λ1 =
λ2 =
λ3 =0 ……..(1)
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 614
ISSN 2229-5518
The Stability of DNA binding with Bisintercalating
Anthracyclin: A Theoretical Study
Gazala Roohi Fatima, Dr Irfan Ali Khan, Dr Seema Srivastava, Rushi Ghizal
Temperature
—————————— ——————————
than any other class of chemotherapeutic agents. Their main adverse effect is cardio toxicity, which considerably
IN the recent years the study of small molecules
interaction with DNA is an investigation area of high
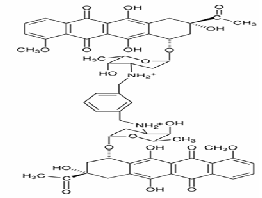
tropical interest. There are number of techniques dedicated to support drug-DNA interactions which are increasing continuously. Over the past several years, the interaction of aromatic heterocyclic molecules to nucleic acids has received significant interest due to their relevance in a variety of biological applications including cancer chemotherapy [7]. Thus, a number of experimental studies are conducted to understand the nature and thermodynamics of heterocyclic aromatic molecules and nucleic acid interaction [6]. A bisintercalating anthracyclin (WP762) (Fig. 1), has been studied for its intercalative interaction with DNA.
The anthracyclin antibiotics including daunorubicin and doxorubicin (Adriamycin), have broad utility in the treatment of several malignancies. These compounds are used to treat many cancers, including leukemia’s, lymphomas, breast, uterine, ovarian, bladder cancer, and lung cancers. The anthracyclines are among the most effective anticancer treatment ever developed and are effective against most types of cancer
limit their usefulness. Although, the efforts are being done to synthesize anthracyclines which have fewer toxic side effects. But the efforts have not succeeded yet and the parent compounds remain the best chemotherapeutic agents of this class [2,4]
In these dunorubicin-DNA complexes, each drug molecule was intercalated at either end of a hexanuceotide, with the daunosamine moieties of two drug molecules pointing towards one another, lining the minor groove [10]. The structural data indicate that the fit of the p-xylenyl linker in the minor groove was not optimal, and could be improved [13,14]. A new bisintercalating anthracycline (WP762) has been designed, in which monomeric units of daunorubicin have been linked through their amino groups on the daunosamine moieties using an m-xylem linker. M- xylem linker was designed to be of appropriate length (approximately 7Å) that might fit better into the minor groove of DNA. In present work binding of WP762 with herring sperm DNA is studied. For implementing present study, the experimental models of Portugal et al.(2005)[1] have been used.
————————————————
• Gazala Roohi Fatima,Research scholor, Integral University, Lucknow, PH-
9415263346. E-mail:gazala03@gmail.com
• Dr Irfan Ali Khan, Registrar,IintegraU university, Lucknow, India
• Dr Seema Srivastava, Integral University, Lucknow, India
• Rushi Ghizal,Research school, Integral University, Lucknow, India
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 615
ISSN 2229-5518

λ1 = ![]()
![]()
λ2 =
λ3 =0 ……..(1)
Figure 1: Chemical structure of Bisintercalating anthracyclin (W P762)
On the basis of spectroscopic and calorimetric study for the complex formation between WP762 and DNA, as mentioned above, Portugal et al.(2005) [1] recommended that the drug binds extremely tightly with the herring sperm DNA that resulted in thermal stabilization of the complex. The binding of WP762 with DNA was intercalative and was non-cooperative that resulted in significant perturbation of the conformation of the DNA as well as thermal stabilization [1]. Although, the system remains a highly co-operative one hence the co-operative transition theory could be applied to elucidate the melting profile and temperature dependence of thermo-dynamical parameter, such as heat capacity. Thus, we used modified Zimm and Bragg theory [18]. All the theories are an adoption of the Ising –Model which was initially given to study the ferromagnetic transition in linear chain of spins. Similarly as a spin can exist in two states either spin up or spin down, exactly a residue can exist in ordered or disordered state in a long polymer chain. Since a one dimensional system can not show any type of phase transition due to finite nature of interactions, the Ising model was modified by introducing boundary state. This boundary state is the first ordered state in the sequence of ordered states. Once residue make a transition then its continuance in the second state becomes easier. Now, one can assign different partition function to different states.
