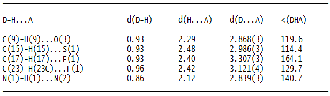International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 1024
ISSN 2229-5518
Synthesis, Characterization, Biological Acvtivity and Crystal Structures of 2(3’,4’-Dimethoxy Phe- nyl imino)–3–o-Fluorophenyl Carboxamido-4,5- Trimethylene Thiophene(I) and 2(3’,4’,5’- Tri- methoxy Phenyl imino)-3-o-Fluorophenyl Carbox- amido-4,5-Trimethylene Thiophene (II)
S Subhadramma*, Naveen Chandra**, J Saravanan***
Abstract —The title compounds (I and II) have been prepared, characterized and crystallized to determine their crystal structures. The compounds I and II crystallize in space groups, Pna2 1 and P2 1 /c, respectively. The former has cell parameters: a = 8.3701(5), b =
19.8697(15), c = 12.3072(9) Å (orthorhombic system) with four asymmetric molecules in the unit cell. The molecule exhibits extensive
hydrogen bonding in the crystal lattice with C-H---O, C-H---F, N-H---N and C-H---S interactions with bridge distances vary from 2.839(3) to
3.307(3) Å. The predominant extensive hydrogen bonding is responsible for the arrangement of molecules in the unit cell. The intra and inter molecular hydrogen bonding play a greater role in the stability of the molecules in the lattice. Similarly compound II crystallizes in space group, P21/c with unit cell parameters: a = 12.0085(5), b = 9.1071(4), c = 20.8623(8) Å, β = 103.723° (3) with four asymmetric molecules in the unit cell. The compound II contains one extra –OCH3 group compared to compound I. Compound II also displays an extensive hydrogen bonding network of the type C-H---O, C-H---F, N-H---N and C-H---S with varying hydrogen bond distances from
2.808(2) to 3.441(2) Å. Both the compounds exhibit antimicrobial and antibacterial activities.
Index Terms— Activity, Antibacterial, Characterization, Crystal, Dimethoxy, Fluoro Phenyl, Hydrogen bonding, Structure
—————————— ——————————
1 INTRODUCTION
hiophenes belongs to the family of well-known heterocy- clic compounds. Due to their wide range of theraphatic properties substituted thiophenes and their bihetetrocy-
cles have received considerable importance [1]. A number of
Schiff bases and thiophenes derivatives have been synthe-
sized, characterized and reported to have significant and di-
verse biological activities such as antimicrobial [2], analgesic
[3], anti-inflammatory [4], antioxidant [5], antitumor [6] and
local anesthetic [7] activities. It may be noted that thiophenes can be fused with different heterocyclic nuclei giving rise to newer compounds with enhanced biological activities. Com- pounds such as thiopyrimidines have occupied pivotal posi- tions among the thiophenes derivatives. Several of these de- rivatives showed antiallergic [8], antibacterial [9], antidepres-
————————————————
*, author, is research scholar at Dr mgr educational and research institute University, Chennai and also an associate professor in physics at Vijaya college, Bangalore, India, Email ssb909@yahoo.com
**, corresponding author, is a professor at St. Joseph's college (Autono- mous), PG & Research Centre, Landford Road, Bangalore, India, Email:dr_naveen_chandra@yahoo.com
***, corresponding author, is Professor and HOD, Dept.of Pharmaceutical Chemistry at PES College of Pharmacy,Hanumanthnagar,Bangalore, India, Email:drjayes@gmail.com
sant [10], antidiabetic [11], analgesic and anti-inflammatory [12] activities. In view of these compounds there is an ambi- tion to synthesize certain tetrahydrobenzothiophene deriva- tives and evaluate them for their antimicrobial, antifungal and anti-bacterial activity. Here we report preparation, characteri- zation, crystal structure elucidation and anti-bacterial activity of the two title compounds I and II. It has been found that both the structures show an extensive hydrogen bonding net- work required for the stability of the molecules in the crystal- line lattice.
