International Journal of Scientific & Engineering Research Volume 2, Issue 9, September-2011 1
ISSN 2229-5518
Studies on preparation and characterizations of CaO-Na2O-SiO2-P2O5 bioglass ceramics substituted with Li2O, K2O, ZnO, MgO, and B2O3
M.R. Majhi, Ram Pyare and S.P.Singh
Abstract--The bioactive glass 45S5 (Hench glass), having composition 45 SiO2 - 24.5 Na2O - 24.5 CaO -6 P2O5 (wt %) were prepared with substituted Li2O, K2O, ZnO, MgO, and B2O3 by conventional melting process in an electric globar furnace at 1400±10 OC. The Controlled crystallization were carried out to convert the bioglasses to their corresponding bioglass ceramics. Nucleation and crystallization regimes were carried out by differential thermal analysis. The X-ray diffraction patterns of the bioactive glass-ceramics were show the presence of two main crystalline phases of sodium calcium silicate (Na2CaSi3O8, Na2CaSi3O9). The effect of introduction of B2O3 in place of SiO2, to the bioactive glass (45S5) lead to the formation of a new crystalline phase of calcium sodium borate (Na2CaB5O10) and the effect of introduction of ZnO and MgO in place of CaO, to the bioactive glass 45S5 there is no additional crystalline phases were developed other than two main crystalline phases of sodium calcium silicate (Na2CaSi3O8, Na2CaSi3O9). The bioactivity of the prepared glass and glass ceramics were done by infrared absorption and reflection spectrometry before and after immersion in the simulated body fluid for different periods of time at 37.8 OC. The Chemical durability of bioglass and bioglass ceramics were determined by pH measurement methods and it was found that pH of the solution varies with change in compositions after immersed in SBF solution from 1 to 30 days.
Keywords - Bioactive glass; pH Measurement; SBF; Chemical durability; Crystallization
—————————— ——————————
Bio- materials implanted into bone defects are generally
encapsulated by fibrous tissue isolating them from the surrounding bone [1] .This is the normal response of the body towards inert materials. However, some ceramics, such as Bio glass [2], glass–ceramic A–W [3] and sintered hydroxyapatite [4] form bone-like apatite on their surfaces in the living body and bond to living bone through this apatite layer. This bone-bonding ability is called bioactivity. These bioactive ceramics are already used clinically as important bone-repairing materials. Their bone-bonding ability is achieved by the formation of a biologically active apatite layer after reaction of the ceramics with the simulated body fluid[5,6] .A controlled surface reaction of the ceramic is an important factor governing its bioactivity, as well as its biodegradability[7,8].In the present study, the introduction of MgO in the bioglass composition improves the chemical durability because of their presence in network forming sites by forming structural units such as MgO4 [9,10] ,which shows that MgO causes obvious phase separation in silicate melts and glasses .The addition of zinc oxide to the bioactive glass–ceramic would control the reaction between the glass–ceramic and the surrounding body fluid. Zinc oxide was selected to control the reactivity since zinc is an essential trace element that
----------------------------------------------------------------------
Ram Pyare is a Professor, Dept. of Ceramic Engg,
IT.BHU -Varanasi (India) email:pyare_ram55@yahoo.co.in
S.P.Singh is a Professor, Dept. of Ceramic Engg,
IT.BHU -Varanasi (India) email:spsinghceram@gmail.com
has stimulatory effects on bone formation [12]. The zinc ions released from the glass–ceramic may enhance bone regeneration. With regards to ceramics designed to release zinc ions, [12, 13, 14] recently developed calcium phosphate ceramics containing zinc. However, they used polycrystalline ceramics, so the range where zinc can be incorporated is limited and the behavior of their materials is expected to be different from the glass-based materials. The bio-glass (45S5) of 5–10% B2O3 for SiO2 substituted the various bioactive glass compositions to form glass– ceramics has minor effect on the final ability of the material to form a bone bond[11].The objective of this work is to study the effect of the addition of Li2O, K2O, ZnO, MgO, and B2O3 to CaO-Na2O-SiO2-P2O5 glass– ceramics on their bioactivity, density ,compressive strength, XRD, DTA and FTIR analysis were done during present investigation.
