International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012 1
ISSN 2229-5518
Treatment of Distillery Spentwash Using AFBBR and Color Removal of Treated Spentwash Using Adsorbtion
Lakshmikanth R, Arjun S Virupakshi
Abstract - Spentwash was pretreated with anaerobic floating bed baffled wall reactor (AFBBR) with a capacity of 4.5 litters. Reactor used to obtain a treated spentwash on which adsorption experiment was carried out. Adsorbent used was activated Bagasse. Its adsorptive proper ties were determined by using standard Melanoidin solution which was synthesized in laboratory. Adsorbents showed a best fit for Langmuir isotherm undergoing monolayer. A 10 g dosage and 90 min contact time was chosen as optimum value. Rector performed well and shown 69 % efficiency in removing COD at 2000 mg/L COD loading rate at 24 hr of HRT. Adsorption experiment had shown 40% efficiency in reducing melanoidin concentration and 68.9 % efficiency in reduction of color from treated spent wash. Combined treatment of spentwash in anaerobic floating bed baffled wall reactor and adsorption resulted in 81
% efficiency in treating COD load at 2000 mg/L COD loading rate at 24 hr of HRT.
Index Terms- Distillery, Spentwash, Melanoidin, Adsorption, Reactor, Bagasse.
————————————————————
1 INTRODUCTION
HEworld’s total water sources are estimated to be 1.36
× 108 Μ ha-m. Among these water sources, about 97.2%
is saline water and mainly available in oceans, and only
2.8% is available as fresh water. Of these 2.8% of fresh water, about 2.2% is available as surface water and 0.6% as ground water. Out of this 2.2% of surface water, 2.15% is fresh water in glaciers and icecaps and only of the order of
0.01% is available in lakes and streams, the remaining 0.04% being in other forms. Out of 0.6% of stored ground water, only about 0.25% can be economically extracted with the present technology [1].
Sources of wastewater in a distillery are stillage, fermenter wastewater known as a spent wash, fermenter and condenser cooling water. The spent wash is a potential water pollutant, because of its highly colored nature and unpleasant odor. It blocks sunlight from penetration into rivers and streams, thus reducing oxygenation of the water by photosynthesis and hence becomes detrimental to aquatic life. And it has a high pollution load which would result in eutrophication of contaminated water courses [2].

Lakshmikanth R is currently pursuing masters degree program in environmental engineering in KLES College of Engineering and Technology, Belgaum, Karnataka, India, PH- +91 97 1196 1726. E-mail: jungle3rlk@gmail.com
Arjun S Virupakshi is Asst. Prof. in Dept. of Civil Engineering, KLES College of Engineering and Technology, Belgaum, Karnataka, India, PH- +91 99 7200 8555. E-mail: arjunvirupakshi@gmail.com
Spentwash from a distillery industry has a large amount of a brown pigment. The color is barely degraded by the conventional treatments methods and even it may become darker during anaerobic treatments, due to re- polymerization of compounds. The color causing pigments are Melanoidins from Maillard reaction of sugars with proteins, Phenolics from the feedstock, Caramels from overheated sugars, and Furfurals from acid hydrolysis. Among the four above mentioned color causing pigments, the major contribution to the color is by Melanoidins and these are having anti-oxidant properties causing toxicity to many microorganisms involved in wastewater treatment processes [2].
A lab-scale anaerobic hybrid reactor was operated in a continuous mode to study anaerobic biodegradation of distillery spent wash. The study demonstrated that at optimum hydraulic retention time (HRT), 5 days and organic loading rate (OLR), 8.7 kg COD/m3.d, the COD removal efficiency of the reactor was 79% [3]. Reactor having coconut coir could treat distillery spent wash at 8 d hydraulic retention time with organic loading rate of 23.25 kg COD m−3 d−1 leading to 64% COD reduction with biogas production of 7.2 m3 m−3 d−1 having high methane yield. [4]. The reactor was started with an OLR of 0.5 g COD/L. d. and was loaded up to 16 g COD/L. d. During startup the HRT was reduced from 48 - 8 h. The optimum HRT was found as
6 h. After the startup the loading was increased at constant HRT of 6 h by increasing the COD concentration of the feed. A maximum COD removal efficiency of 89.4% was achieved. The COD removal rate linearly increased with increase in OLR [5].
