International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 104
ISSN 2229-5518
Thermal expansivity of geophysical minerals at high temperatures
Monika Panwar, S.K. Sharma, Sanjay Panwar
—————————— ——————————
Thermal expansivity is a very important parametric quantity for interpreting the thermal and elastic properties of minerals at high temperatures because it has been emphasized [1] that most of the serious errors in the calculations of thermodynamic function arise due to uncertainty of thermal expansivity at high temperatures. Many researchers [2-5] have been made to estimate the temperature dependence of thermal expansivity to keep in mind its linear as well as non- linear dependence. Thermal expansivity is necessary parameter for solving many problems of material science and geophysics and many thermal and elastic properties can also be derived from it. Thermal expansivity may also be defined as
![]() (1)
(1)
where ![]() ,
, ![]() and
and ![]() are the volume, temperature and pressure respectively. Method of analysis is seen in section 2, result and discussion in section 3 and conclusion are presented in section 4.
are the volume, temperature and pressure respectively. Method of analysis is seen in section 2, result and discussion in section 3 and conclusion are presented in section 4.
Anderson [6] used the following relationship to estimate the value of thermal pressure
![]() (2)
(2)
————————————————
• Sanjay Panwar, Department of Physics, MMEC, Maharishi
![]() (3)
(3)
![]()
where , , and ![]() are thermal pressure, thermal expansivity and isothermal bulk modulus and
are thermal pressure, thermal expansivity and isothermal bulk modulus and ![]() is room temperature or reference temperature. Thermal pressure is a physical quantity of central importance for investigating the thermo elastic properties of materials at high temperatures [6-8]. The volume expansion of solids due to the rise in temperature is directly related to thermal pressure [9, 10]. The equation of state (EoS) is effectively important in studying the properties of solids under high pressure and high temperature. The EoS yields pressure–volume– temperature (P–V–T) relationship for solids and helps in estimating a variety of properties under different conditions of pressure and temperature
is room temperature or reference temperature. Thermal pressure is a physical quantity of central importance for investigating the thermo elastic properties of materials at high temperatures [6-8]. The volume expansion of solids due to the rise in temperature is directly related to thermal pressure [9, 10]. The equation of state (EoS) is effectively important in studying the properties of solids under high pressure and high temperature. The EoS yields pressure–volume– temperature (P–V–T) relationship for solids and helps in estimating a variety of properties under different conditions of pressure and temperature
![]() (4)
(4)
where ![]() is the volume,
is the volume, ![]() the temperature and
the temperature and ![]() is initial value of temperature taken here equal to 300 K. In writing Eq. (2) and (3), it has been assumed that the thermal pressure is a function of temperature only [1]. At atmospheric pressure i.e. at P (V, T) = 0, we have
is initial value of temperature taken here equal to 300 K. In writing Eq. (2) and (3), it has been assumed that the thermal pressure is a function of temperature only [1]. At atmospheric pressure i.e. at P (V, T) = 0, we have
![]() (5) It should be mention that Stacey reciprocal K-primed (SRKP)
(5) It should be mention that Stacey reciprocal K-primed (SRKP)
equation of state (EoS) [11-13] is valid for both isothermal
bulk modulus as well as adiabatic bulk modulus. The Stacey
reciprocal K-primed (SRKP) equation of state (EoS) [11] can be written as follows
![]() (6) Eq. (6) on integration gives an expression for bulk modulus
(6) Eq. (6) on integration gives an expression for bulk modulus
which has further been integrated to obtained [11]
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 105
ISSN 2229-5518
![]()
Now, replacing ![]() by
by ![]() , Eq. (5) can be written as:
, Eq. (5) can be written as:![]()
where ![]() is the isothermal bulk modulus,
is the isothermal bulk modulus, ![]() is the first pressure derivative of isothermal bulk modulus at
is the first pressure derivative of isothermal bulk modulus at![]()
![]()
atmospheric pressure i.e. and is an important
(7)
(8)
modulus (![]() ) have been predicted from Eq. (10). Results obtained shown the consistency with the experimental data as shown in Table -2, 3, and 4 for MgO, Al2 O3 and Mg2 SiO4 .