2 PREPARATION AND CHARACTERIZATION OF COM- POUNDS
2.1 Synthesis of 2-amino 3-o-fluoro phenyl -4,5- trimethylene thiophene
A mixture of o-fluoro cyanoacetamide and cyclopentanone taken in the ratio 1:1 with 2ml of glacial acetic acid and 2g of ammonium acetate was taken in 80ml of pure and dry ben- zene in a round bottom flask and the reaction mixture was refluxed on a water bath involving clean stark apparatus for about ten hours. The reaction mixture was cooled, poured into a separating funnel and the mixture was washed successively with 10% sodium carbonate solution followed by double dis-
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 1025
ISSN 2229-5518
tilled water. The organic layer was transferred to a round bot- tom flask and distilled to remove any traces of benzene which was then collected. The syrupy residue obtained was then transferred to a 100 ml capacity conical flask. To this flask 1.3 g of sulfur was added followed by 30 ml of ethanol and reaction mixture was thoroughly stirred at a temperature between 45-
50°C in the presence of diethyl amine until the sulfur goes into the solution. The solvent was removed by evaporation under vacuum.
2.2 Synthesis of Compound I and II
Compound I was synthesized with equimolar mixture (0.05 mole) of 2-amino-3–o-fluoro phenyl-4,5-trimethylene
thiophene and 3,4-dimethoxy benzaldehyde was refluxed with a few drops of glacial acetic acid in ethanol for about two hours and then the reaction mixture was cooled to obtain the crystals. The compound was recrystallized from isopropanol. Compound II was synthesized by taking 3,4,5- trimethoxy benzaldehyde with 2-amino-3–o-fluoro phenyl -4,5- trimethylene thiophene by taking equimolar mixture of these two reactants. The reaction mixture was cooled and recrystallized from isopropanol.
2.3 Characterization of compounds I and II
2.3.1 FT-IR spectra
The formation of the title compounds I and II has been further characterized by FT-IR and 1H NMR spectra. In addition to this information substantial proof for the occurance of the title compounds has been provided by differences in their melting points and Rf values by TLC method from that of the parent compound. The IR spectra predicts that the formation of Schiff bases of the two compounds. The presence of specific IR peaks at 1532 cm-1 in both the Shciff bases indicate the N=CH- peak. The other important peaks include 3535 (--NH-), 3170 (Arom- CH), 2924 (Ali-CH), 1627(C=O) 1543 (C=N), 1298(C-O);
1267(C-N), 749(C-S).
2.3.2 1H NMR
The results of the proton NMR of the two compounds I and II are given as: 8.89 (s, 1H, -N=CH- ); 8.30 (s,1H, CH ArH phenyl ring); 7.7-7.6 (m,2H,CH,ArH, phenylring ), 3.8( s, 6H , -OCH3 for dimethoxy gps) , 3.8 - 3.6 ( s, 9H , -OCH3 for trimethoxy gps), 2.55(m,4H, -CH2- of cyclopentane ring); 1.95(m,2H, - CH2- of cyclopentane ring).
2.3.3 Mass Spectra
The mass spectrum of both the compounds I and II showed molecular ion peaks at 408 & 438, respectively which is one more than the actual molecular weight of compounds and is the M+1 peak of the compounds I and II.
3 CRYSTAL STRUCTURE DETERMINATION OF COMPOUNDS I AND II
Nice parallelo piped crystals of the title compounds with moderately yellow color were grown from a solution of the compounds in isopropanol at room temperature (25ºC) by slow evaporation technique [20]. A suitable crystal was select- ed after examining it under an analytical polarizing micro- scope for its uniform extinction for data collection. Unit cell parameters were obtained from a set of different weighted
intensity reciprocal lattice points. These parameters were re- fined and based on these unit cell parameters a set of weighted reflection intensities were collected from different parts of the reciprocal space after applying corrections for Lorentz and polarization effects [20]. These intensities were normalized and used in the structure determination of the molecule by direct method of phase determination [20]. The structures of the molecules were obtained and refined by the several cycles of the least-squares method by incorporating isothermal and anisothermal parameters for non-hydrogen. All the positions of hydrogen atoms are geometrically fixed and their parame- ters were not refined. The final R with value of R=0.0358 with weighting factor of 0.0782 for compound I and R=0.0392 with weighting factor of 0.0959 were obtained. The refinement of the structures was consistent because the parameters obtained are within the allowed permissible range. SHELXL97 was used in the structure determination and refinement of the structure. The molecular ORTEP thermal ellipsoid plots are depicted in Figs. 1a and 1b and the crystal packing of the asymmetric molecules are given in Figs. 2a and 2b for the compounds I and II, respectively. The data collection parame- ters and other associated parameters both compunds for are given in Tables 1A and 1B.
