International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 876
ISSN 2229-5518
“Study of adsorption isotherms and thermodynamics for heavy metal removal from aqueous solutions using Canna indica biomass”
Archana Dixit, Savita Dixit, C.S. Goswami
Abstract- Surface water bodies like lakes, ponds, and streams anchorage the survival of aquatic life flora and fauna and maintain ecological balance. Due to urbanization population explosion, and industrialization, the water sources are getting polluted, present paper is an attempt to evaluate the adsorption of heavy metals like cadmium (Cd), chromium (Cr), zinc (Zn), and lead (Pb) by the dry biomass of Canna indica commonly called keli. Very
less literature is available on the adsorption capacity of this plant though is found in excess in Indian climate. Living C.indica plants have minor capacity to absorb heavy metals from waste water. The present experimental study was conducted to assess the adsorption capacity of dry biomass of C.indica to compare and identify their potential to improve the water quality by removing the impurities. The paper critically evaluates the water – purifying capacity of dry – biomass of C.indica. It also evaluates the extent up to which heavy metals can be removed by the dry biomass of this terrestrial plant in a given period of time.
Keywords - Plants, Dry biomass, C.indica, heavy metals, adsorption, isotherm, thermodynamics.
1 INTRODUCTION
Waste water is generated from residential and industrial day today activities but it must be treated before it is released into surface water bodies or to environment. So that it does not cause further pollution of water sources. Waste water comes from variety of sources. Everything that you flush down your toilet or rinse down the drain is waste water. Waste water can also come from agricultural and industrial sources.
Surface water bodies are the major sources of water to
human beings which satisfies many day today purposes. Water Quality of surface water bodies is subjected to the natural degradation, the process of eutrophication and the impact of human activities, [1]. Natural sources of water are depleting fast and are polluted due to industrialization and urbanization in haphazard manner.
Pollution of the aquatic bodies by (synthetics and organic)
pollutants like pesticides, polyaromatic hydrocarbons,
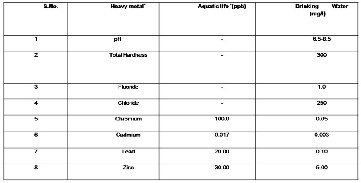
heavy metals, etc, have caused imbalance in the natural functioning of the ecosystem. All of these pollutants and heavy metals cause severe damage to the living system at various levels. The World Health Organization [2] have prescribed standards for heavy metals and other parameter in both aquatic life and drinking water as shown in (Table1)
The Potential toxic metal elements such as cadmium, chromium, lead, Copper, Zinc etc. are identified to cause health hazards in animals [3] , [4] these heavy metals are reported to be toxic and found associated with the occurrence of several health effects. Considering its effect on human being and aquatic life, appropriate treatment of heavy metals from waste water is of utmost importance. Increasing awareness of ecological hazard of toxic metals from urban and industrial sources has involved considerable interest in the study of levels and fate of heavy metals in the aquatic environment [5].
———————————————
Archana dixit is a Research scholar, from department of Chemistry, Maharani Laxmibai Girls College, Bhopal,India, PH-08871117510. Email: dixit.archana07@gmail.com
Savita Dixit and C.S. Goswami are Professors, from Department of Chemistry, Maulana Azad National Institute of Technology, Bhopal,India. and from Department of Chemistry, Kamal Radha Girls College, Gwalior, India. Email: savitadixit1@yahoo.com
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 877
ISSN 2229-5518
Table 1 Maximum permissible limit for the studied parameters (WHO standards)
Table 1 Maximum permissible limit for the studied parameters
(WHO standards)
Various techniques are being used to purify the water, like the installation of ozonizer, aerator, filtration units, sedimentation tanks, electro dialysis, reverse osmosis, addition of chemicals etc. All of these methodologies used are quite costly and energy intensive, none of them could claim to treat all the heavy metals in economically feasible manner [6]. Various waste biomaterials such as grape stalk waste [7], green coconut shell powder [8], chaff [9], and crab shell particles [10] have been studied for the removal of heavy metal ions [11] from the effluents.