) have been predicted from Eq. (10). Results obtained shown the consistency with the experimental data as shown in Table -2, 3, and 4 for MgO, Al2 O3 and Mg2 SiO4 .
As it is clear from Eq. (3) to investigate the values of thermal expansivity, the values of thermal pressure, ![]() and
and ![]() are necessary. By using the concept
are necessary. By using the concept ![]() Eq. (9) takes the form
Eq. (9) takes the form
![]() (11)
(11)
parameter which is first pressure derivate of isothermal bulk modulus at infinite pressure i.e. ![]() . Which can be determine by using [13-17]
. Which can be determine by using [13-17]![]()
where ![]() is the first pressure derivative of isothermal bulk modulus at atmospheric pressure i.e.
is the first pressure derivative of isothermal bulk modulus at atmospheric pressure i.e. ![]() and at room temperature.
and at room temperature.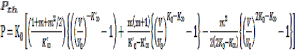
Pallavi Sinha et al. [18] used the following data to calculate data
(9)
where![]()
and other parameter are having their usual meaning and the following relationship have been used to be investigate the value of isothermal bulk modulus ![]() by Pallavi Sinha et al. [18]
by Pallavi Sinha et al. [18]![]()
where all the parameters are having their as usual meaning. The predicted values through Eq. (11) are compared with experimental data Anderson [6] in Table-2, 3 and 4 for MgO, Al2 O3 , Mg2 SiO4 . Comparison between results obtained and experimental data show the good agreement for all geophysical minerals. Using the Eq. (10) and (11), Eq. (3) becomes
(12) The results obtained through Eq. (12) are shown in Table-2, 3
and 4 with the experimental data for MgO, Al2 O3 , and
Mg2SiO4 respectively. Consistency the results obtained from
Eq. (12) and experimental data validate the present approach. For direct vision ![]() vs
vs ![]() are plotted in Figures-1, 2 and 3 for MgO, Al2 O3 , Mg2 SiO4 respectively. It is clear from these figures that the results obtained through Eq. (11) are competent with experimental data [6].
are plotted in Figures-1, 2 and 3 for MgO, Al2 O3 , Mg2 SiO4 respectively. It is clear from these figures that the results obtained through Eq. (11) are competent with experimental data [6]. ![]() vs
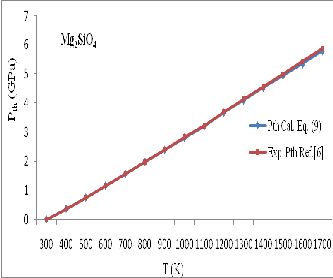
vs ![]() are plotted in Figures- 4, 5 and 6 for MgO, Al2 O3 , and Mg2 SiO4 respectively. An excellent agreement between results obtained through Eq. (12) and experiment data shows the validity of present model.
are plotted in Figures- 4, 5 and 6 for MgO, Al2 O3 , and Mg2 SiO4 respectively. An excellent agreement between results obtained through Eq. (12) and experiment data shows the validity of present model.![]()
(10)
In the present study we introduce a new model to predict the values of thermal expansivity for geophysical minerals such as MgO, Al2 O3 , and Mg2SiO4. It is conclude that the results
We have computed the values of volume expansion by using
the Eq. (8) values of input parameter used in the calculation are shown in Table -2, for geophysical minerals MgO, Al2 O3 and Mg2 SiO4 . The values of ![]() have been taken from Anderson [6] to calculate volume expansion through Eq. (8) is shown in Table-1 and the results obtained through it. A close agreement has been found for all geophysical minerals used in the present study. The values of isothermal bulk
have been taken from Anderson [6] to calculate volume expansion through Eq. (8) is shown in Table-1 and the results obtained through it. A close agreement has been found for all geophysical minerals used in the present study. The values of isothermal bulk
obtained in the present study support the validity of the present model.
