International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 1909
ISSN 2229-5518
Study on synthesis of methyl oleate catalyzed by ceric ammonium sulfate
Xu Long, Li Jian, Yang Lina*, Dong Jiali, Sun Yumeng
(School of Petroleum and Chemical Technology, College of Chemistry, Chemical Engineering and Environmental Engineering, Liaoning Shihua University, Fushun 113001, China)
molar ratio of methanol to oleic acid is 1.6, and the amount of catalyst is 6%(wt), it should be noted that the catalyst can be reused for 5 times without obvious loss of its activity.
Index Terms—Methyl oleate;Ammonium ceric sulfate;Catalytic;Esterification
—————————— ——————————
ITH the fossil fuels becoming depleted, coal, oil and natural gas prices continue to rise, the environmental pollution problems caused by fossil fuel combustion
and vehicle exhaust emissions gradual deterioration, make human urgent need to find new, clean alternative energy [1]. Biodiesel (fatty acid methyl ester) become the generation products to petroleum products due to their biodegradable, renewable, clean and non-toxic, burning completely and many other advantages. At the same time, methyl oleate in the oleo- chemical industry occupies an important position, can replace fatty acid as a raw material of many oleochemicals, can be used as detergents, emulsifiers, wetting agents and intermedi- ate stabilizer. Usage of methyl oleate is gradually increasing, be used as pesticide adjuvant can improve the penetration of pesticides, more quickly and effectively eradicate pests, role play the obvious synergism on insecticide. It is obtained by methanol and oleic acid role in the concentrated sulfuric acid after esterification, mainly in the industrial production. But there are many byproducts, equipment corrosion and serious environmental pollution problems. Since liquid acid and products are the same as the liquid phase, it will be very diffi- cult to separate them completely, therefore people carried out a lot of improvements in the catalyst and production process, such as use solid acid[2], ionic liquids[3,4] and mesoporous mo- lecular sieves[5,6] etc. instead of sulfuric acid, but there is not reports about ceric ammonium sulfate as catalyst for the syn- thesis of methyl oleate. The ceric ammonium sulfate was used
Oleic acid (AR, Tianjin HENG XING Chemical Reagent
Co., Ltd.) ; Methanol(AR, Tianjin GUANG FU Fine
Chemical Plant); Ceric ammonium sulfate(AR, Beijing
Chemical Reagent); Sodium hydroxide(AR, LIAONING
XINXING Reagent Co., Ltd.); Ethanol(AR, LIAONING
XINXING Reagent Co., Ltd).
DF-101S collector constant temperature heating magnetic stir-
rer(GONGYI YUHUA Instrument Co., Ltd.); AUY220 electron- ic balance(SHANG HUA GUANG ZHENG Medical Instru- ment Co., Ltd.); Magnetic stirrer(XIANG SU TAI XIAN Ana- lytical Instrument Factory).
Some amount of oleic acid, methanol and catalyst were put into a single necked flask with a reflux condenser in a thermo- static heating magnetic stirrer, after the reaction at needed conditions the reaction system was cooled to room tempera- ture.
The acid value before and after the reaction was determined based on GBT1668-2008. and the esterification conversion is calculated as follows:
oleinic acid conversion% =
The initial acid of the reaction - The edd acid of reaction
as catalyst for the synthesis of methyl oleate in this study, the![]()
The initial acid of the reaction
×100%
results show that its catalytic activity is excellent and the reus-
V − V
ing ability of the catalyst is outstanding.
= 0 t
V0
×100%
V 0 , V t —Volume of the NaOH-ethanol solution consumed for the reaction system before and after the reaction respectively.
————————————————
• Xu Long postgraduate students engaged in the production of clean fuels in
Liaoning Shihua University, China. E-mail: xulongliaogong@126.com
• Professor Yang Lina* engaged in the production of clean fuesl in Liaoning
Shihua University, China. E-mail: lnqdsd@yahoo.com.cn
can be identify in the intricacies of various factors, there are
rules of analysis the impact of indicators of various factors. We
IJSER © 2013
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 1910
ISSN 2229-5518
therefore adopted orthogonal experimental design method, select L9 (34) table, test each factor and horizontal are shown in Table 1, testing program and the results are shown in table 2.
TABLE 1
ORTHOGONAL LEVEL OF FORM FACTORS
![]()
Factors![]()
![]()
![]()
![]()
![]()
h % ℃
1 1.2 2 2 70
2 1.4 3 4 75
3 1.6 4 6 80
![]()
Note: the amount of catalyst = (mass of catalyst / mass of oleic acid) × 100%
TABLE 2
100
90
80
70
30
20
10
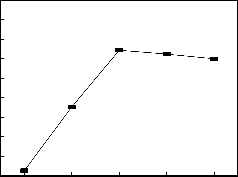
65 70 75 80 85
Reaction temperature/℃
EXPERIMENTAL PROGRAM AND RESULTS OF ANALYSIS
![]()
Factors Conversion
Fig. 1 The affection of reaction temperature to the conversion
![]()
NO.![]()
A B C D %
The impact of reaction time on the conversion of esterification
1 1 1 1 1 29.87
2 1 2 2 2 36.67
3 1 3 3 3 78.00
4 2 1 2 3 43.33
5 2 2 3 1 24.00
6 2 3 1 2 86.21
7 3 1 3 2 71.52
8 3 2 1 3 82.91
9 3 3 2 1 55.33
R1 59.29 48.24 66.33 36.40
R2 51.18 58.97 56.22 75.91
R3 69.92 73.18 57.84 68.08![]()
R 18.74 24.94 10.11 39.51
Table 2 shows the influence extent order of each factor: D>B>A>C, and the optimal combination obtained by orthog- onal experiment is D2 B3 A3 C1 , the optimal conditions reaction
is temperature 75℃, reaction time 4h, molar ratio 1.6, 2% of
the amount of catalyst in the esterification.