IJSER © 2012
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012 2
ISSN 2229-5518
Degradation of Melanoidins and caramel was confirmed
by UV and FTIR spectral analysis. Decrease in OD of Melanoidins and caramel at their max and appearance of new peaks and changes in functional groups of compounds in the IR spectra with respect to control suggest their degradation. K1 was the best Melanoidins (77%) as well as caramel (54%) degrader followed by other three isolates (Ku3, Rtb2 and EB4) [6]. Batch adsorption of recalcitrant melanoidin using the abundantly available coal fly ash was carried out. It had low specific surface area (SBET) of 1.7287 m2/g and pore volume of 0.002245 cm3/g. Maximum color removal was achieved around pH 6, whereas increasing sorbent mass from 10g/L to 200 g/L enhanced color reduction from 25% to 86% at 298 K [7]. 19 carbon samples prepared by acid and thermal activation of various agro- residues viz. bagasse, bagasse Xyash, sawdust, wood ash and rice husk ash for color removal from biomethanated distillery eZuent. Phosphoric acid carbonized Bagasse B (PH) showed the maximum color removal (50%) [8].
2 MATERIALS AND METHODOLOGY
2.1 Construction of AFBBR
Acrylic sheet was used in fabrication of AFBBR. Acrylic sheet was cut in different pieces, each matching the required size. Cut pieces of acrylic sheet was joined and water sealed using a market available bonding solutions. Design arrangements are as follows,
a) Rector was constructed with inner dimensions of 230 mm height, 200 mm length and 150 mm width. Such that the effective volume of the reactor is 4.5 Liters.
b) Rector was separated into two chambers by providing a baffled wall at a distance of 150 mm from the inner inlet.
c) First chamber was filled with poly propylene pall rings, which floats when spent wash is filled and provides a surface for the microbe attachment to facilitate microbial activity.
d) Second chamber acts as a settling zone. It provides comparatively an additional time for the sludge to settle from the treated spent wash.
e) Top portion of the reactor was made tapered towards the baffled wall end up to 200 mm height from the bottom of reactor, to concentrate generated gas to gas outlet.
f) Inlet was kept 10 mm below the outlet, providing a
30 mm free board above the wastewater level. Such as to make water seal, to trap back entry of air into the reactor. Two sludge outlets are provided at bottom most possible point at each opposite face of wall
g) Feed tank made up of poly propylene, was kept such
that to maintain 2 feet head and connected to inlet of
the reactor. Spent wash flow into the reactor was
controlled by provided gunmetal valve.
h) Outlet was connected to water seal arrangement made using CPVC fittings, further lead to the rector effluent collection tank made up of poly propylene, via hose pipe.
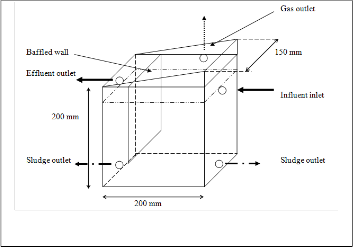
i) A gas nozzle was provided at the top most part of the reactor. It is connected to a hose pipe which at the other end was immersed in a measuring jar containing water. Such that the volume of gas generated will be equal to the volume of water displaced from the measuring jar.
Fig 1: Schematic diagram of AFBBR
2.2 Working principle of AFBBR
a) Anaerobic condition was maintained in rector, as anaerobic treatment is suggestive for the spentwash [2].
b) An air tight rectangular tank was fabricated using acrylic sheet, providing an underflow baffled wall. It helps in sludge settling, before spent wash leaves the reactor [9].
c) Poly propylene pall rings are used as floating bed. It provides a surface for the microbial attachment and growth [10]. Because of its high surface to volume ration it provides maximum surface area for the microbial activity by consuming a lesser volume in the reactor.
d) The whole study was conducted in ambient environment at temperature 280 C - 300 C.
e) Cow dung was used as a source of microbes for the acclimatization.
f) Diluted spent wash was used with the initial COD concentration of 1000 mg/L and further on with increment of COD concentration of 1000 mg/L for every different loading rate.
g) Optimum HRT was established after conducting
several trials varying hydraulic retention times.
IJSER © 2012
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012 3
ISSN 2229-5518
h) Effluent samples were analyzed after every optimum
HRT interval.
i) Chemical parameters of the sample were analyzed in the laboratory using the guidelines from the “Standard Methods for the examination of Water and Wastewater”, published by the American Public Health Association and American Water Works Association [11].