Table 1 – Values of input data used in calculations: isothermal bulk modulus ![]() (GPa) and pressure derivative of isothermal bulk modulus
(GPa) and pressure derivative of isothermal bulk modulus ![]() are at T = 300 K and atmospheric pressure.
are at T = 300 K and atmospheric pressure.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 106
![]()
![]()
![]()
ISSN 2229-5518
Parameters | (GPa) | (GPa) | (GPa) | m |
MgO | 162 | 4.15 | 2.49 | 0.400 |
Al2 O3 | 252 | 3.99 | 2.39 | 0.401 |
Mg2 SiO4 | 127 | 5.40 | 3.24 | 0.400 |
![]()
![]()
![]()
Table 2 — Values of volume thermal expansion, thermal pressure (![]() ), and thermal expansivity (
), and thermal expansivity (![]() ) for MgO as a function of temperature
) for MgO as a function of temperature
T (K) |
Eq. (8) |
Exp. Ref.[6] | (GPa) Eq. (11) | (GPa) Exp. Ref.[6] |
| (10-5 K-1) Exp. Ref. [6] |
300 | 1.0000 | 1.0000 | 0.00 | 0.00 | 0.00 | 0.00 |
400 | 1.0034 | 1.0033 | 0.54 | 0.54 | 3.39 | 3.40 |
500 | 1.0071 | 1.0073 | 1.13 | 1.12 | 3.59 | 3.59 |
600 | 1.0111 | 1.0112 | 1.75 | 1.73 | 3.77 | 3.77 |
700 | 1.0153 | 1.0153 | 2.38 | 2.35 | 3.91 | 3.92 |
800 | 1.0196 | 1.0196 | 3.02 | 2.98 | 4.05 | 4.05 |
900 | 1.0241 | 1.0240 | 3.67 | 3.61 | 4.17 | 4.18 |
1000 | 1.0287 | 1.0284 | 4.32 | 4.24 | 4.29 | 4.30 |
1100 | 1.0334 | 1.0332 | 4.96 | 4.87 | 4.41 | 4.41 |
1200 | 1.0382 | 1.0380 | 5.61 | 5.50 | 4.52 | 4.53 |
1300 | 1.0431 | 1.0428 | 6.26 | 6.12 | 4.63 | 4.64 |
1400 | 1.0482 | 1.0476 | 6.90 | 6.74 | 4.74 | 4.75 |
1500 | 1.0533 | 1.0528 | 7.54 | 7.36 | 4.86 | 4.87 |
1600 | 1.0586 | 1.0581 | 8.18 | 7.97 | 4.98 | 4.98 |
1700 | 1.0644 | 1.0635 | 8.87 | 8.58 | 5.14 | 5.15 |
1800 | 1.0696 | 1.0688 | 9.45 | 9.20 | 5.23 | 5.24 |
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 107
ISSN 2229-5518
![]()
![]()
![]()
Table 3 — Values of volume thermal expansion, thermal pressure (![]() ), and thermal expansivity (
), and thermal expansivity (![]() ) for Al2 O3 as a function of temperature
) for Al2 O3 as a function of temperature
T (K) |
Eq. (8) |
Exp. Ref.[6] | (GPa) Eq. (11) | (GPa) Exp. Ref.[6] |
| (10-5 K-1) Exp. Ref. [6] |
300 | 1.0000 | 1.0000 | 0.00 | 0.00 | 0.00 | 0.00 |
400 | 1.0018 | 1.0018 | 0.45 | 0.45 | 1.80 | 1.80 |
