In accordance with our earlier investigation of the reaction of phenyl hydrazine [1] and pyrazine [7] with hexacyanoferrate (II), phenonthroline has also been shown to react with [Fe (CN)6]4- according to Equations [1-3].
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 299
ISSN 2229-5518
Use of Nitrogen Containing Heterocyclic ligand; Phenanthroline to study the kinetics of uncatalysed ligand substitution reaction of hexacyanoferrate (II)
Prof. (Dr.) Amarika Singh*, Anupma Singh, Isha Rizvi, Dharmendra K. Srivastava, Siraj Ahmad
ABSTRACT-The kinetics and mechanism of uncatalysed substitution of co-ordinated cyanide in hexacyanoferrate (II) by a nitrogen donar heterocyclic ligand, phenanthroline at 528nm (max of a dark coloured compound [Fe (CN)5Phen]3- as a function of [Fe (CN)6]4- and [Phen] under the conditions, pH= 3.00.02, temperature = 25.0 0.10oC , lonic strength (I) = 0.03 M(KNO3).The reaction is a first order each in [Fe (CN)6]4- and [Phen] at low [Phen] concentration. The rate of the reaction in determined from the slopes of absorbances versus time plots. As [Phen] increases the rate of the reaction increases, passes through a maximum and them falls, suggesting that the course of the reaction is different at low [Phen] and high [Phen]. The effect of temperat ure and pH on the initial rate have also been explained and studied. The repetitive spectral scans is also provided as an evidence for exchange of cyanide ions by [Phen] in [Fe(CN)6]4-. Activation parameters have also been evaluated and provided in support of
the proposed mechanistic scheme. The compositon of the complex was established as 1:1 by the mole ratio method.
Keywords: Nitrogen heterocycle, Phenathroline, hexacyanoferrate (II), ligand substitution, kinetics.
—————————— ——————————
Phenanthroline (Scheme 1) is a heterocyclic organic com- pound. As a bidentate ligand in co-ordination chemistry, it forms strong complexes with most metal ions. It is used in transition metal chemistry for determination of metals, as an indicator for alkyl lithium reagents. It is used in metallocene industry and co-ordination of organometallic complexes. Complexes of the type [Fe (CN)5 INH3-] [1] and [Fe (CN)5
PhNHNH2]3- [2] have been obtained either through photo- chemical aquation of [Fe (CN)6]4- or by Hg+2 assisted com- plexes of the type Fe (CN)5 L (L= N3- , Ph NO, amines) have been prepared by substitution in pentacyano amino ferrate (II) or mercury catalysed substitution in hexacyanoferrate (II) mo- lecular complexes of Pyrazine (Pz) and [Ru (CN)6]4- having molecular formula [Ru (CN)5 Pz]3- [7] and N-methyl Pyrazine and [Fe (CN)6]4- [8] have been reported.The thermal decom- position of hexacyanoferrate (II) ion is a slow reversible pro- cess according to equation 1. The pentacyanoaquo complex produced has been reported [9] to react with aromatic nitroso compounds giving intensely coloured products. There in lim- ited information on the kinetics and mechanism concerning substitution in hexacyanoferrate (II) [10-13]. Exchange of la- belled cyanide between [Fe (CN)6]4- and free cyanide is ex- tremely slow, but under U.V. light reversible aquation takes place [14].
————————————————
Author name is Anupma Singh, currently pursuing Ph. D. degree program in Chemistry from Institute of Engineerig & Technology, constitute college of G.B.T.U., Lucknow.
The Co-author name is Isha Rizvi, Currently persuing Ph. D. from Insti- tute of Engineering & Technology under the supervision of Prof. Amarika Singh, Dean, constituent college of G. B. T. U., Lucknow.
Most of the substituted cyano complexes of lron (II) are metal assisted dissociation of hexacyanoferrate (II) [11, 15] followed by reaction with the incoming ligand. Many complexes of the pentacyano (ligand.) ferrate (II) type have been prepared by substitution in pentacyano (amino) ferrate (II) or by metal cat- alysed substitution in hexacyanoferrate (II) [2,8,11,12,16-21].