2.3 Adsorption experiment preparations
a) Adsorbent was prepared using Bagasse. Raw Bagasse was collected from the same industry where spentwash collected. Bagasse was converted to activated carbon using acid activation method as explained bellow,
b) Bagasse was earlier dried and sieved to obtain a fraction of 425 µm.
c) Activation carried out with phosphoric acid in a ratio
of 4:3.
d) The acidified mixture was kept in an air oven maintained at 150±50 C for 12 hr.
e) The resulting char was washed with distilled water and then soaked overnight in 1% sodium carbonate solution to remove the residual acid.
f) The soaked material was again washed with distilled water the following day.
g) Final material which is activated carbon is dried at
1050 C for 24 hr before conducting any adsorption experiment [8].
Melanoidins solution was prepared by mixing 4.5 g of glucose, 1.88 g of glycine and 0.42 g of sodium bicarbonate with 100 ml distilled water and then heated for 7 hr at 950
C. After heating, 100 ml of distilled water was added [7]. The prepared solution had an initial chemical oxygen demand value of 21000 mg/L from which diluted solutions were prepared for 1000, 2000, 3000, 4000, 5000 and 6000 mg/L. The solution pH was adjusted by adding 0.1 M NaOH and 0.1 M NaCl solutions.
2.4 Adsorption experiment procedure
a) Standard melanoidin solution was diluted to 1000 mg/L concentration.
b) 16 conical flasks were taken and each flash was filled with 100 ml of above mentioned diluted sample. Flasks were labeled from 1 to 16. 16 conical flasks are divided into 4 batches. Each batch was having 4 conical flasks.
c) In every batch 2.5g, 5g, 7.5g and 10g activated bagasse were added to the conical flasks.
d) 1st batch was agitated for 30 min, 2nd batch was agitated for 60 min, 3rd batch was agitated for 90 min and 4th batch was agitated for 120 min.
e) After providing an above mentioned contact time
solutions from each conical flask were filtered using Whatman 40 filter paper and the absorbance of the solutions was determined at wavelength 475 nm using UV-Visible spectrophotometer.
f) Optimum dosing and contact time obtained from this were used in the further studies.
Percentage color removal (Rt) was calculated using the formula,
Rt = (Abs0 - AbsF) x 100 / Abs0 (1)
Where, Abs0 is the initial absorbance while Absf is the final absorbance
Melanoidins uptake (qe) was calculated by,
qe = (C0 - CE) x V / m (2)
Where, qe is the specific uptake in mg/g at equilibrium, C0 and CE are the initial and final concentration in mg/L respectively, V the volume in liters of Melanoidins solution and m is the mass of adsorbent.
E. Adsorption experiment on Treated spent wash
a) 100 ml treated spentwash from AFBBR was taken in conical flask.
b) 10 g activated bagasse was added to the conical flask and agitated for 90 min.
c) Solution was filtered with Whatman 40 filter paper and the absorbance of the solutions was determined at wavelength 475 nm using UV-Visible spectrophotometer.
d) Experiments were repeated till constant readings were obtained.
3. RESULTS AND DISCUSSION
3.1 Performance of rector in COD reduction
From the observations and results it can be inferred that, AFBBR is effective in treating distillery spent wash and it has shown 69 % COD reduction at 2000 mg/L COD loading rate at 24 hr of HRT. It is capable of treating spent wash up to 50 % for COD loading rate of 5000 mg/L. Reactor performance deteriorated at 6000 mg/L COD loading rate (Figure 2).
3.2 Performance of rector in BOD reduction
BOD value of treated effluent was determined at optimum COD reduction state. It was found that rector was efficient in reducing BOD up to 68 % at optimum loading rate i.e. 2000 mg/L COD. There was reduction in BOD up to
40 % at 6000 mg/L COD(Figure 3).
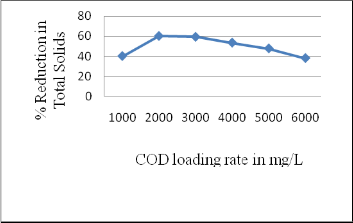
3.3 Performance of rector in Total Solids reduction
Total Solids value of treated effluent was determined at optimum COD reduction state. It was found that rector was efficient in reducing Total Solids up to 60 % at optimum loading rate i.e. 2000 mg/L COD. There was a reduction
IJSER © 2012
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012 4
ISSN 2229-5518
upto 38 % in Total solids at COD loading rate of 6000
mg/L(Figure 4).