The bioactive glass 45S5 (Hench glass), having composition 45 SiO2 - 24.5 Na2O - 24.5 CaO - 6 P2O5 (wt
%) were prepared by substituted with Li2O, K2O, ZnO, MgO, and B2O3. Different compositions of bioactive glasses were prepared as shown in Table 1. For preparation of 100 grams of bioactive glasses fine-grained quartz is used as the source of silica, sodium carbonate (Na2CO3):, calcium carbonate (CaCO3):, ammonium dihydrogen orthophosphate (NH4H2PO4):,aluminium oxide (Al2O3) ,boric acid (H3BO3):, potassium carbonate (K2CO3):,Zinc Oxide (ZnO) and magnesium carbonate (MgCO3) are used as a the source of sodium oxide (Na2O), calcium oxide (CaO), phosphorus
pentaoxide (P O ), boron trioxide (B O ), potassium
2 5 2 3
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 9, September-2011 2
ISSN 2229-5518
oxide (K2O) ,zinc oxide (ZnO) and magnesium oxide (MgO) respectively. Bioactive glasses were prepared by conventional method. For preparing bioactive glasses amounts of materials are weighed using an electronic balance and mixed homogeneously with an agate pestle- mortar. Premixed batch is melted in platinum crucibles, at
1400±10°C, for 4 hours using globar furnace. After 4 hours of melting the glass was taken out from the furnace and, it was poured on an aluminum sheet and then cooled to room temperature .After crushing the glass, it was remelted in the furnace for another two hours to ensure complete homogeneity of glass. After homogenization and remelting, it was taken out from the furnace and poured on a hot rectangular mould kept on aluminum sheet and annealed at 480 oC to remove the thermal stresses and strains from the glass. Further, the glass was cooled to room temperature with controlled rate of cooling and the annealed glass samples were preserved for their properties and structural determinations such as density, compressive strength, DTA, XRD and FTIR spectrometry.
The density were studied by the Arcmedic’s principle method
and compressive strength of the sintered bioglass ceramic samples having size of 3x2x1 cm3 dimension were subjected to compression test. The test was performed using Instron Universal Testing Machine at room temperature (cross speed of 0.05 cm/min and full scale of 5000 kgf)).
2.3. Differential thermal analysis
Differential Thermal Analysis measurement was carried out on powdered bioglass samples which were examined up to 1000 OC using a powdered alumina as a reference material (SETARAM Instrumentation, France) and the heating rate was 10oC min−1. The DTA data were used to obtain the proper heat treatment temperatures to obtain the corresponding glass–ceramic derivatives with high crystallinity. Briefly, these results showed
that the values obtained were for glass nucleation temperature and the crystallization temperatures.
The bioglass samples were thermally heated in two-step
regime, at the deduced temperatures .Each bio glass sample was heated slowly to the first nucleation temperature for the formation of sufficient nuclei sites and after holding for 4 hrs, it was then further heated to reach the second chosen crystal growth temperature for performing the perfect crystal growth. After a second hold
for 6 hrs time, the specimen was left to cool inside the muffle furnace to room temperature at a rate of 20oC per hour.
The crystalline phase was identified by using X-ray
diffraction analysis, the heat-treated bioglass ceramic samples were ground to 75 microns and the fine powder was subjected to XRD test using Cu-Kα radiation (λ =
1.5405A° ) in a 2θ range between 20o and 80o. Step size and measuring speed were set to 0.02◦ and 1o /min;
respectively, with a tube voltage of 40 kV and current of
35mA. The JCPDS-International Centre for diffraction
Data Cards were used as a reference.
container at 37.8 oC with pH= 7.4 in an incubator at static condition for time period 1 to 30 days. The SBF solution was
prepared as described by Kokubo et al. [7]. The samples were prepared in to a disc shape by mixed with KBr powder in the ratio 1:100 (sample: KBr respectively) and the mixtures were subjected to a load of 10 tons/cm2 in an evocable die to produce clear homogeneous discs. The Infrared transmittance and reflectance spectra of the bio glasses and their ceramic derivatives were measured at room temperature in the frequency
range of 4000–400 cm−1 using a Fourier transform infrared spectrometer, (VARIAN scimitar 1000, USA) and (Is10 Nicolet, USA). The test results were compared with functional groups [49, 50].