In accordance with our earlier investigation of the reaction of phenyl hydrazine [1] and pyrazine [7] with hexacyanoferrate (II), phenonthroline has also been shown to react with [Fe (CN)6]4- according to Equations [1-3].![]() Slow (1)
Slow (1) ![]() (2)
(2)
![]() (3)
(3)
The uncatalysed reaction takes about 24 hours to attain maxi- mum absorbance. The stoichiometry of the complex has been established as 1:1 by the mole ratio [22] and slope method [23].
Effect of [Fe (CN)6]4- on the initial rate of unanalyzed reaction
between[Fe (CN)64-] and Phenanthroline was studied taking
[Fe (CN)64-] = (1.0-8.0) x 10-3M. The reaction was found to ex-
hibit first order behavior in [Fe (CN)64-] in the concentration range studied. The plot of initial rate (Vi) versus [Fe (CN)64-] in a straight line as shown in Figure 1 (r20.995,sd0.223).
The effect of [Phen] on the initial rate for uncatalysed ligand
IJSER © 2013
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 300
ISSN 2229-5518
substitution reaction between [Fe (CN)64-] and [Phen] was
studied by varying Phenanthroline concentration from 0.4x10-
3M to 14.0x10-3M. The plot of initial rate versus [Phen] is
shown in Figure 2. It is seen that the initial rate increases with
increasing the concentration of phenanthroline. It rises till and remains constant between 9x10-3M to 12x10-3M and then starts declining at further increase in [Phen].
The dependence of initial rate on pH in co-ordinated cyanide substitution from [Fe (CN)64-] by [Phen] was studied in the pH vange 1.5-7.0. The initial rate is represented in Table 1. In the beginning i.e. pH range 1.5-3.0 the rate increases and at still higher pH the rate decreases. This behavior shows the exist- ence of protonated and deprotonated forms of [Fe (CN)6] 4- and [Phen], all having different reactivities. From species distribu- tion of [Fe (CN)64-] shown in Figure 3, it can be seen that in the lower pH range there exist mono, di, tri and tetra protonated species of [Fe (CN)64-]. The initial increase in the rate between pH 1.5 – 3.0 is due to inter conversions of highly protonated forms of hexacyanoferrate (II) into lower protonated forms. The rate law for the pH range 3.0-7.0 can be expressed accord- ing to equation (4) where deprotonated forms of [Fe (CN)64-] and [Phen], [PhenH]+ or both are prominent reacting species.
![]() (4)
(4)
Here subscript 'T' refers to the total concentrations of the react-
ing species and kf (kf= Rate / [Fe (CN)64-] [Phen] ) in the for-![]()
ward bimolecular rate constant. In the above mentioned pH region [Fe (CN)64-] exists predominantly in deprotonated form and phenanthroline exists as Phen and PhenH+, Therefore equation (4) can be transformed to equation (5).
The effect of temperature on the initial rate of the unanalyzed
reaction between hexacyanoferrate (II) and Phenanthroline was studied at different temperatures ranging from 25-450C and the activation parameters were calculated by Arrhenius and Erying equations. The value of Ea, H and S are
55.74.8KJmol -1, 53.63.6KJmol-1 and–147.25.1JKmol-1 respec-
tively. Higher temperatures were avoided due to decomposi- tion of the complex [Fe (CN)5 Phen]3-. The evaluated activation parameters show the dissociation type mechanism. The high value of Ea shows the rupture of metal ligand bond, which supports the proposed mechanism.
The effect of ionic strength on the rate of the uncatalysed reac- tion between [Fe (CN)64-] and [Phen] was studied in the range
0.01-0.3 M using KNO3. The initial rate of the reaction was
found to decrease with increase in ionic strength of the medi-
um that indicates a negative salt effect. The observed rate de- pendence on the reactants, temperature, pH and lonic strength allowed us to propose the following mechanism (Scheme 2).