500 | 1.0039 | 1.0040 | 0.98 | 0.98 | 1.98 | 1.98 |
600 | 1.0063 | 1.0063 | 1.56 | 1.55 | 2.12 | 2.12 |
700 | 1.0088 | 1.0088 | 2.17 | 2.15 | 2.23 | 2.23 |
800 | 1.0114 | 1.0114 | 2.79 | 2.76 | 2.32 | 2.32 |
900 | 1.0143 | 1.0142 | 3.47 | 3.43 | 2.43 | 2.43 |
1000 | 1.0169 | 1.0168 | 4.09 | 4.01 | 2.48 | 2.48 |
1100 | 1.0197 | 1.0195 | 4.74 | 4.64 | 2.54 | 2.54 |
1200 | 1.0227 | 1.0223 | 5.41 | 5.27 | 2.61 | 2.61 |
1300 | 1.0257 | 1.0252 | 6.07 | 5.90 | 2.67 | 2.67 |
1400 | 1.0288 | 1.0279 | 6.75 | 6.53 | 2.74 | 2.74 |
1500 | 1.0318 | 1.0308 | 7.42 | 7.16 | 2.79 | 2.79 |
1600 | 1.0349 | 1.0337 | 8.08 | 7.79 | 2.84 | 2.84 |
1700 | 1.0381 | 1.0364 | 8.74 | 8.42 | 2.89 | 2.89 |
1800 | 1.0412 | 1.0394 | 9.38 | 9.01 | 2.93 | 2.93 |
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 108
ISSN 2229-5518
![]()
![]()
![]()
Table 4 — Values of volume thermal expansion, thermal pressure (![]() ), and thermal expansivity (
), and thermal expansivity (![]() ) for Mg2 SiO4 as a function of temperature
) for Mg2 SiO4 as a function of temperature
T (K) |
Eq. (8) |
Exp. Ref.[6] | (GPa) Eq. (11) | (GPa) Exp. Ref.[6] |
| (10-5 K-1) Exp. Ref. [6] |
300 | 1.0000 | 1.0000 | 0.0000 | 0.00 | 0.00 | 0.00 |
400 | 1.0029 | 1.0028 | 0.3602 | 0.36 | 2.88 | 2.88 |
500 | 1.0060 | 1.0059 | 0.7496 | 0.75 | 3.05 | 3.05 |
600 | 1.0094 | 1.0094 | 1.1571 | 1.16 | 3.20 | 3.20 |
700 | 1.0128 | 1.0129 | 1.5628 | 1.57 | 3.30 | 3.30 |
800 | 1.0164 | 1.0164 | 1.9819 | 1.98 | 3.41 | 3.41 |
900 | 1.0201 | 1.0199 | 2.3955 | 2.40 | 3.51 | 3.51 |
1000 | 1.0239 | 1.0238 | 2.8149 | 2.83 | 3.61 | 3.61 |
1100 | 1.0275 | 1.0277 | 3.1970 | 3.22 | 3.66 | 3.66 |
1200 | 1.0320 | 1.0320 | 3.6716 | 3.69 | 3.83 | 3.83 |
1300 | 1.0361 | 1.0363 | 4.0912 | 4.13 | 3.93 | 3.93 |
1400 | 1.0406 | 1.0407 | 4.5330 | 4.56 | 4.06 | 4.06 |
1500 | 1.0449 | 1.0451 | 4.9461 | 5.00 | 4.16 | 4.17 |
1600 | 1.0492 | 1.0498 | 5.3466 | 5.43 | 4.26 | 4.26 |
1700 | 1.0541 | 1.0547 | 5.7929 | 5.87 | 4.41 | 4.41 |
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 109
ISSN 2229-5518
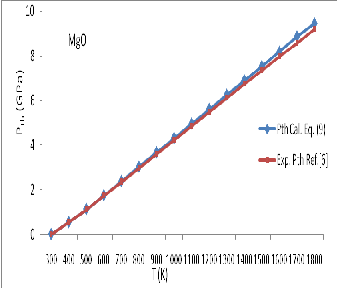
Figure 1: Comparison of theoretical and experimental value of temperature dependence of volume expansion ratio calculated by various Eqs. for MgO