Water can be purified by natural means
such as phytoremediation technique i.e. using plant for purification but in present study dry biomass of plant (C.indica) is used to overcome many inconvenience cased due to living plants. Plants have the ability to assist the breakdown of human and animal derived waste water and remove disease causing microorganisms and pollutants [12]. Their ability to absorb pollutants has been recognized worldwide in the treatment of water [13]. Vietmeyer [14] suggested that Eichhornia crassipes could be used for removal of nutrients and heavy metals from sewage and sludge ponds [15] studied on Hydrilla verticillata and observe the Bio- absorption of copper from wastewater.
Manuscript will be helpful in showing the water purifying capacity of dry biomass this terrestrial or semi aquatic plant (C.indica) and also will evaluate the best results of adsorption shown in varied time period.
2. MATERIALS AND METHODS
The present study is concentrated on the adsorption of four heavy metals viz. chromium, Cadmium, lead and zinc by dry biomass of C.indica for which the experiments are conducted. The plants of C.indica were taken from sahapura lake drainage basin and washed with Milli-Q water to eliminate the remains
of lake sediments and particulate matter, and then the plants are cut into pieces and sun dried. After being completely dried
/ dehydrated they are grinded into powder. The powder was grounded to pass through 2 mm sieve. The samples for analyzing various parameters were prepared by standard method [16] of 10, 50 and 100 mg/l concentration. In 100 ml of each of the samples of Heavy metal i.e. chromium, Cadmium, lead and zinc,1gm of the C.indica powder was added and then put into shaker at 60 rpm and at temperature of 280C, for varied time periods viz. 15 to 120 min and a control sample for all the test was also taken. And then the samples were filtered with whatmann no 40 filter paper after attaining their reaction period. All the experiments were set up in duplicate for all parameters a control set was also studied and there was no powder (dry biomass) added. All the parameter were analyzed by the method as mentioned in APHA i.e. Heavy metals with atomic absorption spectrophotometer.
3. Result and Discussion
Results presented in Table -1- (I), (II) indicate the effect of various dosage of adsorbent (powdered C.indica) at two different contact duration’s on equilibrium concentration, reaction rate constant and percent removal of Cd. Cr, Zn and Pb ions content when dosage vary from 0.5 gm/l to 4.0 gm/l. It also represents the data for the model of Freundlich and Langmuir adsorption isotherm for removal of Cd. Cr, Zn and Pb ions. For Freundlich adsorption isotherm the logarithmic values of equilibrium concentration (Ceq) and per gram removal (x/m) of Cd. Cr, Zn and Pb are given. For Langmuir isotherm model, the inverse values of equilibrium concentration i.e. 1/Ceq and removal per gram liter, i.e. 1/qe are considered.
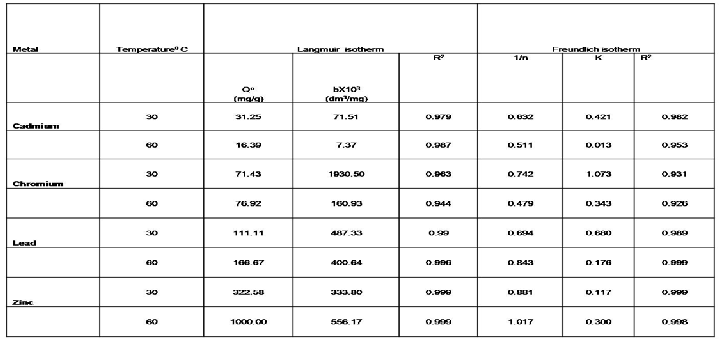
Table 2 represents the Langmuire and Freundlich isotherm
parameters for Cd. Cr, Zn and Pb ion . Here the ϴ0 value
(adsorption capacity) is the slope of the straight line and ‘b’ is the intercept on X-axis is the energy of adsorption. The freundlich isotherm parameter K is the intercept on X- axis and 1/n is the slop of straight line.
Fig.-1 represents adsorption of Cd, Cr, Pb, and Zn ion on
dry biomass of C.indica at various contact time from 15 to 120 minutes.
Fig. 2 - indicates Freundlich adsorption isotherm model i.e. the logarithemic values of equilibrium concentration and x/m (where x= removal of Cd. Cr, Zn and Pb ions amd m= weight of adsorbent) is plotted, which gives straight line.
Fig. 3- represents the data for Langmuir isotherm model
for the removal Cd. Cr, Zn and Pb using powdered C.indica as an adsorbent. The inverse values of equilibrium concentration (Ceq) Vs removal/ gm/1(qe) for heavy metals (Cd. Cr, Zn and Pb) is plotted for that.