From equation (4) and (5) we get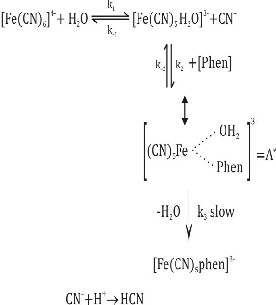
(5)![]()
(6)
Here, ![]() , subscript 'f' denotes the concentration of Phenanthroline in the unprotonated form, kPhen, kPhenH+ and KPhenH+ refers to the rate constants due to reac-
, subscript 'f' denotes the concentration of Phenanthroline in the unprotonated form, kPhen, kPhenH+ and KPhenH+ refers to the rate constants due to reac-
Scheme 2
The rate law based on the above scheme can be expressed by equation (7)
![]() (7)
(7)
tions of phen and PhenH+ and protonation constant of Phe-
If k-2 >>k3, the formation of [A]
is due to a fast equilibrium and
nanthroline respectively. The plot of the left hand side of equa- tion (6) versus [H+] yields a straight line (Figure 4). r2 0.9952 and its slope and intercept give the value of kPhenH+=37.85.50 mol-1Ls-1 and kPhen= 58.95.12 mol-1 Ls-1. The values resolved rate constants are in fairly good agreement with the experi- mentally observed values at high and low pH values respec- tively (Table 1).
for the condition where, [Fe (CN)5H2O]3- < [Phen], the equilib-
rium concentration of activated complex [A] (or ion pair) is
evaluated by using the following algebraic manipulation and finally given by equation (8).![]()
![]()
Or
IJSER © 2013
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 301
ISSN 2229-5518
Where ![]()
And 'f' refer to the free concentration of [Fe (CN)5H2O]3-,
Or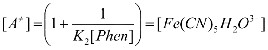
Or![]()
![]()
Or
(8)
Applying steady state approximation to the intermediate [Fe
(CN)5H2O]3-, one gets
![]() (9) Substituting equation (9) in equation (8) we get
(9) Substituting equation (9) in equation (8) we get
![]() (10) Considering equations (9) and (10), the rate law given is ex- pressed as equation (11)
(10) Considering equations (9) and (10), the rate law given is ex- pressed as equation (11)
![]() (11)
(11)
If in Equation (11) k2 and k1 are comparable (both are fast), the following two conditions may exist. (i) At low [Phen], k-1 [CN-]
> k2 [Phen]. The second term of denominator in equation (11) is greater than the third term and second term comprises mul- tiple and small concentration terms. Thus, both can be ne- glected in comparsion to k-1 [CN-]. Therefore, Equation (11) is reduced to equation (12). ![]() (12)
(12)![]()
Equation (12) shows an increase in rate with increase in [Phen]; (ii) At high [Phen], k2 [Phen] >> k-1 [CN-]. This implies that the third term in the denominator in Equation (11) is greater than the first and second terms and consequently the rate law can be expressed by equation (13)
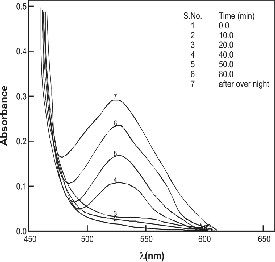
served experimentally (Figure 2). Finally, the repetitive spec- tral scan (Figure 5) of the unanalyzed substitution reaction between [Fe (CN)64-] and [Phen] have been recorded at defi- nite times in the wavelength range 450 to 650 nm under the specified reaction conditions in order to understand the nature of the product formed during the course of the reaction and provide better understanding of the proposed mechanism. The repetitive spectral scan (Figure5) shows that a peak con- tinuously grows with time at 528 nm. This is attributed to the formation of the final product, [Fe (CN)5Phen]3- during the course of the reaction. The mole ratio studies performed by us show the formation of a 1:1 complex, [Fe (CN)5Phen]3- as the final product of the reaction, as shown by other researchers [25].