The glass and glass-ceramic samples in the form of palate
in the size range 1cm diameter, were subjected to the action of simulated body fluid , prepared by Kokubo et al[7] at 37.8 oC and pH 7.4 for different time periods (1 to
30 days). The pH of solution were measurement at different time periods by the help of pH meter to find out
their chemical durability.
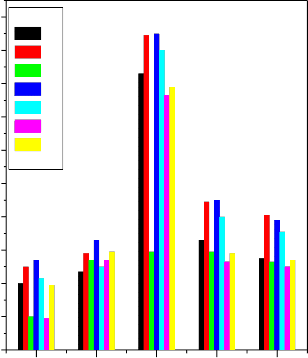
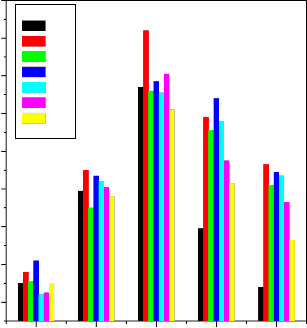
Results presented in figure 1 show that the density and the
compressive strength increases with increasing the concentration of Li2O and K2O in the place of Na2O in bio-glass composition No.(2) and (3) respectively. Similarly density and the compressive strength of the bio glass composition No.(4) and (5) increases with increasing ZnO and MgO in the place of CaO. Similar trend was observed while B2O3 is substituted for SiO2 in bio-glass composition No.(6) and (7) respectively.
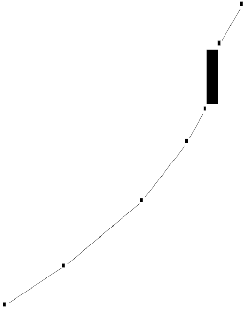
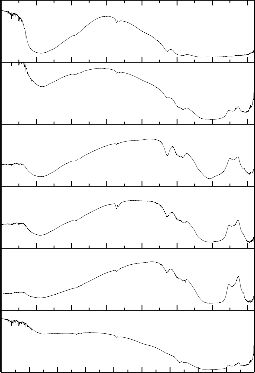
The differential thermal analysis (DTA) curve reveals
information about the transformations that have occurred, such as glass transitions and crystallization temperature. The differential thermal analysis (DTA) curves of bioactive glasses has been shown in figure.2.
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 9, September-2011 3
ISSN 2229-5518

160
7
Tg=519
T =723 OC
C
![]()
150
T
C
6
Tg=518OC
=721 OC
T =726 OC
![]()
C
5
140
T =521 OC
g
T =724OC
![]()
C
4
T =520 OC
g
130
![]()
3
T =592 OC
C
120
T 491
g
T
C
2
=598 OC
![]()
110
T =499 OC
g
T
C
1
=719 OC
![]()
2.2 2.3 2.4 2.5 2.6 2.7 2.8
![]()
![]()
![]()
![]()
![]()
T =515 OC
g
0 200 400 600 800 1000
O
Density(gm/cc)
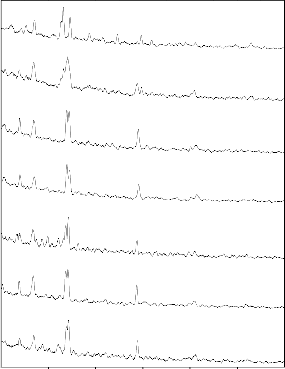
Fig.1 .Variation of Density and compressive strength of bio- glass ceramics Samples from Sl.No. (1-7)
Temperature( C)
Fig .2. Differential Thermal Analysis (DTA) Curves of
Bioactive Glasses Ceramics samples from Sl.No (1-7)
7
9.6
9.4
G-1
G-2
6
5
9.2
9.0
8.8
G-3
G-4
G-5
G-6
G-7
3
8.6
8.4
8.2
2
1
8.0
7.8
7.6
1 day 3 days 7 days 15 days 30 days
20 30 40 50 60 70 80
2 (degree)
Reaction Time(days) in SBF Solution
[-(Na2CaSi3O8,) ],[-(Na2CaSi3O9 )],[- Fig. 4. Variation of pH with different time
(Na2CaB5O10)],[- (Li2CaMg(PO4)2 ] periods of bio glass samples at initial pH =7.4
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 9, September-2011 4
ISSN 2229-5518
9.4
9.2
9.0
8.8
GC-1
GC-2
GC-3
GC-4
GC-5
GC-6
GC-7
8.6
8.4
8.2
8.0
7.8
1 day 3 days 7 days 15 days 30 days
Reaction time(days) in SBF solution
Fig. 5. Variation of pH with different time periods of bio glass ceramic samples at initial pH = 7.4
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 9, September-2011 5
ISSN 2229-5518
30 days
30 days
15days
15 days
7 days
7 days
3 days
3 days
1 days
1 day
before
Before
4000 3600 3200 2800 2400 2000 1600 1200 800 400
Wavenumber ((cm-1)
4000 3600 3200 2800 2400 2000 1600 1200 800 400
Wavenumber (cm-1)
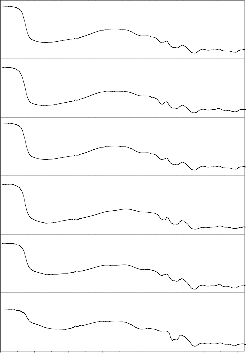
Fig.6.FTIR transmittance Spectra of Bio glass before and after SBF treatment (G-2)