Adsorption of Heavy metals
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 878
ISSN 2229-5518
Cadmium - Results after treating cadmium standard solution of 10 ppm concentration at different time are very encouraging. Adsorption % of 10 ppm 'Cd' solution in 15 – 120 min. it was found in the range of 60 to 96.5 % (Fig.1) it shows that the dry biomass adsorbed almost whole of the Cd metal ion. Here in this case also the adsorption concentration was directly proportional to increasing time. Cadmium has been recognized as a toxic metal for a long time but it attracted attention due to the spread of the disease 'itai-itai' in Japan. The exposure to cadmium for extended periods results in respiratory distress and uncontrolled cough and gastrointestinal pain. Its permissibility is 0.017 ppb for aquatic life and 0.003 mgL-1 for drinking water as per WHO.
Chromium- Dry biomass of C.indica efficiently adsorbed Cr from 10, 50, 100 mg/l concentration of Chromium solution at varied time period. The adsorption percent increased with increasing time (Fig 1-a). i.e. the adsorption shown by the material at normal condition of temperature and pressure at various time period starting from 15 min. to 120 min. the average percentage adsorption of chromium is 73.25 % . The results show that initially the adsorption rate was high and after duration of 2 hr. it slowly got reduced but overall the
adsorption was remarkably satisfying. Cr is very much toxic and carcinogenic for living beings.
Lead - The lead metal concentration was remarkably reduced by the dry biomass from the sample from 10 ppm to 1.5 ppm. There was the adsorption of about 89.58 % on an average. With the increasing time period the original concentration in the solution decreased with slow rate and this concentration increased with very slow rate with increasing time and remained almost stationary after 60 min attaining adsorption of 97 %. Lead is very much toxic and carcinogenic for living beings. Its permissible limit is 25.00 ppb aquatic life and 0.10 mg/l for drinking water as per WHO.
Zinc- Results obtained by treating 10 mg/l of zinc soln. for varied time period showed marked decrease in its concentration (Fig.1) with the contact period of only 1/2 hr. and then the values showed linear trend after 1 hr. The % adsorption of Zn metal ion of 10 mg/l concentration calculated for period of ½ hr., 1hr., and 2 hr., were 42 %, 58 % and 60% respectively. As we know, zinc is not very much toxic up to permissible limit (30.0 ppb for aquatic life and 5.0 mg L-1 for drinking water as per WHO). It is one of trace element for regulating plant metabolism.
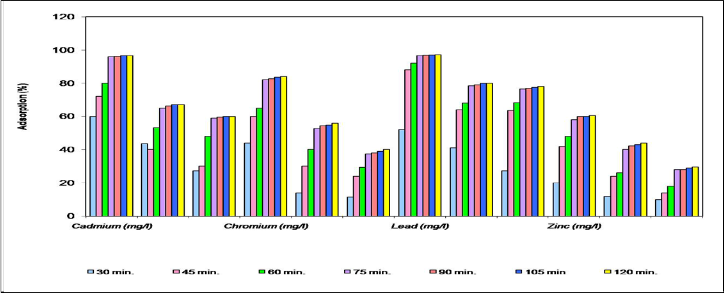
Fig. 1 Percent adsorption of heavy metals by dry biomass of C.indica
Adsorbent : Powdered C.indica Temperature : 28±1 0C