The impact of reaction temperature on the conversion of ester- ification is studied in fig.1 at such conditions: amount of cata- lyst 2%, reaction time 2.5h, molar ratio1.6.
It is seen from fig.1 that as the reaction temperature elevated, oleic acid conversion is increasing before 75℃ then declined
slightly when the temperature is higher. This is because the esterification reaction is reversible, when the temperature is
below 75 ℃, the reaction is kinetically controlled, elevated
temperatures conducive to positive reaction direction, the
conversion increased; When the temperature is higher than 75
℃, reaction is controlled by thermodynamics, elevated tem-
perature conducive to endothermic reverse reaction, the con- version is lower therefore the best reaction temperature is 75℃
.
is studied in fig.2 at such conditions: amount of catalyst 2%, reaction temperature 75℃, molar ratio1.6.
100
90
80
70
60
50
40
30
20
10
1 2 3 4
Reaction time/h
Fig. 2 The affection of reaction time to the conversion
It is seen from fig.2 that as the reaction time increased, oleic acid conversion increased gradually, but the conversion changes slowly when the reaction time is longer than 3.5 h, then the system has basically reached the equilibrium.
The impact of molar ratio of the raw material on the conver- sion of esterification is studied in fig.3 at such conditions:
amount of catalyst 2%, reaction temperature 75℃, reaction
time 4 h.
It is seen from fig.3 that esterification of oleic acid is increased
with molar ratio of the increase, When the molar ratio is 1.6,
reaches a maximum, and then decreased. This is because as
the mole ratio increases, relatively lower concentrations of
oleic acid, the reaction rate is reduced, thus conversion de- clined, therefore the optimal molar ratio is 1.6.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 1911
ISSN 2229-5518
100
90
80
70
60
50
40
30
20
10

100
90
80
70
60
50
40
30
20
10
Fig. 3 The affection of molar ratio to the conversion
The impact of the amount of catalyst on the conversion of es- terification is studied in fig.4 at such conditions: reaction tem-
perature 75℃, molar ratio1.6, reaction time 4h.
100
90
80
70
60
50
40
30
20
10
1 2 3 4 5 6 7 8
the Amount of catalyst/%
Fig. 4 The affection of the amount of catalyst to the conversion
It is seen from fig.4 with the increase of the catalyst amount, its conversion increases significantly. When the amount of ceric ammonium sulfate is 6%, its conversion reached 84.37%, however, further increase of catalyst amount, will not change the conversion significantly, so the amount of catalyst is se- lected as 6%.
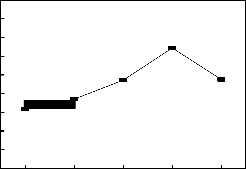
Reusing ability of the catalyst is studied under the optimal experimental conditions, the catalyst was collected by filtra- tion after the reaction, then the catalyst is used for new reac- tion, every reaction result for each cycle of reaction was shown in fig.5.
It is shown in fig.5 that the catalyst’s activity hasn’t obvious decrease even after 5 cycles of reactions; therefore such cata- lyst’s reusing ability is outstanding.
Fig. 5 The affection of catalyst can be reused to the conversion
Ceric ammonium sulfate has good catalytic activity and reus- ing ability in the reaction of oleic acid and methanol, the pro- cess is simple, and no pollution basically produces. The opti-
mal reaction conditions are: the reaction temperature 75℃,
reaction time 4h, molar ratio of 1.6, ceric ammonium sulfate
catalyst dosage 6%(wt), at such conditions the conversion can reach 86.3%, and the catalytic performance has no significantly changes even after five times of reactions.
[1] Farrell A E, Plevin R J, Turner B T, Jones A D, Ohare M, Kammen D M. Etha- nol can contribute to energy and environmental goals[J]. Science, 2006,
311(5760): 506-508.
[2] Guo HF, Yan P, Hao XY, Wang ZZ. Influences of introducing A1 on the solid super acid SO42-/SnO2 . Materials Chemistry and Physics[J]. 2008, 112(3):
1065-1068.
[3] Zhang L, Xian M, He Y, et al. A Bronsted acidic ionic liquid as an efficient and environmentally benign catalyst for biodiesel synthesis from free fatty acids and alcohols. Bioresource Technology[J]. 2009, 100(19): 4368-4373.
[4] Han M, Yi W, Wu Q, Liu Y, Hong Y, Wang D. Preparation of biodiesel from waste oils catalyzed by a Bronsted acidic ionic liquid. Bioresource Technolo- gy[J]. 2009, 100(7): 2308-2310.
[5] Shu Q, Yang B, Yuan H, Qing S, Zhu G. Synthesis of biodiesel from soybean oil and methanol catalyzed by zeolite beta modified with La3+. Catalysis Communications[J]. 2007, 8(12): 2159-2165
[6] Chung KH, Park BG. Esterification of oleic acid in soybean oil on zeolite cata- lysts with different acidity. Journal of Industrial and Engineering Chemis- try[J]. 2009, 15(3): 388-392.
IJSER © 2013 http://www.ijser.org