
The catalyst is derived from trunk of Musa bulbisiana colla
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 70
ISSN 2229-5518
Transesterification of dimethyl malonate with a
novel catalyst derived from Musa balbisiana colla
Swarnali Pathak and Dibakar Chandra Deka
Abstract— Malonate esters are important synthons that can be transformed into variety of building blocks in organic syntheses. Trunk of Musa balbisiana colla triggered successful transesterification of dimethyl malonate with a series of higher alcohols efficiently. The catalyst is obtained from seedy variety of banana plant Musa balbisiana colla which is popularly known as kolakhar in the Assamese community of the north-eastern region of India and is used as an additive in many traditional cuisines. This work highlights the transesterification of dimethyl malonate with different types of alcohols resulting in the products from moderate to good yields. This newly developed catalyst can be considered as green catalyst as it is heterogeneous, natural, biodegradable, non-toxic, easily obtainable, inexpensive and environmentally safe. We are hopeful that it will contribute a lot in the field of organic synthesis.
Index Terms— Banana plant, Dicarboxylic esters, Dimethyl malonate, Heterogeneous, Kolakhar, Musa balbisiana colla, Transesterification.
—————————— ——————————
n the present scenario, dicarboxylic esters are of great inter- est amongst researchers as they are entirely bio-renewable
plays an important role here as it shows a simple route for the synthesis of more complex products from more easily accessi-
IJSER
and green chemicals that can replace petroleum based sol-
vents [1]. Due to their rich content of oxygen atoms, many
diesters can be blended with fuels. They can be used as an
additive to enhance the BCN value (blended cetane number)
of the fuel and diminishing the probability of particulate mat-
ter emissions [2]. They can play a vital role as intermediates in
the synthesis of fine chemicals, drugs, plasticizers, food pre-
servatives, pharmaceuticals and cosmetics [1]. Malonic acid
and its derivatives like malonic esters can act like platform
molecules as they possess the potential to be transformed into useful building blocks and serve as a valuable tool for the syn- thesis of various complex compounds and pharmaceutics, plasticizers, perfumes etc. [1], [3], [4], [5]. Malonic ester is also
used for the synthesis of carboxylic acid by malonic ester syn- thesis. Petrochemical based production routes to malonates and malonate derived compounds are dependent on nonre- newable feedstock accompanied by deficiency in oxygen con- tent [6]. Conventional procedures for production of dicarbox- ylic ester involve a stirred batch or continuous reactor in pres- ence of H2 SO4 as a homogeneous catalyst. Due to known dis- advantages of the traditional liquid acids (corrosiveness, sepa- ration problem and short life span), much attention has been focused on the development of easily recoverable, recyclable, non-toxic, inexpensive, environmentally benign solid hetero- geneous catalysts with cleaner operations. Transesterification
————————————————
• Swarnali Pathak is currently pursuing Ph. D., Dept. of Chemistry, Gauhati
University, Guwahati, Assam, India. E-mail: swarnaliaims@gmail.com
• Dibakar Chandra Deka is Professor and Head, Dept. of Chemistry, Gauhati
University, Guwahati, Assam, India.
ble compounds [7]. A literature survey shows that malonate
esters have been generally synthesized by transesterification
reaction. Transesterification is an important synthetic process
used as an alternative method to synthesize a large variety of
carboxylic esters [8]. It’s a single pot equilibrium reaction
which gets accelerated in presence of a catalyst. Transesterifi-
cation has wide applications in industrial field as well as in
academic research [9], [10], [11], [12]. Transesterification is
more advantageous than esterification owing to the high sta-
bility and solubility of the esters in most organic solvents, whereas carboxylic acids have often low solubility in organic solvents [13]. Transesterification has tremendous application in biodiesel industries [12], [14], [15], [16], [17], [18], paint in-
dustry and is important in the synthesis of biologically active compounds and drugs [7], [19]. It is an essential part for the synthesis of polyethylene terephthalate [8]. Various esters are used for transesterification reactions but rather few generally applicable methods are known about the transesterification reaction carried out with malonic acid esters.
A number of useful transesterification methods have been reported in the literature, catalyzed by tin oxide-modified mesoporous SBA-15 [20], amino functionalized SBA-15 [21], Mg-Al calcined hydrotalcite [22], aluminophosphate and alu- minophosphate modified with different transition metals ( V, Fe, Co, Ni, Cu) [23], zinc perchlorate hexahydrate [24]. Diffi- cult preparation steps and economic consideration limit the applicability of many heterogeneous catalysts. Here, we wish to report the transesterification of dimethyl malonate cata- lyzed by trunk of Musa balbisiana colla which is environmen- tally safe, non-toxic, heterogeneous, economical which comes at almost zero cost. Post harvesting of banana plant is also free of cost. This catalyst has been successfully applied for bio- diesel production from yellow oleander (Thevetia peruviana) seed oil. Fuel properties conform to standards set for ASTM
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 71
ISSN 2229-5518
D6751, EN14214, BSII and BSIII, and in certain aspects it is found to be better [25].
