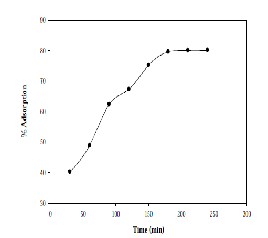
Figure 1: Time Vs% Adsorption.
International Journal of Scientific & Engineering Research, Volume 3, Issue 8, August-2012
ISSN 2229-5518
The Removal and Equilibrium studies of Cadmium by Natural Clay as adsorbent K.Mariadas, G.Kalyani, H.Joga Rao, Y.Prasanna Kumar, P.King
The equilibrium adsorption isotherms of Langmuir, Freundlich, were obtained for the quan titative description of the cadmium uptake. The effect of pH, agitation time, metal ion concentration, adsorbent dose on biosorption equilibrium was studied demonstrating the importance of this parameter for an accurate evaluation of the biosorption proces s. The results indicated that the Bentonite clay is a suitable adsorbent for the removal of Cadmium from wastewaters.
—————————— ——————————
Any change in physical, chemical or biological properties of water is known as water pollution. Heavy metals play an important role in water pollution. The heavy metals are continuously released into the aquatic eco system from natural process such as volcanic activity and weathering of rocks. The removal of heavy metals from our environment especially wastewater is now shifting from the use of conventional adsorbent to the use of biosorbent. The presence of heavy metals in the environment is of major concern because of their toxicity and threat to human life and the environment. Among the toxic heavy metals, mercury, lead and cadmium, called the big three are in the limelight due to their major impact on the environment. Arsenic, chromium copper and zinc are also toxic; lead and cadmium are potent neurotoxic metals .Due to their extreme toxicity, metal ions are now-a-days among the most important pollutants both in surface water and in groundwater. Since levels of metals in the environment have increased because of industrial pollution [1, 2], the elimination of such ions from water is essential to protect public health. In addition, these toxic elements can seriously affect plants and animals, causing a large number of afflictions.
Heavy metals such as lead, mercury, arsenic, copper, zinc and cadmium are highly toxic when adsorbed into the body. Cadmium, one of the earliest metals recognized and used by humans, has a long history of beneficial use to humankinds, but now been recognized as toxic and as posing a widespread threat to humans and wildlife. The sources of human exposure to Cd include atmospheric, terrestrial and aquatic routes
Treatment of Cadmium from polluted water and
wastewater has received a great deal of attention.
Cadmium is a toxic element in large excess, being
carcinogenic [3]. These facts prompt the setting up of experimental methods that enable the most rapid and efficient removal of Cadmium from a contaminated medium to be obtained. In this connection one must have in mind that Cadmium is usually found together with metals such as Zinc and mercury.
Mining and metallurgical waste are the most considerable sources of environmental pollution by heavy metals. Due to the health hazard presented by heavy metals, development of effective and economic removal technologies is necessary. Moreover, mercury is a strong chalcophile, like Zinc and cadmium, and the three metals are found as their sulfide minerals. On the other hand, adsorption studies from multicomponent systems are of interest with a view to environmental preservation, as most natural systems are multicomponent ones.
Previously, the removal of Cadmium either alone or from multicomponent systems [5– 17] has been investigated using activated carbon or similar carbons [18]. The carbonaceous material is an aforesaid unique and versatile adsorbent that is extensively used in water treatments [19]. In the present work, the kinetics of adsorption of Cd2+ in aqueous solution onto activated carbon has been studied. The main objective has been to determine the foreseeable influence on kinetics of the presence of Cd2+, Hg2+, or both ions in the Cd2+ solution and of the variation of this solution‘s ionic strength and pH.
In the present paper it is proposed to apply bentonite as sorbent of Cadmium. Environmental parameters affecting the sorption process such as pH, contact time, metal ion
IJSER © 2012
International Journal of Scientific & Engineering Research, Volume 3, Issue 8, August-2012
ISSN 2229-5518
concentration, sorbent concentration and sorbent size were evaluated. The equilibrium sorption data were evaluated by Langmuir and Freundlich isotherm models.
All the chemicals used in the experiments were of analytical grade and they were used without further purification. CdSO4.7H2O (Loba Chemie, Mumbai; minimum assay 99%) was used as the source of Cadmium and all the solutions were made in distilled water. The solutions of Cadmium were made from a stock solution containing 1000 mg of Cadmium in 1 L. For experiments to be carried out at different pH values, the pH of Cadmium solutions was adjusted by addition of 0.1 M H2SO4 and 0.1 M NaOH solutions.