In brief the theory consists of writing an Ising
matrix for a two phase system, the fused (A+B) state (say)
and disordered state. The Ising matrix M can be written as :
M = 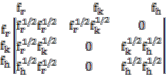
Where f r , f h and f k are corresponding base pair partition functions contributions in the three states. i.e. ordered, disordered and boundary or nucleation. The eigen values of M are given by:
Since we are dealing with a finite system hence the effect of initial and final states becomes important. The
contribution of the first segment to the partition function is given by:
U=(f r 1/2,0,0) ……..(2)
Where the column vector V gives the state of the
last segment,
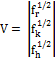 ………….(3)
………….(3)
The partition function for a N-segment chain is given by
Z = UMN-1V ………(4)
On substituting the values from eq.1, 2, 3 and 5 the partition function becomes:
The matrix T which diagnolizes M consists of the column vectors given by:
MX = λ X …………(5)
Where: X=![]() ………(6)
………(6)
By substituting the values of M from Eq. 5, we get:
T = 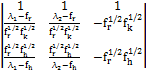
Similarly, we get T-1 from the matrix equatio :
YM = λY …………(7) Where, Y = [Y1 , Y2 , Y3 ]
Again by substituting the values of M from eqn. 1
in eqn. 7, we get;![]()
T-1=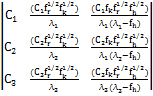 ....(8) The normalization constants are:
....(8) The normalization constants are:
C1 =
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 616
ISSN 2229-5518
C2 = ![]()
And C3 = 0 …..(9)
If we let Λ = T-1MT be the diagonalized form of M, the partition function can be written as:
Z = UTΛN-1T-1V …..(10)
On substituting the values from equations 1, 2, 3, 6, 8 and 9 in eqn. 10, the partition function becomes:
Z=C1 λ1 N+C2 λ2 N .....(11)
The fraction of the segments in the disordered form is given by
Qr = ![]() [δlnZ/δlnf r ]/N Solving the above equation, we get :
[δlnZ/δlnf r ]/N Solving the above equation, we get :
Qr = ![]() +
+![]() +
+![]() …(12) Where
…(12) Where
P = ![]() , s=
, s= ![]() ,
,
σ =![]()
A=[(f r -f h )2+4f kf r ]-2
Here s is propagation parameter, which for
simplicity is assumed to be one. In fact in most of the
systems it is found to be close to unity.
If Ar and Ah are absorbance in disordered and
ordered states respectively, the total Absorption can be written as :
A = Qr Ar +(1-Q r )A h ........(13)
The extension of this formalism to specific heat is straightforward. The specific heat is related to the molar enthalpy and entropy changes in the transition from state I to II. From well known thermodynamic relations, free energy and internal energy are F = -KT ln Z and U = -T2(![]() ) (
) (![]() ), respectively. Differentiating internal energy with respect to temperature we get the specific heat :
), respectively. Differentiating internal energy with respect to temperature we get the specific heat :
Cv =![]()
= Nk(![]() )2 (
)2 (![]() ) ...(14)
) ...(14)
where ΔH is the molar change in enthalpy about the
transition point, S is entropy which is equal to
S = exp[(![]() ){
){![]() -
-![]() }],
}],
Tm is the transition temperature, and![]() =
= ![]() and σ =
and σ =![]() σ- is the nucleation parameter and is a measure of the energy expanded/released in the formation (uncoiling) of first turn of the ordered/disordered state. It is related to the uninterrupted sequence lengths. The volume heat capacity Cv has been converted into constant pressure heat capacity Cp by using Nernst- Lindemann approximation,
σ- is the nucleation parameter and is a measure of the energy expanded/released in the formation (uncoiling) of first turn of the ordered/disordered state. It is related to the uninterrupted sequence lengths. The volume heat capacity Cv has been converted into constant pressure heat capacity Cp by using Nernst- Lindemann approximation,
Cp –C v = 3RA0 (![]() …..(16)
…..(16)
Where A0 is a constant often of universal value [3.9×10-9
(Kmol)/J-1] and Tm is the melting temperature.