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014
ISSN 2229-5518
879
Size: 200 fA Contact time : 30 Minutes
| lnitiiiiConc. | F nalCone. | Removal | LoaCea | Loaae | 1/Cea | 1/ae |
| mgll | Ceq(mgll) | xlm =qe (mg/g) | | | | |
Cd | 5 | 0.21 | 2.395 | .678 | 0.379 | 4.7619 | 0.418 |
| 10 | 2 | 4 | 0.301 | 0.602 | 0.5 | 0.250 |
| 50 | 20 | 15 | 1.301 | 1.176 | 0.05 | 0.067 |
| 100 | 60 | 20 | 1.778 | 1.301 | 0.0166 | 0.050 |
| 500 | 350 | 75 | 2.544 | 1.875 | 0.0028 | 0.013 |
c.- | 5 | 0.32 | 4.84 | .495 | 0.685 | 3.125 | 0.207 |
| 10 | 0.42 | 9.79 | .377 | 0.991 | 2.3809 | 0.102 |
| 50 | 24 | 38 | 1.380 | 1.580 | 0.0416 | 0.026 |
| 100 | 70 | 65 | 1.845 | 1.813 | 0.0142 | 0.015 |
| 500 | 362 | 319 | 2559 | 2.504 | 0.0027 | 0.003 |
Pb | 5 | 0.27 | 4.865 | .569 | 0.687 | 3.7037 | 0.206 |
| 10 | 22 | 8.9 | 0.342 | 0.949 | 0.4545 | 0.112 |
| 50 | 20.8 | 39.6 | 1.318 | 1.598 | 0.0480 | 0.025 |
| 100 | 62.6 | 68.7 | 1.797 | 1.837 | 0.0159 | 0.015 |
| 500 | 352 | 324 | 2547 | 2.511 | 000284 | 0.003 |
Zn | 5 | 4 | 3 | 0.602 | 0.477 | 025 | 0.333 |
| 10 | 6.6 | 6.7 | 0.820 | 0.826 | 0.1515 | 0.149 |
| 50 | 35.8 | 32.1 | 1.554 | 1.507 | 0.0279 | 0.031 |
| 100 | 77.5 | 61.25 | 1.889 | 1.787 | 0.0129 | 0.016 |
| 500 | 446 | 277 | 2.649 | 2.442 | 0.0022 | 0.004 |
Adsorbent : Powdered C.indica Temperature : 28±1 oc
I£ER 2014 http://WWW.ISer.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014
ISSN 2229-5518
880
Size: 200 fA Contact time : 60 Minutes
| | Final Cone. | Removal | Log Ceq | Logqe | 1/Ceq | 1/qe |
| | Ceq(mgfl) | xlm =qe(mglg) | | | | |
Cd | 5 | 0.55 | 2.225 | -0.2596 | 0.3473 | 1.818 | 0.4494 |
| 10 | 5.6 | 2.2 | 0.7481 | 0.3424 | 0.1785 | 0.4545 |
| 50 | 36.8 | 6.6 | 1.5658 | 0.8195 | 0.0271 | 0.1515 |
| 100 | 75 | 12.5 | 1.8750 | 1.0969 | 0.0133 | 0.08 |
| 500 | 460 | 20 | 2.6627 | 1.3010 | 0.0022 | 0.05 |
Cr | 5 | 4.8 | 2.6 | 0.6812 | 0.41497 | 0.2083 | 0.3846 |
| 10 | 4.2 | 7.9 | 0.6232 | 0.8976 | 0.2381 | 0.1265 |
| 50 | 45.6 | 27.2 | 1.6589 | 1.4345 | 0.0219 | 0.0367 |
| 100 | 91.5 | 54.25 | 1.9614 | 1.7344 | 0.0109 | 0.0184 |
| 500 | 458 | 271 | 2.6608 | 2.4329 | 0.0022 | 0.0036 |
Pb | 5 | 4.8 | 2.6 | 0.6812 | 0.4149 | 0.2083 | 0.3846 |
| 10 | 6 | 7 | 0.7781 | 0.8450 | 0.1667 | 0.1428 |
| 50 | 38.8 | 30.6 | 1.5888 | 1.4857 | 0.0258 | 0.0326 |
| 100 | 78 | 61 | 1.8920 | 1.7853 | 0.0128 | 0.0163 |
| 500 | 462.5 | 268.8 | 2.6651 | 2.4293 | 0.0024 | 0.0037 |
Zn | 5 | 5 | 2.5 | 0.6989 | 0.3979 | 0.2 | 0.4 |
| 10 | 9.5 | 5.25 | 0.9777 | 0.7201 | 0.1053 | 0.1904 |
| 50 | 50 | 25 | 1.6989 | 1.3979 | 0.02 | 0.04 |
| 100 | 98.5 | 50.75 | 1.9934 | 1.7054 | 0.0102 | 0.0197 |
| 500 | 458 | 271 | 2.6608 | 2.4329 | 0.0022 | 0.0036 |
I£ER 2014 http://WWW.ISer.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 881
ISSN 2229-5518
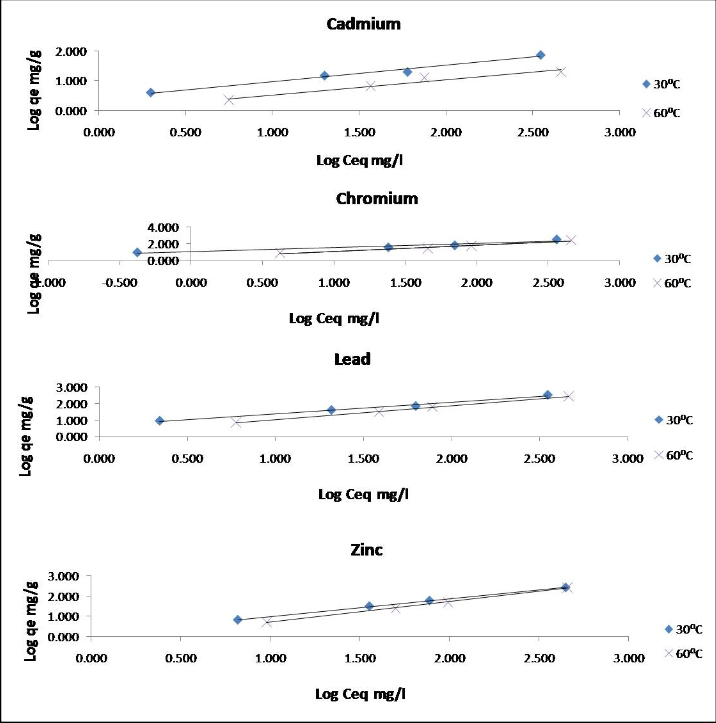