The catalyst is derived from trunk of Musa bulbisiana colla
O O
atalyst 20% wt. of ester O O
locally known as athia kal, the seedy variety of banana plant
which is considered to be the best as far the quality of kolakhar
C
+ ROH
O O
( )
Ref lux R O O R
is concerned. Kolakhar is obtained from the ash of different parts of banana plant and is a very popular food additive in the north-eastern region. The main objective of this study is to investigate the general applicability of the catalyst for the transesterification of dimethyl malonate with a series of struc- turally varied alcohols.
Dimethyl malonate and amyl alcohol were purchased from Loba Chemie and other alcohols viz., methanol, ethanol, buta- nol, heptanol, benzyl alcohol were purchased from Merck Ltd. Alcohols were dried over Na2 SO4 prior to use. The catalyst was also dried in oven at 120 °C for 2 hours as it is hygroscop- ic in nature and presence of moisture can retard the rate of the reaction.
We followed the traditional procedure for the preparation of
R=Ethyl, Butyl, Amyl, Heptyl, Benzyl
1:20 molar ratio
Fig.1. Transesterification of dimethyl malonate with various alco- hols.
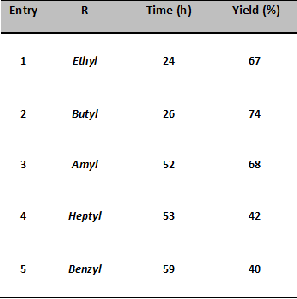
TABLE 1
TRANSESTRIFICATION OF DIMETHYL MALONATE USING CATALYST DERIVED FROM THE TRUNK OF MUSA BALBISIANA COLLA
the catalyst known as kolakhar. Parts of banana plant (Musa balbisiana colla) were cut into pieces and air dried under sun for several weeks. The dry material was burnt into ashes and stored in a plastic container. Chemical and spectroscopic in- vestigation of the catalyst shows the presence of chloride, car- bonate. Major components present are K+, CO3 2-, Na+, Cl- along with other metals viz., Al, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Cd, in trace amount (ppm level)[26].
A 1:20 molar ratio mixture of dimethyl malonate ester and alcohol together with the catalyst derived from trunk of bana- na plant (20% wt. of ester) without a co-solvent were stirred in a two neck round bottomed flask with a magnetic stirrer. Re- actions were carried out under reflux. The progress of the re- action was monitored by TLC. After completion of the reac- tion, the reaction mixture was partitioned between petroleum ether and water. The organic layer was washed with (10%, 10 ml) brine solution and dried over Na2 SO4 . Solvent was re- moved under vacuum and crude product was chromato- graphed on silica gel using light petroleum ether (bp. 40 -60
C) and ethyl acetate as the eluent. Products were identified by IR and NMR. 1H and 13C NMR were recorded in CDCl3 at 300 and 75 MHz, respectively using Bruker Advance III
300MHz/54mm NMR spectrometer. FT-IR spectra were ob- tained on a Perkin Elmer RX I FT –IR spectrometer.
The scheme of the reaction is shown in Fig. 1 and results of various reactions carried out are summarized in Table 1.
All yields are isolated product yields.
1, 3-Diethyl propanedioate (Diethyl malonate): 1H NMR (300 MHz, CDCl3 ): δ 1.255-1.302 (6H, t, CH3 ), 3.358 (2H, s, OOCCH2 COO), 4.169-4.240 (4H, m, -OCH2 -). 13C NMR (75
MHz, CDCl3 ): 14.02, 41.65, 61.47, 166.61. FT-IR (thin film on
KBr, cm-1): 1029.99, 1149.57, 1265.30, 1739.79, 2858.51, 2924.
Dibutyl propanedioate (Dibutyl malonate): 1H NMR (300
MHz, CDCl3 ): δ 0.883-.934 (6H, t, CH3 ), 1.302-1.419 (4H, m, - CH2 -), 1.561-1.653 (4H, m, -CH2 -), 3.345 (2H, s, OOC- CH2 COO), 4.102-4.148 (4H, t, -OCH2 -). 13C NMR (75 MHz, CDCl3 ): 13.61, 18.98, 30.45, 41.66, 65.32, 166.69. FT-IR (thin film
on KBr, cm-1): 1026.13, 1068.56, 1458.18, 1658.78, 1735.93,
2862.36, 2927.94.