Figure 3: Comparison of theoretical and experimental value of temperature dependence of volume expansion ratio calculated by various Eqs. for
Mg 2 SiO 4
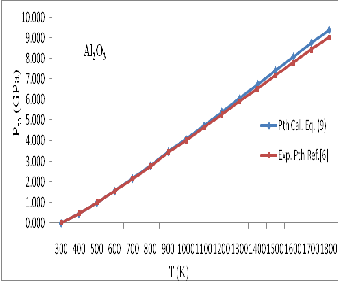
Figure 2: Comparison of theoretical and experimental value of temperature dependence of volume expansion ratio calculated by various Eqs. for
Al2 O 3
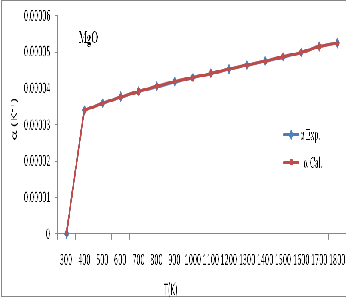
Figure 4: The graph between thermal expansivity ( ![]() ) and temperature for
) and temperature for
MgO between calculated values and experimental data
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 110
ISSN 2229-5518
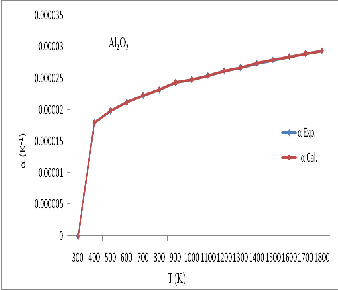
Figure 5: Thermal expansivity ( ![]() ) for Al 2 O 3 calculated in present study and experimental data
) for Al 2 O 3 calculated in present study and experimental data
[5] Fang Z H, Physica B 403 (2008) 1971.
[6] Anderson O L, Equations of State of Solids for Geophysics and Ceramic science, Oxford University Press, New York (1995).
[7] Wang Z, Lazer P, Saxena S K, Physica B 293 (2001) 408.
[8] Wang Z, Schott B, Lazer P, Saxena S K, J. Alloys Compd. 315 (2001) 51. [9] Kushwah S S, Shanker J, Physica B 253 (1998) 90.
[10] Shanker J, Kushwah S S, High Temp. High Press. 33 (2001) 207. [11] Stacey F D, Geophys J. Int. 143 (2000) 621.
[12] Stacey F D & Davis P M, Phys. Earth Planet. Int. 142 (2004) 137. [13] Stacey F D, Rep. Prog. Phys. 68 (2005) 341.
[14] Kushwah S S, Bhardwaj N K, J. Phys. Chem. Sol. 70 (2009) 700. [15] Srivastava H C, Physica B 404 (2009) 251.
[16] Kushwah S S, Shrivastava H C & Singh K S, Physica B 388 (2007) 20. [17] Kushwah S S, Tomar Y S, Ind. J. Pure and App. Phys. 49 (2011) 99-103. [18] Sinha Pallavi, Srivastava S.K. & Verma Nidhi, Physica B 406 (2011) 2488-
2491.
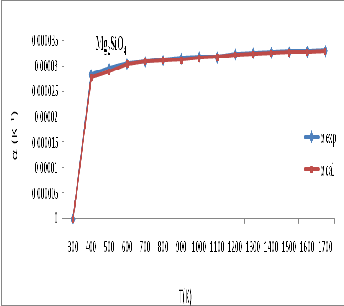
Figure 6: Thermal expansivity ( ![]() ) for Mg 2 SiO 4 calculated in present study and experimental data
) for Mg 2 SiO 4 calculated in present study and experimental data
[1] Xia X & Xiao J, J. Phys. Chem. Sol. 54 (1993) 629.
[2] Singh K S & Chauhan R S, Physica B 315 (2002) 74. [3] Srivastava S K, Physica B 355 (2005) 32.
[4] Fang Z H, J. App. Phys. 102 (2007) 013523.
IJSER © 2015 http://www.ijser.org