Fig..7: FTIR transmittance Spectra of Bio glass ceramics before and after SBF treatment (GC-2)
###
10
8
30 days
6
30 days
2
X Axis Title
###
10
0 2 4 6 8
X Axis Title
8
15 days
6
4 15 days
2
X Axis Title
###
10
0 2 4 6 8
X Axis Title
8
7 days
6
4
7 days
2
3 days
X Axis Title
###
10
0 2 4 6 8
X Axis Title
8
6
4 3 days
2
1 day
X Axis Title
###
10
0 2 4 6 8
X Axis Title
8
6
1 day
2
Before
X Axis Title
###
10
0 2 4 6 8
X Axis Title
8
6
Before
4
4000 3500 3000 2500 2000 1500 1000 500
2
4000 3600 3200 2800 2400 20000 1600 12200 800 4 400 6 8
W avenumber(cm-1)
Wavenumber(cm
-1)
X Axis Title
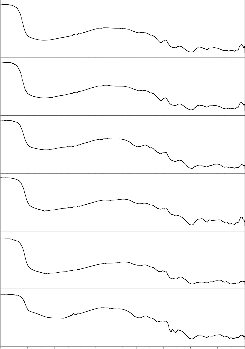
Fig.8.FTIR reflectance Spectra of Bio glass before and after
SBF treatment (G-2)
Fig..9: FTIR reflectance Spectra of Bio glass ceramics before and after SBF treatment (GC-2)
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 9, September-2011 6
ISSN 2229-5518
Sl.No | SiO2 (wt%) | Na2O (wt%) | CaO (wt%) | P2O5 (wt%) | Li2O (wt%) | K2O (wt%) | ZnO (wt%) | MgO (wt%) | B2O3 (wt%) |
1 | 45 | 24.5 | 24.5 | 6 | - | - | - | - | - |
2 | 45 | 19.5 | 24.5 | 6 | 2.5 | 2.5 | - | - | - |
3 | 45 | 14.5 | 24.5 | 6 | 5 | 5 | - | - | - |
4 | 45 | 24.5 | 19.5 | 6 | - | - | 2.5 | 2.5 | - |
5 | 45 | 24.5 | 14.5 | 6 | - | - | 5.0 | 5.0 | - |
6 | 40 | 24.5 | 24.5 | 6 | - | - | - | - | 5 |
7 | 35 | 24.5 | 24.5 | 6 | - | - | - | - | 10 |
Table. 2. Correlation between Wave number at which Transmittance Bands emitted and Functional Groups in Bioactive Glasses and their Ceramic Derivatives after immersing in Simulated Body Fluid (SBF) [44]
Wavenumber(cm-1) | Functional groups |
400-500 | Si-O-Si (bend) |
500 - 560 | P-O (Bend) (Crystalline) |
560 - 600 | P-O (Bend) (Amorphous) |
720 - 840 | Si-O-Si (Tetrahedral) |
860 - 940 | Si-O (Stretch) |
1000 - 1100 | Si-O-Si (Stretch) |
1100 - 1200 | P-O (Stretch) |
1400 - 1530 | C-O (Stretch) |
Table. 3. Correlation between Spectral Frequencies and Functional Groups in a Bioactive Glass and their Ceramic Derivative and the Steps of Surface Changes by immersing in Simulated Body Fluid (SBF) [43]
Wavenumber(cm-1) | Vibrational mode | Surface reaction stages |
860 - 940 | Si-O (Stretch) | Stage 1 and 2 |
720 - 840 | Si-O-Si (Tetrahedral) | Stage 3 |
560 - 600 | P-O(Bend) (Amorphous) | Stage 4 |
500 - 560 | P-O(Bend) (Crystalline) | Stage 5 |
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 9, September-2011 7
ISSN 2229-5518
The differential thermal analysis (DTA) curves of bioactive glasses show the glass transition temperature (endothermic effects) in the range of 491-525 0C and crystallization temperature (exothermic effects) in the range of 592-7260C. The substitution of Na2O with Li2O and K2O (bioactive glass 2 and 3) decreases of glass transition and crystallization temperature, with d the substitution of CaO with ZnO and MgO (bioactive glass 4 and 5) cause increase of glass transition and crystallization temperature, while the substitution of SiO2 with B2O3 (bioactive glass 6and 7) cause increases of glass transition temperature and crystallization temperature [27].