Dipentyl propanedioate (Diamyl malonate): 1H NMR (300
MHz, CDCl3 ): δ 0.901 (6H, t, CH3 ), 1.249-1.325 (8H, m, - CH2 CH2 -), 1.625-1.644 (4H, m, -CH2 -), 3.368 (2H, s, OOC- CH2 COO), 4.115-4.157 (4H, t, -OCH2 -). 13C NMR (75 MHz, CDCl3 ): 14.06, 22.21, 27.86, 28.08, 41.65, 65.62, 166.68. FT-IR
(thin film on KBr, cm-1): 1049.28, 1064.71, 1273.02, 1739.79,
2862.36, 2939.52.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 72
ISSN 2229-5518
Diheptyl propanedioate (Diheptyl malonate): 1H NMR (300 MHz, CDCl3 ): δ 0.864-.885 (6H, m, CH3 ), 1.287 (12H, m, - CH2 CH2 CH2 -), 1.596-1.639 (8H, m, -CH2 CH2 -), 3.642 (2H, s, OOCCH2 COO), 4.036-4.080 (4H, t, -OCH2 -). 13C NMR (75
MHz, CDCl3 ): 14.04, 22.58, 25.88, 28.91, 29.10, 31.71, 41.64,
65.38, 174.05. FT-IR (thin film on KBr, cm-1): 1045.42, 1065,
1273.02, 1411.89, 1712.19, 2870.08, 2943.37.
Dibenzyl propanedioate (Dibenzyl malonate): 1H NMR
(300 MHz, CDCl3 ): δ 3.669 (2H, s, OOCCH2 COO), 5.377 (4H, s,
-OCH2 -), 7.266-7.909 (10H, m, C6 H5 ). 13C NMR (75 MHz, CDCl3 ): 40.8, 66.65, 126.94, 127.62, 128.96, and 136.02, 166.49.
FT-IR (thin film on KBr, cm-1): 1454.33, 1735.93, 2962.66, and
3039.81.
In this study, the catalytic activity of the catalyst derived from the trunk of Musa balbisiana colla was explored for the trans- esterification of dimethyl malonate with a variety of higher alcohols. The catalyst shows good activity towards transesteri- fication of dicarboxylic ester to its higher esters especially with butanol. However, with the increase in chain length its activity decreases. This will be a very good option for the transesterifi-
cation of dimethyl malonate with lower chain alcohols. This
[11] Brindaban C. Ranu, Pinak Dutta, and Arunkanti Sarkar, “A Simple and Effi- cient Procedure for Transesterification Catalyzed by Indium Triiodide,” J. Org. Chem., 63, 6027-6028, 1998.
[12] Tanmay Chatterjee, Debasree Saha, Brindaban C. Ranu, “Solvent-free trans- esterification in a ball-mill over alumina surface,” Tetrahedron Letters, 53, 4142-
4144, 2012.
[13] Masazumi Tamura, S. M. A. Hakim Siddiki, and Ken-ichi Shimizu, “CeO2 as a versatile and reusable catalyst for transesterification of esters with alcohols
under solvent-free conditions,” Green chem., 15, 1641-1646, 2013.
[14] Nicholas E. Leadbeater and Lauren M. Stencel, “Fast, Easy Preparation of
Biodiesel Using Microwave Heating,” Energy & Fuels, 20, 2281-2283, 2006.
[15] Mamoru Iso, Baoxue Chen, Masashi Eguchi, Takashi Kudo, Surekha
Shrestha, “Production of biodiesel fuel from triglycerides and alcohol using immobilized lipase,” J. of Molecular catalysis B; Enzymatic, 16, 53-58, 2001.
[16] Dong Fang, Jinming Yang and Changmei Jiao, “Dicationic Ionic Liquids as
Environmentally Benign Catalysts for Biodiesel Synthesis,” ACS catal., 1, 42-
47, 2011.
[17] Karen Wilson, Chris Hardacre, Adam F. Lee, Janine M. Montero and Lee Shellard, “The application of calcined natural dolomitic rock as a solid base catalyst in triglyceride transesterification for biodiesel synthesis,” Green Chem.,
10, 654-659, 2008.
[18] Ambarish Datta and Bijan Kumar Mandal, “Biodiesel Production and its
Emissions and Performance: A Review,” International Journal of Scientific & En- gineering Research, 3, 6, 1-6, 2012.
[19] Puran Singh Rathore, Jacky Advani, Sonika Rathore, Sonal Thakore, “Metal nanoparticles assisted amine catalyzed transesterification under ambient con- ditions,” Journal of Molecular Catalysis A: Chemical, 377, 129-136, 2013.