Bentonite is obtained from ONGC Rajahmundry, which is used by them as drill mud. It consists of different impurities. In order to remove the impurities it is washed with 5% H2O2 solution to remove the carbon matter in the clay as it interferes the adsorption of the Cadmium ions onto it. It is then boiled, and filtered. It is washed with distilled water to remove any impurities and dried in hot air oven at 50 °C for 12 hours. The dried bentonite is in the form of flakes and in order to use it for adsorption it is powdered by grinding in a ball mill. The powder was then sized by passing it through a set of sieves ranging from size
72 to 200 meshes.
4.44g of 99% Cadmium Sulphate (CdSO4.7H2O) was dissolved in 1 L of distilled water to prepare 1000 ppm of Cadmium stock solution. Synthetic samples of different concentrations of Cadmium were prepared from this stock solution by appropriate dilutions. For example 20 ppm of Cadmium solution was prepared by diluting 20ml of
1000ppm Cadmium stock solution with distilled water in
1000 ml volumetric flask and was made up to the mark.
Similarly solutions with different metal concentrations as
10ppm, 50ppm, 75ppm and 100ppm were prepared.
Batch adsorption experiments were carried out by shaking
2 gms of activated carbon prepared from coconut shells with 50 ml aqueous solution of Cadmium Sulphate of the desired concentration at various retention times at room
temperature in orbital shaker. Continuous mixing was provided during the experiments with a constant agitation speed of 200 rpm. Adsorption studies were carried out at room temperature with initial concentration range from 5 to
100 ppm, adsorbent dosage range from 1 to 6 gm and size
of the adsorbent ranges from 500 – 150μm. After shaking, the samples were withdrawn at suitable time intervals, filtered through membrane filter and then analyzed for Cadmium concentration with an atomic absorption spectrophotometer (Perkin Elmer 3100). For the isotherm studies, 2 gm of activated carbon was put into 50 ml solutions of various concentrations of Cadmium. The flasks were shaken for 5 hrs to attain equilibrium. A known volume of the solution was removed and filtered for Cadmium analysis. The amount of Cadmium adsorbed was determined by the difference between the initial and the equilibrium concentrations. Similar batch experiments were carried out using activated carbon prepared from used tea powder and brick powder.
The optimum agitation time is determined by agitating 50 ml of aqueous solution containing 20 mg/L of Cadmium with 0.5 g of adsorbent and with the finest size of the adsorbent in time intervals of 30 min to 6 hr. The data are plotted in Fig-1 and from this plot, the optimum time for Cadmium adsorption is found to be 3 hr. It is noticed that the rate of percentage removal is higher in the initial stages because adequate surface area of the adsorbent is available for the adsorption of Cadmium. As time increases, more amount of Cadmium gets adsorbed onto the surface of the adsorbent and surface area available decreases. Normally, the adsorbate forms a thin layer over the surface, which is only one molecule thick. When this monomolecular layer covers the surface, the capacity of the adsorbent is exhausted. The percent removal of Cadmium is found to increase with time up to 3 hrs and later the change is observed to be marginal with further increase in time. Hence the optimum time of contact is taken as 3 hrs.
IJSER © 2012
International Journal of Scientific & Engineering Research, Volume 3, Issue 8, August-2012
ISSN 2229-5518

Figure 1: Time Vs% Adsorption.
The data drawn with the 50 ml of the aqueous solution having a concentration of 20 mg/l of Cadmium is exposed to different dosages of adsorbent of 75μm are plotted in Fig-2. It is evident from Fig-2 that the fraction of the metal removed from the aqueous phase increases with an increase in the adsorbent amount. Such behavior is obvious since the metal uptake capacity of the adsorbent increases as its dosage was increased. This is so because the number of active sites available for metal uptake would be more as the amount of the adsorbent increases.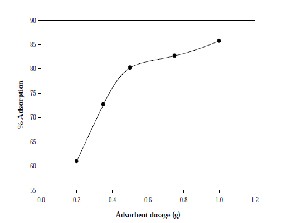
Figure 2: Adsorbent Dosage Vs% Adsorption.
For an adsorbent dosage of 0.5 g, 20 mg/L of Cd concentration and 50 ml of aqueous solution is contacted with different sizes of adsorbent varying from 75μm to
212μm. The data obtained are plotted in Fig-3; it is observed that the percentage removal of metal decreased with increase in the size of the adsorbent. This phenomenon is expected since as the size of the adsorbent decreases, surface area of the powder increases, thereby the number of active sites on the adsorbent is better exposed to the adsorbate. Hence, the metal uptake would be increased.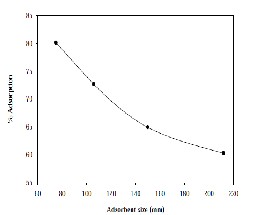
Figure 3: Adsorbent size Vs% Adsorption.