Double distilled water was used throughout to prepare all solutions. All the chemicals used in this study were of analyti- cal reagent grade. The solution of K4[Fe(CN)6]. 3H2O (AR,E. Merck, Germany) was prepared by weighing a desired amount and kept in dark in coloured bottles to prevent photo- chemical dissociation. The fresh solutions of Phenanthroline (S.D. Fine Chemicals) were prepared in double distilled water everyday before starting the kinetic run. due to its. (A.R. Glaxo) was used to maintain ionic strength in reaction medi- um. The pH of the reaction mixture was adjusted to a desired value using buffer solutions prepared by adding KCl to HCl or potassium hydrogen phthalate (A.R. Qualigens Fine chemi- cals) and hydrochloric acid /NaOH as reported in the litera- ture [24].
All the reactant solutions were equilibrated at 25oC by keeping them inside environmental chamber in order to maintain the desired temperature within the accuracy of ± 0.1oC Genesis model No. 10UV was used to record the spectra of the reac- tion. The pH measurements were made on a digital pH meter of Sio-global pH meter. After equilibrating the reactants, 2 ml of each reactant was mixed in the sequence, Phenanthroline and buffer. The reaction was finally started by adding 2 ml of hexacyanoferrate (II) to the above mixture. This reaction mix- ture was shaken thoroughly and quickly transferred to a 10 mm cuvette in the temperature controlled cell compartment of the spectrophotometer. The progress of the reaction was sub- sequently followed by monitoring the increase in absorbance at 528 n.m. due to formation of the product [Fe(CN)4Phen]3- (Figure 5).
(13)
In terms of equation (13), we expect a decrease in the rate of formation of product with increase in [Phen], which was ob-
IJSER © 2013
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 302
ISSN 2229-5518
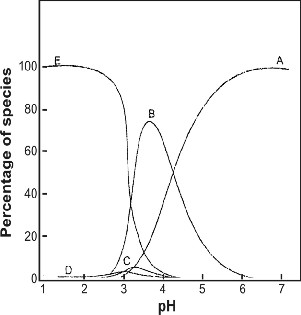
Figure 1 Plot of initial rate of the uncatalysed ligand exchange reaction versus [Fe(CN)6]4- at [Phen] = 2.5x10-3 M, pH =
3.00.02, Temp. =250.10C, I = 0.03M (KNO3).
ligand exchange Reaction between [Fe(CN)6]4- and [Phen] at
[Fe(CN)6]4- =5.0x10-3 M, pH = 3.00.02, temp. = 250.10C, I = 0.03
M (KNO3).
and [Phen H]+
with [Fe(CN)6]4-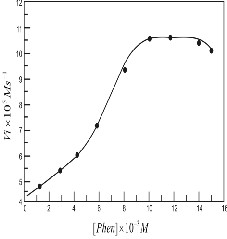
Figure 3 Species distribution of [Fe(CN)6]4- as a function of pH, A= [Fe(CN)6]4- B=H [Fe(CN)6]3-, C= H2 [Fe(CN)6]2-, D= H3 [Fe(CN)6]- and E = H4 [Fe(CN)6].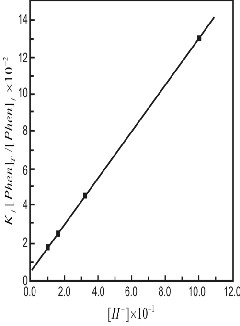
Figure 5 A repetitive spectral scan of the reaction mixture during a typical kinetic run at [Fe(CN)6]4- = 4.5x10-3 M, [Phen]=
2.0x10-4 M, pH = 3.00.02, temperature = 250.10C, I = 0.03 M
(KNO3).