[20] Pallavi Shah, Arumugamangalam V. Ramaswamy, Karoly Lazar,Veda
Ramaswamy, “Synthesis and characterization of tin oxide-modified mesopo-
IJSER
protocol can build a new synthetic route due to the versatility
and environmentally benign character of catalyst.
[1] Varsha Brahmkhatri and Anjali Patel, “Synthesis and Characterization of 12- Tungstosilicic Acid Anchored to MCM-41 as well as Its Use as Environmen- tally Benign Catalyst for Synthesis of Succinate and Malonate Diesters,” Ind. Eng. Chem. Res., 50, 13693-13702, 2011.
[2] Aikaterini Serdari, Euripides Lois, and Stamoulis Stournas, “Impact of Esters of Mono- and Dicarboxylic Acids on Diesel Fuel Quality,” Ind. Eng. Chem. Res., 38, 3543-3548, 1999.
[3] Kazumasa Matsuo, and Mitsuru Shindo, “Efficient Synthesis of Dissymmetric
Malonic Acid S, O-Esters via Monoalcoholysis of Symmetric Dithiomalonates under Neutral Conditions,” Organic Letters, 13, 16, 4406–4409, 2011.
[4] Wu Du, William K. Hagmann and Jeffrey J. Hale, “A ‘one-pot’ synthesis of a-
1, 2, 4-oxadiazolo esters from malonic diesters and amidoximes under sol- vent-free conditions,” Tetrahedron Letters, 47, 4271–4274, 2006.
[5] Naohiko Kanai, Hiroki Nakayama, Norihiro Tada, and Akichika Itoh, “Tan- dem Oxidation/Rearrangement of ß-Ketoesters to Tartronic Esters with Mo- lecular Oxygen Catalyzed by Calcium Iodide under Visible Light Irradiation with Fluorescent Lamp,” Organic Letters, 12, 9, 1948-1951, 2010.
[6] Junxia Liu, Zhongtian Du, Yanliang Yang, Tianliang Lu, Fang Lu, and Jie Xu,
ChemSusChem, 5, 2151 – 2154, “Catalytic Oxidative Decarboxylation of Malic
Acid into Dimethyl Malonate in Methanol with Dioxygen,” 2012.
[7] J. Otera, “Transesterification,” Chem. Rev., 93, 1449-1470, 1993.
[8] Fathallaah Bazi, Hanane EI Badaoui, Samira Sokori, Soumia Tamani, Mo-
hamed Hamza, Said Boulaajaj, and Said Sebti, “Transesterification of Methylbenzoate with Alcohols Catalyzed by Natural Phosphate,” Synthetic Communications, 36, 1585–1592, 2006.
[9] Tongshou Jin, Suling Zhang and Tongshuang Li, “Transesterification of ß- ketoesters with alcohols catalyzed by montmorillonite K-10,” Green Chemistry,
4, 32-34, 2002.
[10] Bernard Jousseaume, Christian Laporte, Marie-Claude Rascle and Thierry
Toupance, “Dichlorodistannoxane transesterification catalysts, pure Lewis ac-
ids,” Chem. Commun., 1428-1429, 2003.
rous SBA-15 molecular sieves and catalytic activity in trans-esterification reac- tion,” Applied Catalysis A: General, 273, 239–248, 2004.
[21] S. Saravanamurugan, Sujandi, Dae-Soo Han, Jeong-Boon Koo, Sang-Eon Park, “Transesterification reactions over morphology controlled amino- functionalized SBA-15 catalysts,” Catalysis Communications, 9, 158-163, 2008.
[22] Ganapati D. Yadav, Anup A. Kadam, “Selective engineering using Mg–Al calcined hydrotalcite and microwave irradiation in mono-transesterification of diethyl malonate with Cyclohexanol,” Chemical Engineering Journal, 230,
547–557, 2013.
[23] A.V.Vijayasankar, N. Nagaraju, “Preparation and characterisation of amor-
phous mesoporous aluminophosphate and metal aluminophosphate as an ef- ficient heterogeneous catalyst for transesterification reaction,” C. R. Chimie, 14,
1109-1116, 2011.
[24] Servet TURAL, “Zinc Perchlorate Hexahydrate Catalyzed Mono- and Bis
Transesterification of Malonic Esters,” Turk J Chem, 32, 169 – 179, 2008.
[25] Dibakar Chandra Deka, Sanjay Basumatary, “High quality biodiesel from
yellow oleander (Thevetia peruviana) seed oil,” Biomass and Bioenergy, 35,
1797-1803, 2011.
[26] D. C. Deka, N. N. Talukdar, “Chemical and spectroscopic investigation of
Kolakhar and its commercial importance,” Indian J. of Traditional Knowledge, 6,
1, 72-78, 2007.
IJSER © 2014 http://www.ijser.org