Fig. 2 Freundlich adsorption isotherm
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014

ISSN 2229-5518
882
cadmium
'110
........
'110
..........
ao
..E.... ao cu
0.4500
0.300 :
0.200 ----------- -.
g: I
0.000 0.100 0.200 0.300 0.400 0.500
1/Ceql/mg
Chromium
0.600
•
+30"<:
X 60"C
+30"<:
U"
':=i
0.000 0.500 1.000 1.500 2.000
1/Ceq 1/mg
2.500 X 60"C
0.200
0.100
Lead
j ------------- ..•.....
...... 0.000 -=- ==;:==---.---r----.----,----,---..,----.---,
ao
+30"<:
cu 0.000 0.050 0.100 0.150
U"
':=i
ao o.3oo l
0.200 0.250 0.300 0.350 0.400 0.450
1/Ceq 1/mg
Zinc
0.500
X 60"C
Eo.2oo •
-i op=o I
- · · - · - · -
"C'QUa
':U="i
0.1000
1 •- /+=-=/: •E::::::=;::::== =---.-----.----.----.-----,
0.020 0.040 0.060 0.080 0.100 0.120 0.140 0.160
+30"<:
X 60"C
0.000
1/Ceq 1/mg
Fig. 3 langmuir adsorption isotherm
I£ER 2014 http://WWW.IjSer.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 883
ISSN 2229-5518
Table 2 Freundlich & Langmuir isotherm parameters for Cd, Cr, Pb, and Zn
It is evident from the results presented in table 1 that increase temperature leads to gradual reduction in Cd. Cr, Zn and Pb content, this can be contributed to considerable increase in dissociation of adsorbent particles. Increase in temperature restricts adsorption of heavy metal ions to adsorbent molecule.
Increase in percent removal of Cd. Cr, Zn and Pb with
increase in contact time of adsorbent suggest more adsorption of these ions, supporting considerable increase in reaction rate. The equilibrium study has been conducted based on the commonly used monolayer and multi layer adsorption isotherm models of Langmuir and Freundlich. The adsorption data were fitted in two models viz., Freundlich and Langmuir isotherms.
The significance of adsorption isotherms is that they show
how the adsorbate molecules (metal ion in aqueous solution) are distributed between the solution and the adsorbent solids at equilibrium concentration at different temperatures. The purpose is to understand the strength of the solute-surface bond, during the study of adsorption form the solution. The nature of isotherm clearly indicates the dependence of the solvent on the level of adsorption.
Results of Table 1 representing Freundlich isotherm for Cd. Cr, Zn and Pb removal indicate that when contact period is increased from 30 to 60 min. lesser amount of adsorbent is required to attain equivalence concentration, while the same equivalence concentration is attained with more adsorbent at
contact period of 30 minutes. This can be interpreted as more removal of Cd. Cr, Zn and Pb contents with less amount of adsorbent, when longer contact period is permitted. In other words, increase in contact period which affects economics of the adsorption process and enhances the removal Cd. Cr, Zn and Pb contributing components of the waste water.