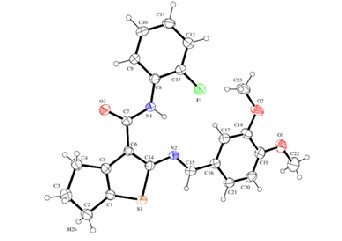
Fig.1a An ORTEP diagram with 50% thermal ellipsoid probability for the compound I. Flourine and oxygen atoms are indicated by green and red colors.
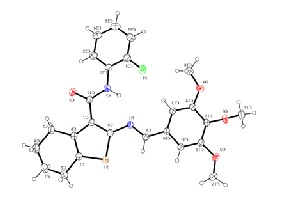
Fig.1b An ORTEP diagram with 50% thermal ellipsoid probability for the compound II.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 1026
ISSN 2229-5518
| -14<=l<=14 |
Reflections collected / unique | 16624 / 3525 [R(int) = 0.0393] |
Completeness to theta | 24.91 99.3 % |
Max. and min. transmission | 0.952 and 0.911 |
Refinement method | Full-matrix least-squares on F2 |
Data / restraints / parame- ters | 3525 / 1 / 273 |
Goodness-of-fit on F2 | 1.028 |
Final R indices [I>2sigma(I)] | R1 = 0.0358, wR2 = 0.0781 |
R indices (all data) | R1 = 0.0497, wR2 = 0.0844 |
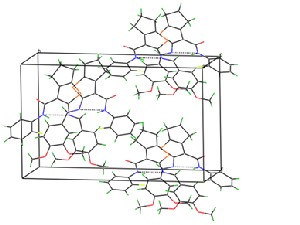
Fig. 2a Crystal packing diagram for the compound I
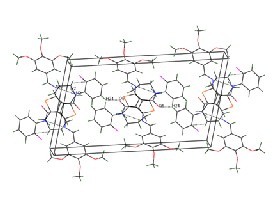
Fig. 2b Crystal packing diagram for the compound II
TABLE 1A
CRYSTAL DATA FOR COMPOUND I
TABLE 2A
CRYSTAL DATA FOR COMPOUND 2
Parameter | Compound I |
Empirical formula | C23 H21 F N2 O3 S |
Formula weight | 424.48 |
Temperature | 293(2) K |
Wavelength | 0.71073 Å |
Space group | Pna21 |
Unit cell dimensions | a = 8.3701(5) Å b = 19.8697(15) Å c = 12.3072(9) Å |
Volume | 2046.8(2) Å3 |
Z, Calculated density | 4, 1.377 Mg/m3 |
Absorption coefficient | 0.195 mm-1 |
F(000) | 888 |
Crystal size | (0.30 x 0.20 x 0.20)mm |
Theta range for data collec- tion | 2.64 to 24.91° |
Limiting indices | -9<=h<=9, -23<=k<=23, |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 1027
ISSN 2229-5518
4 RESULTS AND DISCUSSION
The final atomic fractional coordinate (x104 Å) and their equiv- alent isotropic displacement parameters (Å2x103) of the non- hydrogen atoms are given in Table 2a and Table 2b respective- ly. The bond distances and bond angles are given in Table 3 for the molecules I and II. It is seen from the table that the bond distances and angles in both the compounds I and II are within the normal range with 3 times of e.s.ds. The molecules are packed in the unit cells nicely by inter and intra hydrogen bondings (Table 4A and Table 4B). The fluorine F atom in compound I is involved in hydrogen bonding of the type: C(17)–H(17)---F, 3.307 (3) Å and C(23)–H(23)----F with hydro- gen bond distance 3.121(4)Å. Also sulfur atom is involved in hydrogen bonding of the type C(15)-H(15)---S with hydrogen bridge distance 2.986(3) Å. In the case of compound II, F at- om is involoved in hydrogen bonding of the type: C(15)-H(15)-
--F with hydrogen bond distance 3.143(2)Å and S atom is also involved in the hydrogen bonding of the type: C(9)-H(9)---S with hydrogen bridge distsnce 2.969 (2) Å. In both compounds I and II, both nitrogen atoms are involved in the hydrogen bonding interactions of the type: N(1)-H(1)---N(2) with bridge distances 2.839(3) and 2.808(2) Å, respectively.There are also hydrogen bonding ineractions in compound I of the type: C(9)-H(9)---O(3) with hydrogen bond distance 2.868(3) Å. Whereas in compound II, the two O atoms are involved in hydrogen bondings of the type: C(11)-H(11)---O(3) and C(25)- H(25)---O(5) with hydrogen bond distances 3.441(2) and
2.853(3) Å, respectively. In compound I, due to extensive hy- drogen bonding network, the molecules are arranged in a spi- ral manner in the unit cell. In compound II, the hydrogen bonding scheme is mainly responsible for the packing of mol- ecules parallel to the longest c-axis. The packing consideration in both the molecules due to extensive hydrogen bonding leads to stability of the molecules in the unit cell.