The UV melting studies and Differential scanning calorimeter results show that binding constant for interaction of WP762 with herring sperm DNA is 7.3 × 1012
M-1. High value of binding constant represents that WP762
tightly bounds to DNA[1]. As mentioned earlier each drug molecule was intercalated at either end of a hexanuceotide, with the daunosamine moieties of two drug molecules pointing towards one another, lining the minor groove[10]. One base pair is attached to each of DNA hexagon to add stability to the double helix ends and ensure a stable realistic interaction site [1]. When daunorubicinn drug bind DNA, the structure still remains a very much co-operative. Accordingly two sate theory of order-disorder transition is applicable. The Zimm-Bragg theory is modified so as to ordered and disordered states as the two co-existing sates at the transition point. The transition is mainly described by nucleation parameter σ and change in enthalpy. DSC measurements take all this into account and gives change in enthalpy. The changes in enthalpy and in other transition parameters, such as nucleation parameter, melting point obtained theoretically indicate that the binding of WP762 increases the melting temperature of herring sperm DNA. At saturation the melting temperature of DNA duplex increases by 29.75K in comparison to free DNA. The sharpness of the transition can be observed in terms of half width and a sensitivity parameter as (ΔH/σ). The deviation in half width and sensitivity parameter revealed that the transition is sharp in case of unbound state and goes blunt with WP762 saturation. A range of parameters, which give transition profiles in best agreement with the experimental data for binding of WP762 with DNA, are given in table.![]()
![]()
![]()
![]()
With![]()
={(S-σ)N/( )2} × ( ) × [-2 + { ]
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 617
ISSN 2229-5518
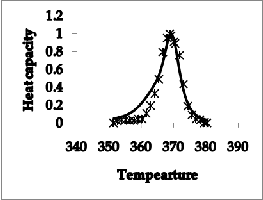
Table1. Transition parameters for W P762 binding to DNA
Figure 2 (b) The transition when DNA was saturated with
WP762 (0.16 mol antibiotic/mol bp)
The heat capacity and transition profile for unbounded DNA and bounded with WP762 are shown in Figure2. Figure 2(a) explains experimental and calculated transition curves for herring sperm DNA at 1 mM (bp) in absence of WP762 and figure 2 (b) shows transition when DNA was saturated with WP762 (0.16 mol antibiotic/mol bp). As anticipated, a co-operative transition profile is obtained with calculated data. The conclusions of theoretical data are consistent with the measured binding enthalpy by portugal et al.[1].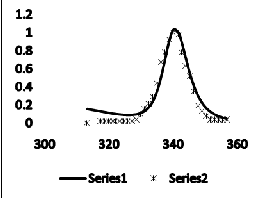
Figure 2(a) The experimental and calculated transition curves for herring sperm DNA at 1 mM (bp) in absence of WP762
The second derivative of the free energy i.e. heat capacity has been calculated by using equation 14. The heat capacity curves with λ-point anomaly and along with their transition profile are shown in figure 2. The results indicate that the theoretically obtained heat capacity profile agreed with the experimentally reported ones. The theoretical data obtained also verified that binding of WP762 with herring sperm DNA is an endothermic process and this increases the melting temperature of the DNA as supported by the calorimetric measurements. The binding and intercalation of WP762 with herring sperm DNA is studied by by using differential scanning calorimeter by Portugal et al.[1]. The binding of drug stabilizes the DNA and change I melting temperature of about 30˚c was obtained. Figure 2(a and b) disclosed that transition of WP762-DNA is significantly broader than the transition of unbounded DNA. Besides affecting the transition enthalpy and melting temperature, binding of WP762 with DNA also changes the nature of transition profile as reflected by the increase in transition width in experimental and calculated data. Further figure