3.4 Performance of rector in Dissolved Solids reduction
Dissolved Solids value of treated effluent was determined at optimum COD reduction state. It has found that rector was efficient in reducing Dissolved Solids upto 62 % at optimum loading rate i.e. 2000 mg /L COD. There was a reduction upto 36 % in Dissolved solids at COD loading rate of 6000 mg/L(Figure 5).
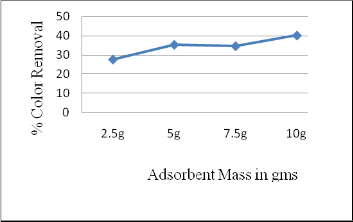
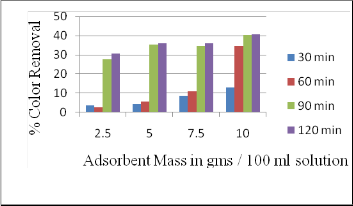
3.5 Effect of Adsorbent Mass
The effect of adsorbent on color removal was investigated for the melanoidin solution concentration of 1000 mg/L with contact time 90 min. It can be inferred that increase in adsorbent mass increases the color removal. Beyond the 10 g, solution will turn into slurry and condition becomes inconvenient for the study. At dosing 10 g color removal was up to 40 %, thus for the further study 10 g dosing was opted(Figure 6).
Fig 4: Overall performance of AFBBR in Total Solids reduction
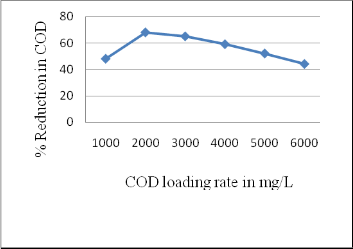
Fig 5: Overall performance of AFBBR in Dissolved Solids
reduction
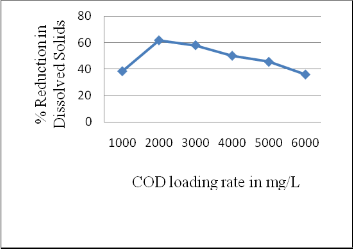
Fig 2: Overall performance of AFBBR in COD reduction at
different loading rate
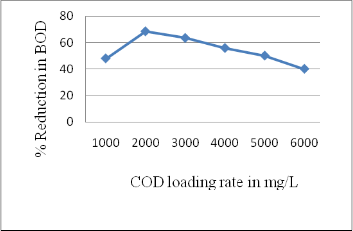
Fig. 6: Effect of adsorbent Mass on color reduction
Fig 3: Overall performance of AFBBR in BOD reduction
IJSER © 2012
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012 5
ISSN 2229-5518
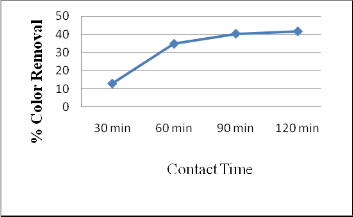
3.6 Effect of Contact Time
Fig. 7: Effect of Contact Time on color reduction
The effect of adsorbent on color removal was investigated for the solution concentration of 1000 mg/L with dosing 10 g. It can be inferred that increase in contact time increases the color removal. For 90 min and 120 min, percentage removal value is 40 % and 40.5 % respectively. This is because; most of the adsorptive site has been occupied within 90 min contact time. Thus 90 min contact time is opted for further studies.
3.7 Determination of optimum dosage and contact time
Optimum adsorbent dosage and effective contact time was determined using 1000 mg/L concentration standard melanoidin solution. An adsorbent dosed were 2.5 g, 5 g,
7.5 g and 10 g. Each dosage was given a contact time of 30 min, 60 min, 90 min and 120 min. Following are the results obtained from the study.
From the below chart it can be inferred that optimum dosing is 10 g with contact time 90 min. While for the 10 g dosing with 120 min contact time there was not much considerable removal. Hence in further studies 10 g adsorbent dosage with 90 min contact time was opted.