IJSER © 2013
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 303
ISSN 2229-5518
![]()
Table 1 Dependence of initial rate on pH under conditions [Fe (CN)64-] = 3x10-3M, [Phen] = 2.5x10-3M, temp. = 25 0.10C, I = 0.03M (KNO3)![]()
5 -1
![]()
PH Vi x 10 M sec
![]()
![]()
![]()
![]()
![]()
This kinectic study of hexacyanoferrate (II) was not investigat- ed ever before it with N-containing heterocyclic ligand i.e. phenanthroline. All the datas indicate that the reaction is a first order type and substitution was taken place by phenan- throline ligand which relaces cyano ligand by following first order kinetics.
The authors are thankfull to Director, Institute of Engineering
& Technology, Constituent college of G. B.T. U., Lucknow, for
providing necessary facility for research work.
[1] Naik, Radhey Mohan;Tiwari, R.K.; Yadav, S.B.S. Singh, P.K.; Agarwal, A.
Progress in Reaction Kinetics and Mechanism 2009, 34, 211.
[2] Naik, R.K.; Tiwari, R.K.; Singh, P.K.; Verma, A.K. Inorganic Reaction Mecha- nism 2007, 6, 217.
[3] Legros, J.J Chem Phys 1964, 61, 217.
[4] Kenney, D.J.; Flynn, T.P.; Calline, J.B. J Inorg Nucl Chem 1965, 20, 75. [5] Dupleseis, J.; Legros, J; Emischwiller, G. Compt. Rend 1971, C 273,452. [6] Asperger, S.; Pavlovic, D. J Chem Soc 1955, 1449.
[7] Naik Radhey, M; Verma Amit K.; Agarwal Abhivav; Asthana Abhas Transi-
tion Metal Chem 2009, 34, 209.
[8] Prasad Surendra Transition Met Chem 2003, 28, 1. [9] Bandisch, Ber 1929, 62, 2706.
[10] Zmikie, A; Cvitila, D.; Pavloic, D.; Murati, I; Reynolds, W.; As perger, S. J Chem Soc Dalton Trans 1973, 1284.
[11] Feng, Y.L.; Narasaki, (II); Tian, L.C; Wu, S.M.; Chen, (II). Y. Anal Sci 1999, 15,
915.
[12] Prasad, S.; Nigam, P.C. Indian J Enviv Protect 1989, 9, 113. [13] Phull, M; Nigam, P.C. Talanta 1981, 28, 519.
[14] Kuhn, D.D.;Young, T.C. Chemosphere 2005, 60, 1222.
[15] Sicilia, D; Rubio, S.; Perez-Bendito. D. Talanta 1991, 38, 1147. [16] Qin, W.; Guohua. C.; Jinhu. V.; Bin, D. Anal Lett 2003, 36, 627.
[17] Reddy, V.K.; Chenniaiah, A.; Reddy, P.R.; Reddy, T.S. Chem Anal (Warsaw).
Pol.) 2003, 48, 733.
[18] Naik, R.M.; Tiwari, R.K.; Singh, P.K.; Yadav, S.B.S. Int J Chem Kinet 2007, 39,
447.
[19] Naik, R.M.; Sarkar, J; Chaturvedi, D.D. Int J Chem Kinet 2005, 37, 22.
[20] Naik, R.M.; Tiwari, R.K.; Singh, P.K.; Tiwari, A.; Prasad,S. Trans Met cham
2005, 30, 968.
[21] Foretic, B.; Burger, N.; Hankonyi, V. Polyhedron 1995, 14, 605. [22] Job. P.; Ann Chem 1928, 9, 133
[23] Williard, M.H.; Merritt, (Jr.) L.L.; Dean, J.A. Instrumental Methods of Analysis; Litton Edu.; Pub Inc: N.Y., 1977, pp 121.
[24] Weast, R.C. CRC Handbook of chemistry and Physics; The chemical Rubber
Co. 49th edn., Ohio, 1969; pp. D.79.
[25] Sousa, J.R; D: ogcnes, 1. C.N.; Carvalho, I.M.M; Temperini, M.l.; Tanaka, A.A.; Moreira, I.S. Dalton Trans 2003, 11, 2231.
IJSER © 2013