3(a) shows Predicted absorbance of DNA solution with
saturating amounts (0.16 mol antibiotic/mol bp) of WP762
added. And figure 3(b) shows absorbance at DNA concentration (20 μM bp). The calculated data is in good agreement with the experimental results of UV melting studies performed by Portugal et al. Figure 4 represents the predicted values of heat capacity at conc. (20 μM bp) in the range 290-380K.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 618
ISSN 2229-5518
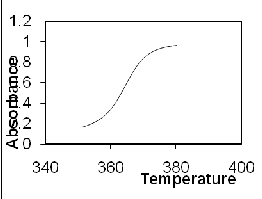
Figure 3. Predicted absorbance of DNA solution with saturating amounts (0.16 mol antibiotic/mol bp) of WP762 added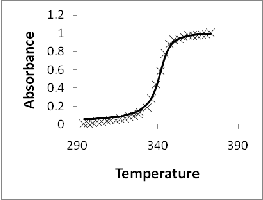
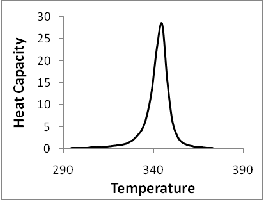
Figure 4. Absorbance at DNA conc(20 μM bp)
The present study concluded that DNA molecule is an extremely co-operative structure when WP762 binds to it, the co-operativity is not much disturbed. Therefore amended Zimm-Bragg theory can be effectively applied to it. It generates the experimental transition profile and λ point heat capacity anomaly effectively. These results allow us to assess the thermodynamic profile of binding process.
Our theoretical data also demonstrate that the binding of WP762 to DNA is an endothermic process. Therefore the theoretical analysis presented in this study can be implicated to understand bimolecular interactions and may also be applied in biomedical industry for drug design and development.
[1] Portugal J,cashman DJ,Ferrer-Mirallels, Przewloka T,Fokt I, Priebe W,Chaires JB,A new bisintercalating anthracycline with picomolar DNA binding affinity. J Med Chem 2005 Dec 29;48(26):8209-19.
[2] Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacological Reviews
2004;56:185–229. [PubMed: 15169927]
[3] Ghazala Yunus, Seema Srivastava, Vishwambhar D. Gupta,
A Theoretical Study on the Interaction of Thionine with Micrococcus Lysodeikticus DNA International Journal of Biophysics 2012, 2(1): 7-11 [4]Binaschi M, Bigioni M, Cipollone A, Rossi C, Goso C, Maggi CA, Capranico G, Animati F.Anthracyclines: selected new developments. Current Medicinal Chemistry - Anti-Cancer Agents 2001;1:113–130. [PubMed: 12678762]
[5] Priebe W. ACS Symposium Series. American Chemical Society; Washington, D.C: 1995. Anthracycline Antibiotics: New Analogues, Methods of Delivery and Mechanisms of Action.
[6] Priebe, W. ACS Symposium Series. Anthracycline Antibiotics: New Analogues, Methods of Delivery and Mechanisms of Action. American Chemical Society; Washington, D.C: 1995.
[7] Ghazala Yunus, Seema Srivastava, Vishwambhar D. Gupta,A Theoretical Study on the Interaction of Thionine with Micrococcus Lysodeikticus DNA, International Journal of Biophysics 2012, 2(1): 7-11 [8] G. Bruylants, J. Wouters and C. Michhaux Differential scanning calorimetry in life science: Thermodynamic stability,moleculer recognition and apllication in drug design Current medicinal chemistry, 2005
[9] Chaires JB, Herrera JE, Waring MJ. Preferential binding of daunomycin to 5′ATCG and 5′ATGC sequences revealed by footprinting titration experiments. Biochemistry. 1990;29:6145–6153 [10]Frederick CA, Williams LD, Ughetto G, van der Marel GA, van Boom JH, Rich A, Wang AH.