The effect of initial concentration of Cadmium on the percentage removal of Cadmium at equilibrium is shown in Fig-4. For a 50 ml of aqueous solution, adsorbent dosage of
0.2g with size 75μm and optimum agitation time of 3hr, the percentage removal of Cadmium from the aqueous solution is observed to increase from 69.28% to 87.92% by varying Cadmium concentrations in the aqueous solution from 10 mg/l to 100 mg/l. These concentrations generally depend on the equilibrium data, which are checked for Langmuir and Freundlich isotherms.
IJSER © 2012
International Journal of Scientific & Engineering Research, Volume 3, Issue 8, August-2012
ISSN 2229-5518
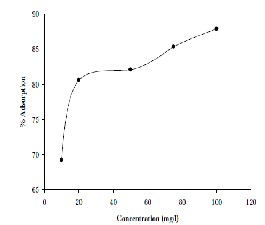
Figure 4: Effect of Initial Concentration on adsorption of
Cadmium on natural bentonite.
pH is an important factor controlling the process of adsorption. The data drawn with the 50 ml of aqueous solution having a concentration of 20 mg/l of Cadmium, with different initial pH varying from 2-8 are exposed to an adsorbent dosage of 0.5 g are plotted in Fig-5. From Fig-5 it is known that maximum % adsorption is obtained at a pH of 5.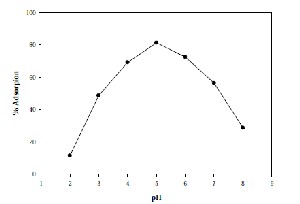
Figure 5: Effect of Initial pH of the solution on adsorption on adsorption of Cadmium on natural bentonite.
The result for the adsorptive removal of Cadmium with respect to the volume of the aqueous solution is shown in Fig.6. For an adsorbent dosage of 0.5g, particle size of 75μm and optimum agitation time of 3 hrs and Co of 20 mg/L, the
percentage removal of Cadmium from the aqueous solution is observed from 84.28 % to 49.26 % as the volume of the Cadmium solution was increased from 25 ml to 120 ml. As the volume of the aqueous solution increases, the amount of Cadmium present in the solution also increases. This implies that the metal uptake by the fixed surface area of the adsorbent would be decreased as the amount of Cadmium in the solution is increased. Such a behavior can be due to the exposure of higher amount of Cadmium to the unaltered surface area of the adsorbent.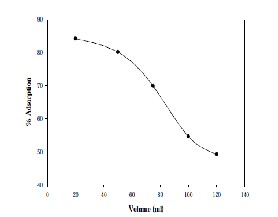
Figure 6: Effect of Initial Volume of the solution on adsorption of Cadmium on natural Bentonite.
The adsorption of a substance from one phase to the surface of another in a specific system, leads to a thermodynamically defined distribution of that substance between the phases as the system reaches equilibrium. The equilibrium between the concentration of the adsorbate in the fluid phase and the concentration of the adsorbate held by a particular adsorbent is generally known as the isotherm for the solute – adsorbent system at a given temperature. There are many equations, which represent equilibrium adsorption data. Of these, two are widely used viz., (1) The Freundlich equation and (2) The Langmuir equation. Since the total adsorption cannot be measured, the relative or apparent adsorption of solute is determined. The customary procedure is to treat a known volume of solution with a known weight of adsorbent. As a result of preferential adsorption of solute, the solute concentration in the liquid is observed to fall from the initial value C0 to a final equilibrium value CE mass solute / volume liquid. The apparent adsorption of solute neglecting any volume change in the solution is the V (C0 – CE) mass solute adsorbed / mass adsorbent. Where ‗V‘ is the volume of the solution per unit mass of adsorbent. This is satisfactory for dilute solutions when the fraction of the original solvent,
IJSER © 2012
International Journal of Scientific & Engineering Research, Volume 3, Issue 8, August-2012
ISSN 2229-5518
which can be adsorbed, is small. The adsorption isotherms, over small concentration gradients, particularly dilute solutions can be frequently given by an empirical expression given by Freundlich
qE K f
CE
n casey,1997
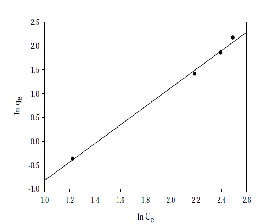
Where CE is the apparent adsorption per unit mass of adsorbent and K and n are constant. The results obtained from experimental data are tested for Langmuir and Freundlich isotherms. It is observed that the adsorption isotherms drawn for adsorption of Cadmium on bentonite from stock solution have followed Freundlich isotherm with a standard deviation of 0.0145. The empirical equation so obtained is given by 0.06275 C0.513. The percentage removal of Cadmium by bentonite is comparable to that by other adsorbents as reported in the earlier studies. It is observed that the % removal of Cadmium by adsorbent bentonite can be better performed at a size of 75μm of bentonite, at a concentration of 100 mg/l, at a pH of 5 and at optimum time of contact 3 hrs.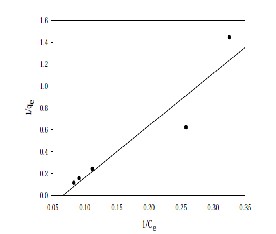
Figure 7: Langmuir Isotherms for Cadmium adsorption on natural bentonite.