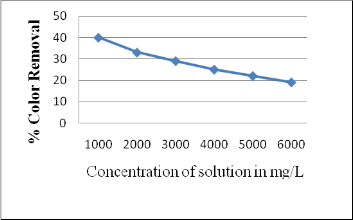
3.8 Effect of Initial Concentration of Solution
Fig
. 9: Effect of Concentration of solution on color reduction
The effect of concentration of solution on color removal was investigated for constant contact time 90 min and dosing 10 g. It can be inferred from the graph that, increase in solution concentration decreases the color removal (40 % for 1000 mg/L concentration to 19 % for 6000 mg/L concentration). This is due to saturation of adsorption sites at higher concentrations.
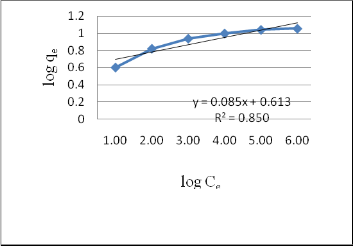
3.9Freundlich Adsorption Isotherm
Fig. 10: Freundlich Isotherm for Melanoidins adsorption on
Activated Bagasse
The well knownFreundlich isotherm is often used for
heterogeneous surface energy systems and is represented by the equation,
Fig. 8: Effect of adsorbent Mass on color reduction
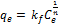
(3)
Where qe (mg/g) is the equilibrium sorption capacity, kf (L/g) is Freundlich constant indicative of sorption capacity, Ce (mg/L) is the equilibrium concentration and n is the Freundlich constant indicative of adsorption intensity [7].
Table 1 shows the Freundlich parameters for activated
Bagasse. The isotherm model does not represent the best fit
IJSER © 2012
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012 6
ISSN 2229-5518
to experimental data (R2< 0.95). But the values of constants are nearer to the values of study conducted by Y. Satyawali et al., 2007 and V. O. Ojijo et al., 2010.
Table 1: Freundlich Constants for Melanoidins adsorption
3.10Langmuir Adsorption Isotherm
3.11 Adsorption of Treated Spentwash using activated
Bagasse
Results obtained from adsorption experiments and AFBBR studies were used in the study of color removal on treated distillery spentwash. From the results of adsorption experiment it was found that 10 g dosage with 90 min contact time is more efficient in reducing the color. And from the AFBBR studies it was found that, rector performed efficiently at 2000 mg/L COD loading rate at 24 hr HRT. Considering the above values for the adsorption of treated spentwash, 10 g activated Bagasse was used on treated spentwash of 2000 mg/L COD loading rate. This resulted following data represented in table 4.5. Values were incorporated after several trails.
Table 3: Activated Bagasse adsorption results
Sl. No. | Parameter | Initial Conc. | Final Conc. | % reduction |
1. | COD in mg/L | 620 | 380 | 38.7 |
2. | Color in absorbance | 0.293 | 0.091 | 68.9 |

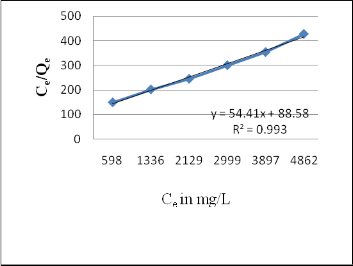
Fig. 11: Langmuir Isotherm for Melanoidins adsorption on
Activated Bagasse
Langmuir isotherm model assumes a number of factors:
monolayer sorption on a set of distinct localized sorption sites; no interaction between adsorbed species; all sites are energetically equivalent; the adsorbent is structurally homogeneous among others and is represented by,
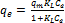 (4)
(4)
Where qe (mg/g) is Melanoidins uptake at equilibrium, qm(mg/g) is the Langmuir maximum sorption capacity, KL (L/mg) is Langmuir adsorption constant and Ce (mg/L) is the concentration at equilibrium [7].
Table 2 shows the Langmuir parameters for activated Bagasse. Langmuir isotherm represent the best fit to experimental data (R2>0.95). But the values of constants are less while comparing to the study conducted by Y. Satyawali et al., 2007 and V. O. Ojijo et al., 2010, this is because of the less adsorptive capacity of the activated Bagasse.
Table 2: Langmuir Constants for Melanoidins adsorption
BEFORE AFTER
Fig. 12: Color comparison of treated spentwash before and after adsorption.
The experimental data obtained from adsorption by activated Bagasse it can be seen that, color removal was achieved up to 68.9 %. This can be visually stated as color was turned from brown to light brown.