Structural comparison of anticancer drug-DNA complexes: adriamycin and daunomycin.Biochemistry 1990;29:2538–2549. [PubMed: 2334681] [11] Cashman DJ, Kellogg GE. A computational model for anthracycline binding to DNA: tuning groove-binding intercalators for specific sequences. Journal of Medicinal Chemistry. 2004;47:1360–1374 [12] Cashman DJ, Scarsdale JN, Kellogg GE. Hydropathic analysis of the free energy differences in anthracycline antibiotic binding to DNA. Nucleic Acids Research. 2003;31:4410–4416
[13] Hu GG, Shui X, Leng F, Priebe W, Chaires JB, Williams LD. Structure of a DNA-bisdaunomycin
complex. Biochemistry 1997;36:5940–5946. [PubMed: 9166763]
[14] Robinson H, Priebe W, Chaires JB, Wang AH. Binding of two novel bisdaunorubicins to DNA studied
by NMR spectroscopy. Biochemistry 1997;36:8663–8670. [PubMed:
9289011]
[15] Dervan PB, Edelson BS. Recognition of the DNA minor groove by pyrrole-imidazole polyamides. Current Opinion in Structural Biology. 2003;13:284–299
[16] G.E. Plum, J. Völker and K.J. Breslauer. Thermodynamic properties of DNA. In.Encyclopedia Reference of Genomics & Proteomics in Molecular Medicine. D. Ganten & K. Ruckpaul, (Eds.) Springer-Verlag, Berlin, Germany, Vol. 1, pp. 1851-1855 (2006)
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 619
ISSN 2229-5518
[17] Z. Xu, D.S. Pilch, A.R. Srinivasan, W.K. Olson, N. Geacintov, and K.J. Breslauer. Modulation of nucleic acid structure by ligand binding: Induction of a DNA-RNA-DNA hybrid triplex by DAPI intercalation, Bioorganic & Medicinal Chemistry 5, 1137-1147 (1997). [18] B.H. Zimm and J.K. Bragg, ”Theory of the phase transition between helix and random coil in polypeptide chains”, Journal of Chemical Physics, Vol. 31, pp 526-535, 1959.
[19] Burnett JC, Kellogg GE, Abraham DJ. Computational methodology for estimating changes in free energies of biomolecular association upon mutation. The importance of bound water in dimertetramer
assembly for beta 37 mutant hemoglobins. Biochemistry 2000;39:1622–
1633. [PubMed:
10677211]
[20] G.E. Plum, J. Völker and K.J. Breslauer. Thermodynamic properties of DNA. In.Encyclopedia Reference of Genomics & Proteomics in Molecular Medicine. D. Ganten & K. Ruckpaul, (Eds.) Springer-Verlag, Berlin, Germany, Vol. 1, pp. 1851-1855 (2006).
[21] D.S. Pilch, S.U. Dunham, E.R. Jamieson, S.J. Lippard, and K.J.
Breslauer. DNA sequence context modulates the impact of a cisplatin
1,2-d(GpG) intrastrand cross-link on the conformational and thermodynamic properties of duplex DNA, J. Mol. Biol. 296, 803-812 (2000)
[22] Booser DJ, Hortobagyi GN. Anthracycline antibiotics in cancer therapy. Focus on drug resistance.
Drugs 1994;47:223–258. [PubMed: 7512899]
[23] Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of
the anthracycline antibiotics adriamycin and daunorubicin. Biochemical Pharmacology 1999;57:727–
741. [PubMed: 10075079]
[24] Chaires JB. Dissecting the free energy of drug binding to DNA. Anti-Cancer Drug Design
1996;11:569–580. [PubMed: 9022746]
[25] Cashman DJ, Scarsdale JN, Kellogg GE. Hydropathic analysis of the free energy differences in
anthracycline antibiotic binding to DNA. Nucleic Acids Research
2003;31:4410–4416. [PubMed:
12888500]
[26] Mansilla S, Pina B, Portugal J. Daunorubicin-induced variations in gene transcription: commitment
to proliferation arrest, senescence and apoptosis. Biochemical Journal
2003;372:703–711. [PubMed:
12656675]
[27] Trent JO. Molecular modeling of drug-DNA complexes: an update. Methods in Enzymology
2001;340:290–326. [PubMed: 11494855]
IJSER © 2015 http://www.ijser.org