as the Zeolite becomes unsaturated again, the adsorption cycle is again carried out.
In order to determine the performance of the system, temperatures were measured in different points of the prototype using thermocouple.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 539
ISSN 2229-5518

Figure 3
4 COEFICIENT OF PERFORMANCE
The coefficient of performance is defined as the ratio of the total refrigeration effect produced in the system to the total energy required for this effect.
C.O.P. = Refrigeration Effect
Total Energy Input
C.O.P. = Q1 = T1
Q2 T2
Where Q1 is the heat exchanged between evaporator and the food particles and Q2 is the heat used during the generation of the refrigerant.
5 TESTING PROCEDURE AND RESULTS
The tests were carried out in the month of march at the Department of Mechanical Engineering, JSS Academy of Technical Education.
The zeolite was found to have a maximum adsorption capacity of about 0.3kg of adsorbate/0.85kg of adsorbent as given in I. Amber et.al, 2013. In the beginning, the Zeolite cylinder was filled with 600gms of zeolite which was connected to the water cylinder containing 500ml of distilled water. The system is made air tight with the transport pipes and valves as shown in figure 3. The system is maintained at a pressure below the atmospheric pressure.
The zeolite container is maintained at complete vacuum whereas the water cylinder is kept at a pressure such that the water boils at room temperature ( 23mbar in this case).
Regeneration of zeolite occurs by heating the zeolite container to around 200 deg Celsius and opening the valves V2 and V3 such that the water lost by the zeolite reaches back to the water cylinder. The desorption curves of zeolite at different pressures and temperature as given in Miguel et.al, (2003), shows that at 250 deg Celsius, 2.5 hours is required for the zeolite to achieve 5% humidity with 84% of the water extracted and it takes 6 hours at 200 deg Celsius to reduce humidity to 10% thereby extracting 66% of water.
The heat losses for the adsorber and the longer time intervals for the regeneration of the zeolite along with the use of steel container as evaporator having slow rate of cooling instead of copper container, were the main reasons for the por performance of the system.
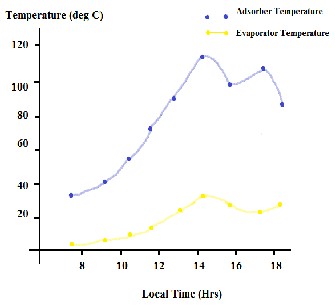
The extraction of heat from the products kept inside the refrigerator leads to the formation of vapors in the evaporator which are adsorbed by the Zeolite thus creating cooling effect called isoteric cooling. The maximum adsorber temperature was 115 deg Celsius which is much below the expected temperature of 200 deg Celsius and the minimum evaporator temperature was 9.8 deg Celsius. Refrigeration effect produced and the COP of the system are dependent on the mass of the refrigerant desorbed. The graph of the temperature recorded at various time intervals is shown in figure 4. The COP of the system can range from
0.32 to 0.6 which is lower in comparison to the vapor
compression refrigeration systems having COP in the range of 1.0 to 1.6.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 540
ISSN 2229-5518
6 CONCLUSION
The system attained a very low COP with long regeneration time intervals and a minimum refrigeration temperature of
9.8 deg Celsius. The adsorber attained a maximum temperature of 115 deg Celsius which is much below the expected regeneration temperature of 200 deg Celsius for zeolite. With continuous improvements in this segment the present mechanical vapor compression systems can be replaced with these environment friendly adsorption systems. The solar technology can be brought into use in these systems in order to regenerate the zeolite making it completely ecofriendly and very less dependent on electricity.
ACKNOWLEDGEMENT
The authors are very grateful to Mr. Dhananjay Trivedi for his guidance in this project along with the Department of Mechanical Engineering, JSS Academy of Technical Education, India for allowing the usage of some of the equipments and materials for the development of this project.
REFERENCES
[1] Belgenin KisaAdresi, A review Adsorption working pair for refrigeration Elsevier 2010.
[2] SiegrrfriedKreussler, DetlefBolz (2000) Experiment on solar adsorption refrigeration, using Zeolite and water.
[3] Aderemi B.O., (2004): Preliminary studies on Synthesis of
Zeolite from local clay, Nigeria Journal of scientific research 4(2)7-12.
[4] El Fadar A. Mimet A Perez-Garcia., (2009a), Study of an adsorption refrigeration system powered by parabolic trough collector and coupled with a heat pipe. Renewable energy, 34 2271-2279.
[5] I. Amber, Clement Folayan, Rekiyah Suleiman, Abdulazeez Yusuf Atta, (2013), Application of synthesized zeolite A from kankara kaolin for solar Adsorption Refrigeration.
[6] N.O. Omisanya, C.O. Folayan, S.Y. Aku, S.S. Adefila, Pelegia Research Library(2012), Performance of a Zeolite-Water adsorption refrigerator.
[7] N.R. SPARKS and C.C. DILLIO, “Mechanical refrigeration”, Mcgraw-Hill Book Co.Inc. pp. 140-64,1959.
[8] “Refrigeration air conditioning data book design volume”, ASRE , pp.5-12to 15,1957.
[9] Dhar P. L., and Singh, S. K., Applied Thermal Engineering,
2001,21(2):119-134.
IJSER © 2015 http://www.ijser.org