The two compounds have been screened for their antimicrobi- al activity. It has been found from the screening results that the two compounds exhibit a potential candidate for their an- tibacterial activity when tested with E.coli. The presence of electron withdrawing group and methoxy groups in both the compounds have made them good antibacterial agents in comparison to standard drug ampicillin. The values obtained have been found to be 16 and 17 mm using cup-plate method against ampicillin which is 21 mm. In this connection, proba- bly the electron withdrawing groups and methoxy groups play a greater role in the antibacterial property of the com- pounds I and II. These values are comparable with the ob- served values by earlier workers [1]. It may be noted here that compound II with its three methoxy groups show a higher antibacterial activity than compound I.
The antibacterial activity may be attributed to the inter and intra hydrogen bonding interactions and also the propensity of the molecules to pack together as dimers involving N-H---N and C-H…O bonding interactions along with C-H---S and C- H---F interactions lead to the possible variation in the nature of packing motifs. These kind of interactions involving O-H---
O, C-H---O and C-H---Cl interactions may be responsible for the biological activity of the molecues which is responsible for molecular basis for drug design as observed in the case of crystal structure of atovaquone [22].
TABLE 2A
ATOMIC COORDINATES (X104) AND EQUIVALENT ISOTROPIC DIS- PLACEMENT PARAMETERS (A2 X 103) FOR COMPOUND I
TABLE 2B
ATOMIC COORDINATES (X104) AND EQUIVALENT ISOTROPIC DIS- PLACEMENT PARAMETERS (A2 X 103) FOR COMPOUND II
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 1028
ISSN 2229-5518
TABLE 3
BOND LENGTHS [A] AND ANGLES [DEG] OF COMPOUNDS I AND II
Compound I | Compound II |
C(1)-C(5) C(1)-C(2) C(1)-S(1) C(2)-C(3) C(2)-H(2A) C(2)-H(2B) C(3)-C(4) C(3)-H(3A) C(3)-H(3B) C(4)-C(5) C(4)-H(4A) C(4)-H(4B) C(5)-C(6) C(6)-C(14) C(6)-C(7) C(7)-O(3) C(7)-N(1) C(8)-C(13) C(8)-C(9) C(8)-N(1) C(9)-C(10) C(9)-H(9) C(10)-C(11) C(10)-H(10) C(11)-C(12) C(11)-H(11) C(12)-C(13) C(12)-H(12) C(13)-F(1) C(14)-N(2) C(14)-S(1) C(15)-N(2) C(15)-C(16) C(15)-H(15) C(16)-C(21) C(16)-C(17) C(17)-C(18) C(17)-H(17) C(18)-O(2) C(18)-C(19) C(19)-O(1) C(19)-C(20) C(20)-C(21) C(20)-H(20) C(21)-H(21) C(22)-O(1) C(22)-H(22A) C(22)-H(22B) C(22)-H(22C) C(23)-O(2) C(23)-H(23A) C(23)-H(23B) | 1.341(3) 1.487(4) 1.715(3) 1.511(4) 0.9700 0.9700 1.546(4) 0.9700 0.9700 1.503(4) 0.9700 0.9700 1.428(3) 1.384(3) 1.481(3) 1.222(3) 1.362(3) 1.362(4) 1.388(4) 1.397(3) 1.375(4) 0.9300 1.358(4) 0.9300 1.368(4) 0.9300 1.363(4) 0.9300 1.362(3) 1.386(3) 1.754(2) 1.274(3) 1.453(4) 0.9300 1.378(3) 1.393(3) 1.367(4) 0.9300 1.365(3) 1.402(4) 1.356(3) 1.371(4) 1.384(4) 0.9300 0.9300 1.429(4) 0.9600 0.9600 0.9600 1.407(3) 0.9600 0.9600 | C(1)-C(6) C(1)-C(2) C(1)-S(1) C(2)-C(3) C(2)-H(2A) C(2)-H(2B) C(3)-C(4) C(3)-H(3A) C(3)-H(3B) C(4)-C(5) C(4)-H(4A) C(4)-H(4B) C(5)-C(6) C(5)-H(5A) C(5)-H(5B) C(6)-C(7) C(7)-C(8) C(7)-C(19) C(8)-N(1) C(8)-S(1) C(9)-N(1) C(9)-C(10) C(9)-H(9) C(10)-C(15) C(10)-C(11) C(11)-C(12) C(11)-H(11) C(12)-O(2) C(12)-C(13) C(13)-O(3) C(13)-C(14) C(14)-O(4) C(14)-C(15) C(15)-H(15) C(16)-O(2) C(16)-H(16A) C(16)-H(16B) C(16)-H(16C) C(17)-O(3) C(17)-H(17A) C(17)-H(17B) C(17)-H(17C) C(18)-O(4) C(18)-H(18A) C(18)-H(18B) C(18)-H(18C) C(19)-O(5) C(19)-N(2) C(20)-C(21) C(20)-C(25) C(20)-N(2) C(21)-C(22) | 1.354(3) 1.497(3) 1.7176(19) 1.515(3) 0.9700 0.9700 1.479(3) 0.9700 0.9700 1.523(3) 0.9700 0.9700 1.512(3) 0.9700 0.9700 1.440(3) 1.383(3) 1.490(3) 1.399(2) 1.740(2) 1.279(2) 1.455(3) 0.9300 1.391(3) 1.395(3) 1.382(3) 0.9300 1.370(2) 1.385(3) 1.376(2) 1.393(3) 1.364(2) 1.384(3) 0.9300 1.405(3) 0.9600 0.9600 0.9600 1.417(3) 0.9600 0.9600 0.9600 1.397(3) 0.9600 0.9600 0.9600 1.217(2) 1.358(3) 1.377(3) 1.393(3) 1.403(3) 1.366(3) |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 1029
ISSN 2229-5518
TABLE 4A
HYDROGEN BONDS [A AND DEG] OF COMPOUNDS I
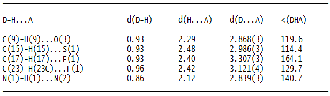
TABLE 4B
HYDROGEN BONDS [A AND DEG] OF COMPOUNDS II

TABLE 5A
ANISOTROPIC DISPLACEMENT PARAMETERS (A2 X 103)
OF COMPOUNDS I
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 1030
ISSN 2229-5518
TABLE 5B
ANISOTROPIC DISPLACEMENT PARAMETERS (A2 X 103)
OF COMPOUNDS II
TABLE 6A
HYDROGEN COORDINATES(X104) AND ISOTROPIC DISPLACEMENT PARAMETERS (A2 X 103) OF COMPOUNDS I
TABLE 6B
HYDROGEN COORDINATES(X104) AND ISOTROPIC DISPLACEMENT PARAMETERS (A2 X 103) OF COMPOUNDS II
TABLE 7
TORSION ANGLES [DEG] OF COMPOUNDS I AND COMPOUND II
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 1031
ISSN 2229-5518
S(1)-C(14)-N(2)-C(15) C(20)-C(19)-O(1)-C(22) C(18)-C(19)-O(1)-C(22) C(17)-C(18)-O(2)-C(23) C(19)-C(18)-O(2)-C(23) C(5)-C(1)-S(1)-C(14) C(2)-C(1)-S(1)-C(14) C(6)-C(14)-S(1)-C(1) N(2)-C(14)-S(1)-C(1) | -1.3(3) 2.6(4) -177.0(3) -5.7(4) 174.8(2) 1.5(2) -176.1(3) -2.09(18) 177.85(19) | S(1)-C(8)-N(1)-C(9) O(5)-C(19)-N(2)-C(20) C(7)-C(19)-N(2)-C(20) C(21)-C(20)-N(2)-C(19) C(25)-C(20)-N(2)-C(19) C(11)-C(12)-O(2)-C(16) C(13)-C(12)-O(2)-C(16) C(12)-C(13)-O(3)-C(17) C(14)-C(13)-O(3)-C(17) C(15)-C(14)-O(4)-C(18) C(13)-C(14)-O(4)-C(18) C(6)-C(1)-S(1)-C(8) C(2)-C(1)-S(1)-C(8) C(7)-C(8)-S(1)-C(1) N(1)-C(8)-S(1)-C(1) | -6.9(2) -0.3(3) 179.11(19) -163.6(2) 17.4(3) 19.5(3) -161.4(2) 91.7(2) -91.4(2) -15.6(3) 165.0(2) 0.72(16) -176.50(18) -1.02(15) 176.64(16) |
5 Conclusion
Substituted thiophenes with electron withdrawing and meth- oxy groups play a vital role in their biological activity as anti- bacterial. The results of the present research indicate that the two molecules are nicely packed in the unit cell with extensive hydrogen bonding network. They exhibit a greater antibacte- rial activity. It may be interesting to see that more different substituted groups on the thiophene molecule could be a fu- ture investigation followed by their three-dimensional struc- ture determination.