The linear plots at different temperatures indicate the applicability of the Freundlich equation the values Kf and n are calculated from the intercept and slope respectively of the linear plots of log q e versus log C eq . The constant K f, which is a measure of sorption capacity, decreases with increase of temperature the decrease in the values of Kf with increase in temperatures shows the decrease in adsorption. It validates the experimental observation about the decrease in percent adsorption with gradual increase in temperature.
The values of l/n are less than unity [17] , [18] for all the four
metal ions, showing the favorable adsorption of Pb2+ ,Cd2+ ,Cr6+
and Zn2+ on C.indica.
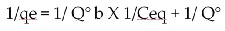
This equation is empirical expression that covers the heterogeneity of the surface and the exponential distribution of sites and their energy value of n and Kf is presented in table 1. The data presented in table 1 give linear natures of the plots presented in Fig.3; confirm applicability of Langmuire model also. Which is governed by the expression.
(Where, qe = amount of Cd. Cr, Zn and Pb adsorb per unit weight of absorbent (mg/g), Ceq = equilibrium concentration of
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 884
ISSN 2229-5518
Cd. Cr, Zn and Pb (mg/l), x = amount of Cd. Cr, Zn and Pb adsorbed (mg/l), m= weight of adsorbent (gm/l), Q° =
Langmuir constant ; related to the capacity of adsorption (mg/g), b = Langmuir constant ; related to the energy of adsorption (dm3/mg).)
The Langmuir constant (b) represents the degree of
adsorption affinity of the adsorbate. The maximum adsorption
capacity (Q) associated with complete monolayer cover is typically expressed in (mg/g). High value of b indicates for much stronger affinity of metal ion adsorption. The Langmuir equation is tested by plotting 1/qe versus l/Cqe for different metal ions present as single species in solution at different temperatures. The straight-line plots indicate the applicability of the model for the present systems. The values of Q0 and b for the metal and nutrient ions were calculated from the slopes and intercepts of the plots through regression analysis and the value of Q° and b decreases with increasing temperature indicating the exothermic nature of adsorption. The applicability of the four linear forms of Langmuir model to the adsorbent was proved by the high correlation coefficients R2>0.8.
The adsorption isotherm studies clearly indicated that the
adsorptive behaviour of heavy metal ions on adsorbents
satisfies not only the Langmuir assumptions but also the Freundlich assumptions, [19] i.e. multilayer formation on the surface of the adsorbent with an exponential distribution of site energy.
The regression analysis and experimental observations show that the experimental data fitted well in both the isotherms. As seen from results the Langmuir model exhibited a slightly better fit to the biosorption data than the Freundlich model as the values of correlation coefficient 'R2, were marginally better for Langmuir isotherm than the values of 'R2, for Freundlich isotherm [20].
Two types of observations were collected after treating the
water with the dry- biomass.
1. Reduction concentration of heavy metals from aqueous
sample.
2. Effect in the rate of adsorption with changing time.
4. CONCLUSION
Observations obtained from the above mentioned batch study concluded that not only living plants of C.indica but also dry biomass of the plant can also be used for reducing heavy metals present in waste water generated from industrial activities. The control samples merely showed any visible difference in the concentration to that of original concentration which signifies that the dry biomass of the plant has good potential for waste water treatment. It is well evaluated that the dry mass of the plant showed the required good results in case of heavy metals is astonishing as it has shown great
reduction from its original concentration for all the studied heavy metals and the results obtained were comparatively
better than that done with the living plants. It was also noticed that many of the studied heavy metals viz. Cadmium, Chromium, Zinc and Lead showed reduction in their concentration with increasing contact period but after 4 hr. they showed stability in reduction percentage. This concludes that the dry biomass powder of C.indica can be effectively utilized for reducing heavy metals and it gives the results in short span of time and so will prove to be a powerful tool among various waste water treatment technologies.
5. REFERENCES
[1] Shrivastava.J.,Managing water quality with aquatic macrophytes. rev. Science and Biotechnology 7,pp.
255-266, 2008 .
[2] WHO, the guideline for drinking water quality recommendations, World Health Organization, Geneva, 2002.