Further, it was observed that, there was further reduction in COD concentration which was up to 38.7 %, thus it can be inferred that use of activated Bagasse in combination with AFBBR resulted in total 81 % (Resulting value of Initial COD concentration before treatment and final concentration after treatment in AFBBR and Adsorption) COD reduction.
IJSER © 2012
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 11, November-2012 7
ISSN 2229-5518
4 CONCLUSIONS
A. Anaerobic Floating Bed Baffled wall Reactor which was used for the treatment of distillery spent was efficient in treatment at organic loading rate of 2000 mg/L of COD at 24 hr of HRT. At this organic loading rate, it performed optimally by reducing 69 % COD, 60
% Total Solids, 62 % Dissolved Solids and 68% BOD.
B. AFBBR performed economically by reducing up to 50
% COD concentration till the organic loading rate was
5000 mg COD/L at 24 hr HRT.
C. Performance of AFBBR deteriorated below 50 % from the organic loading rate 6000 mg/L of COD at 24 hr of HRT.
D. Adsorption experiment conducted on synthetic Melanoidins solution show best fit for Langmuir Isotherm undergoing monolayer adsorption with a maximum adsorption capacity of 0.0188 mg/g.
E. Activated Bagasse was effective in removing Melanoidins up to 40 % and color in treated spent wash up to 68.9 %. Thus this can be used in distillery spent wash treatment, as the Bagasse is readily available at distillery unit and method of activation is economical.
F. Use of activated Bagasse also reduced the COD concentration in the treated spent wash. The combined treatment of spent wash in AFBBR and adsorption yield 81 % reduction in COD concentration.
REFERENCE
[1] H. M. Raghunath, “Hydorlogy; Principle-Analysis-
Design”, New Age International (P) Ltd., Publishers,
2nd Edition, 2006.
[2] Deepak Pant and AlokAdholeya, “Biological
approaches for treatment of distillery wastewater: A
review”, Bioresource Technology, Vol. 98, 2007, pp
2321–2334.
[3] Gupta, Sunil Kumara, Gupta, S. K., Singh, Gurdeep, “Biodegradation of Distillery Spent Wash in Anaerobic Hybrid Reactor”, Water Research, Vol. 41, 2007, pp 721-
730.
[4] Bhavik K. Acharya, SarayuMohana, DattaMadamwar, “Anaerobic treatment of distillery spent wash – A study on upflow anaerobic fixed film bioreactor”, Bioresource Technology, Vol. 99, 2008, pp 4621-4626.
[5] Hampannavar, U.S, Shivayogimath, C.B, “Anaerobic treatment of sugar industry wastewater by Upflow anaerobic sludge blanket reactor at ambient temperature”, International journal of environmental sciences, Vol. 1, 2010, pp 631-639.
[6] NagarajNaik, K.S. Jagadeesh and M.N. Noolvi, “Enhanced Degradation of Melanoidin and Caramel in Biomethanated Distillery Spentwash by Microorganisms Isolated from Mangroves”, Iranica Journal of Energy & Environment, Vol. 1, 2010, pp 347-
351.
[7] V.O. Ojijo, M.S. Onyango, AoyiOchieng and F.A.O.
Otieno, “Decolourization of Melanoidin Containing Wastewater Using South African Coal Fly Ash”, International Journal of Civil and Environmental Engineering, Vol. 2, 2010, pp 17-23.
[8] Y. Satyawali, M. Balakrishnan, “Removal of color from biomethanated distillery Spentwash by treatment with activated carbons”, Bioresource Technology, Vol. 98,
2007, pp 2629-2635.
[9] RamarajBoopathy, “Biological Treatment of Swine Waste Using Anaerobic Baffled Reactors”, Bioresource Technology, Vol. 64, 1998, pp 1-6.
[10] S. CheSheng Chen, Dezhi Sun, Jong-Shik Chung,
“Simultaneous removal of COD and ammonium from landfill leachate using an anaerobic–aerobic moving- bed biofilm reactor system”, Waste Management, Vol.
28, 2008, pp 339-346.
[11] “Standard Methods for Examination of Water and Wastewater”, APHA, AWWA, WPLF, New York, 16th Edition, 1985.
IJSER © 2012
http://www.ijser.org