6 Acknowledgment
The authors thank Dr. Babu Varghese for his help in determining the structures of the molecules and also to utilize the facilities available at Sophisticated Analytical Instrument Facility (SAIF) Center of IIT Madras. The authors also thank Mr. Vamsy for his help in preparing this manuscript.
7 REFERENCES
[1] Raghav Mishra, Isha Tomer, Sachin Kumar, Der Pharmacia Sinica,
2012: 3(3); 332-336
[2] I. C. F. Ferreira, R. C. Calhelha, L. M. Estevinho, M. J. R. Queiroz; Bioorg. Med. Chem. Lett.; 2004; 14; 5831.
[3] S. Shafeeque, S. Mohan, K. S. Manjunatha; Indian J. Hetero. Chem.;
1999; 8(4): 297.
[4] U. V. Laddi, M. B. Talwar, S. R. Desai, Y. S. Somannavar, R. S. Bennur, S. C. Bennur; Indian Drugs; 1998; 35(8); 509.
[5] C. F. R. Ferreira, R. P. Maria-Joao, M . Vilas-Boas, L. M. Estevinho, A.
Begouin, K. Gilbert; Bioorg. Med. Chem. Lett. 2006; 16; 1384.
[6] I. Jarak I, M. Kralj M, L. Suman L, G. Pavlovic G, J . Dogan J, I. Pianta- nida; J. Med. Chem.; 2005; 48;2346
[7] A. K. Gadad, H. Kumar, C. J. Shishoo, I. Mkhazi, C. S. Mahajanshetti; Ind. J. Chem. Soc.; 1994; 33; 298.
[8] E. Gillespie, K. M. Dungan, A. W. Gomol, R. J. Seidehamel; Int. J. Im- munopharmaco.; 1985; 7(5); 655.
[9] E. F. Elslager, P. Jacob, L. M. Werbel; J. Hetero. Chem.; 1972; 9; 775. [10] Chem. Abstr.; 1994; 120; 290120.
[11] Chem. Abstr.; 1977; 87; 117896.
[12] A. Santagati, M. Modica, M. Santagati, A. Garuso; Pharmazie; 1994;
49(1); 6.
[13] K. Gewald, E. Schinke, H. Bottcher; Chem. Ber.; 1966; 99; 94.
[14] Mohan, S. & Saravanan, J. (2002). Indian J. Heterocycl. Chem. 12, 87±88. [15] Mohan, S. & Saravanan, J. (2003). Asian J. Chem. 15, 67±70.
[16] Vasu, Nirmala, K. A., Choudhury, A. R., Mohan, S., Saravanan, J. & Narasimhamurthy, T. (2003). Acta Cryst. C59, o676±o678.
[17] Watkin, D. M., Pearce, L. & Prout, C. K. (1993). CAMERON. Chemical
[18] Lakshmi, V. V., Sridhar, P. & Polasa, H. (1985). Indian J. Pharm. Sci. 47,
202±204
[19] Vasu,a K. A. Nirmala,b Deepak Chopra,c* S. Mohan. d and J. Sara- vanan
[20] X-ray structure determination II edition, A practical guide. George. H.
Stout & Lyle H. Jensen, A Wiley-interscience publication John Wiley & Sons, Chap 4. Pg 75-78, Chap 7. Pg 171, Chap 10. Pg 245-246, Chap 11. Pg 249-278, Chap 16. Pg 344-378, Chap 17. Pg 378-390
[21] G.N. Anil Kumar, thesis 2010
[22] Susanta K. Nayak, Srijita Basu Mallik, Shankar Prasad Kanaujia, Kana- garaj Sekar, K. R. Ranganathan, V. Ananthalakshmi, G. Jeyaraman, S. S. Saralaya, K. Sundararaja Rao, K. Shridhara, K. Nagarajan and Tayur N. Guru Row CrystEngComm, 2013,15, 4871-4884
IJSER © 2013 http://www.ijser.org