[3] Bryan G. W., Heavy metal contamination in the sea.
In R. Johnston (Ed,), Maarine pollution. London:
Academic ,1976.
[4] Sivakumar K., Subbalah, K.V., and Sai Gopal, D.V.R., Studies of certain trace elements in industrial efflutents sediments and their effect on plants physiology pollution Research 20(1), pp. 99-102 2001.
[5] Ahmed M. K., Mehedi M. Y., Haque , M. R., and Ghosh R.K., Concentration of heavy metals in two upstream rivers sediments of the sunderbans man grove forest. Bangladesh, asian Journal of Microbiology, Biotechnology & Environmental Sciences 5(1),pp. 41-47, 2003.
[6] Singh, D. B., Prasad, G. and Rupainwar, D.C., Addsorption technique for the treatment of As (V) rich effluents, colloids. surf, 111, pp.49-56, 1996.
[7] Martinez, M., Miralles, N, Hidalgo, S., Fiol, N.,
Villaescusa, I., and Poch, J, Removal of lead (II) and cadmium (II) from aqueous solutions using grape stalk waste. Journal of Hazardous Materials, 2006; B133: pp.203-211, 2006.
[8] Pino, G.H., Souza de Mesquita, L.M., Torem, M.L.,
and Pinto, G.A.S. Biosorption of cadmium by green coconut shell powder. Minerals Engineering,19: pp.
380-387, 2006.
[9] Han, R., Zhang, J., Zou, W., Xiao, H., Shi, J., and Liu, H. Biosorption of copper (II) and lead (II) aqueous solution by chaff in a fixed bed column. Jour nal of Hazardous Materials, B133: pp. 262-268 2006.
[10] Vijayaraghavan, K., Palanivelu, K., and Velan, M., Biosorption of copper (II) and cobalt (II) from aqueous solutions by crab shell particles. Bioresource Technology, 97: pp.1411-1419, 2006.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 885
ISSN 2229-5518
[11] Ahluwalia S.S., Goyal D., Microbial and plant derived biomass for removal of heavy metals from wastewater, Bioresour. Technol. 98, pp.2243-2257,
2007.
[12] Kadlec, R. H., and knight, R.L., Treatment wetlands
New York . (p. 950) 1996.
[13] Kar. R.N., Sahoo, B.N. and Sukla, L.B, Removal of
heavy metal from mine water using sulfate reducing bacteria. Pollution Research, 11, pp.1-13 , 1992.
[14] Brix, H., and Schierup, H.H. The use of aquatic
macrophytes Cyperus papyrus in receiving domestic waste. Hydrobiologica Bulletin, 2, pp.167-170, 1989.
[15] Vietmeyer, N.D., The beautiful blue devil. Natural
History, 84, pp.64-73, 1975.
[16] Elankumaran, R., Raj, M.B., and Madhyastha, M.N. ,
Biosorption of copper from contaminated water by Hydrilla verticillata casp. and Salvinia sp. Green Pages, Environmental News Sources , 2003.
[17] APHA, Standard methods for the examination of water and waste water (20th ed.). American Public Health Association, American Water Works Association and Water Poll. Control Federation 1995. American Public Health Association. Washington, DC, 1999.
[18] Mugugan, T. and Ganapathi, A. Kinetics and Freundlich Isotherm studies on to removal of Grey BL dye by using Arasu (Ficusrelegosia) Leaf Powder. International J. of Research in Environmental Science and Technology, Vol. 2(4), pp. 104-107, 2012.
[19] Dada, A. O., Olalekan, A. P., Olatunya, A. M. and DADA, O., Langmuir, Freundlich, Temkin and Dubinin–Radushkevich Isotherms Studies of Equilibrium Sorption of Zn2+ Unto Phosphoric Acid Modified Rice Husk IOSR Journal of Applied Chemistry (IOSR-JAC) ISSN: 2278-5736. Volume 3 (1), pp. 38-45,
2012.
[20] Taha A. W., Dakroury A. M., Sayed G. E. and El- Salam S. A., Assessment Removal of Heavy Metals Ions from Wastewater by Cement Kiln Dust (CKD) , Eleventh International Water Technology Conference, IWTC11 2007 Sharm El-Sheikh, Egypt pp. 879-893,
2007.
IJSER © 2014 http://www.ijser.org