Figure 8: Freundlich Isotherms for adsorption of
Cadmium on natural bentonite.
Studies were carried out for the removal of Cadmium metal from synthetic stock solution, by the adsorbent material bentonite clay. Parameters studied include time of contact for adsorption, size of bentonite particles, and dosage of the adsorbent, initial concentration of the solution, initial pH of the solution and volume of the solution. On the analysis of the data the following conclusions are drawn.
The optimum time of contact was found to be 3 hrs.
The increase of the initial concentration of the
Cadmium ion resulted in higher % removal of
Cadmium.
The increase of size of particle resulted in the
decrease of adsorption.
With increase in volume of the solution there was a
decrease in the % removal of Cadmium.
The increase of dosage of adsorbent had resulted in the increase of % removal of Cadmium.
The solution pH is an important parameter, which
affects biosorption of cadmium by the clay. Maximum % removal of Cadmium is observed at a pH of 5.0.
The adsorption isotherms had followed Freundlich
equation with a standard deviation of 0.0145 and resulted in the following equation 0.06275 C0.513.
[1] Periasamy. K and Namasivayam.C, 1994, ―Ind. Eng. Chem. Res‖. 33,317– 320.
IJSER © 2012
International Journal of Scientific & Engineering Research, Volume 3, Issue 8, August-2012
ISSN 2229-5518
[2] Periasamy. K and Namasivayam.C, 1995, ―Sep. Sci. Technol‖. 30, 2223– 2237.
[3] Emsley. J, 1989, ―The Elements‖, Oxford Univ. Press,
Oxford, p. 213.
[4] O‘Neill. P, 1985, ―Environmental Chemistry, second ed‖,
Chapman & Hall, London, p. 215.
[5] Leyva-Ramos.R, Bernal-Jacome.L.A, Mendoza-Barron.J, Fuentes-Rubio.L, Guerrero- Coronado. R.M, 2002,
―Journal of Hazardous Materials, 90, 2738.
[6] Carrott. P.J.M, Ribeiro-Carrot. M.M.L, Nabais. J.M.V and
Prates- Ramalho. J.P, 1997, ―Carbon‖, 35, 403–410.
[7] Wilson .J.A, Pulford I.D and Thomas. S, 2003, ―Environ. Geochem, Health‖ 25, 51–56.
[8] Cheung.C.W, Chan.C.K, Porter.J.F and McKay.G., 2001,
―Environ. Sci. Techol‖, 35, 1511– 1522.
[9] Galiatsatou.P, Metaxas. M, Kasselouri-Rigopoulou.V,
2002, ―Journal of Hazardous Materials‖, 91,187–203.
[10] Babic.B.M, Milonjic.S.K, Polovina.M.J, Cupic.S and
Kaludjerovic.B.V, 2002, ―Carbon‖ 40, 1109–1115.
[11] Seco. A, Marzal, P, Gabaldon.C and Ferrer.J, 1997, ―J.
Chem. Technol. Biotechnol‘. 68, 23–30.
[12] Choi.J.Y and Kim.D.S, 2002, "Environ. Sci. Health A Toxic/Haz. Subst. Environ. Eng‖, 37,701–1719.
[13] Mohan.D and SinghK.P., 2002, ―Water
Research‖,36,304–2318.
[14] Dastgheib. S.A and Rockstraw D.A., 2002, ―Carbon‖,
40, 1853–1861.
15] Juang. R.S and Shao. H.J, 2002, ―Adsorption‖ 8, 71–78.
[16] Mohan.D and Chander.S, 2001, ―Colloids Surf. A
Physicochem. Eng. Aspects‖, 177, 183– 196.
[17] Bansode.R.R, Losso .J.N, Marshall .W.E, Rao R.M and
Portier.R.J, 2003, ―Bioresour. Technol‖, 89, 115–119.
[18] Ricordel .S, Taha .S, Cisse. I and Dorange.G, 2001,
―Separation and Purification Technology‖, 24, 389–401.
[19] Bansal.R.C, Bonnet. J.B and Stoeckli. F, 1988,‖Active
Carbon‖, Dekker, New York , p. iii.
IJSER © 2012