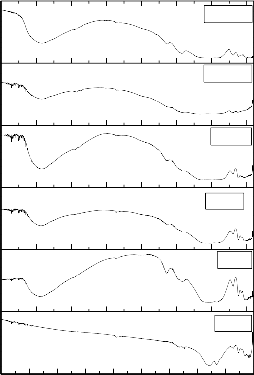
The X-ray diffraction (XRD) pattern reveals information about the
different crystalline phases. The X-ray diffraction (XRD) patterns of Ceramic Derivatives of Bioactive Glasses has been shown in Fig. 3. The X-ray diffraction (XRD) patterns of ceramic derivatives of all the bioactive glasses show the presence of two crystalline phases of sodium calcium silicate of the formula (Na2CaSi3O8, card number: PDF# 12-0684) and (Na2CaSi3O9, card number: PDF# 45-0550) (except ceramic derivative of bioactive glass 3 and
7).The X-ray diffraction (XRD) pattern of ceramic derivative of bioactive glass 3 shows an additional crystalline phase, which is attributed to the formula calcium lithium silicate phase (Li2CaSi3O8), while the X-ray diffraction (XRD) pattern of ceramic derivative of bioactive glass 7 shows an additional crystalline phase which is attributed to sodium calcium borate of the formula ((Na2CaB5O10)], card number: PDF# 38-0827).X-ray diffraction (XRD) patterns of ceramic derivatives of all the bioactive glasses indicate the formation of crystalline phases after the specified heat treatment. Each ceramic derivative of bioactive glass reflects specific crystalline phases depending on the chemical composition of the bioactive glasses and heat treatment conditions. The reason of the ease of crystallization of the bioactive glasses can be correlated with the presence of phosphate and silicate network, and the possible crystalline phase separation even in micro scale of the two crystalline phases depending on heat treatment conditions. It is well known that the addition of P2O5 (a few percent) to silicate glasses promotes volume nucleation and glass-ceramic formation as indicated by Elbatal[46]. have shown that the addition of B2O3 promotes volume crystallization in bioactive glasses but they showed that nucleation rates for CaO - P2O5 - B2O3 glasses were too low for practical glass–ceramic formation[36]. Hench reviewed the nature of crystalline phase separation in oxide glasses and showed that Li2O, K2O ,ZnO and MgO increases the tendency towards crystalline phase separation .ElBatal et al [46] has shown that the heat treatment of the parent bioactive glass 45S5 (bioglass or Hench glass) at a glass transition temperature of 5250C and followed by heating at a crystallization temperature of 7260C produced a ceramic derivative of bioactive glass containing the sodium calcium silicate crystalline phase (Na2Ca2Si3O9)[43,48]. Hench et al. showed that there is a relationship between the local structure of the modifier cations in silicate glasses of the systems (SiO2 – CaO – Na2O and SiO2 – CaO) and the nucleation ability [48]. These glasses can easily nucleate in the volume because they have similar local structures to their crystalline phases. They detected the crystalline phase Na2Ca2Si3O9 in the system SiO2 – CaO - Na2O after heat treatment at a crystallization temperature of
730 oC. It is obvious that the system SiO2 – CaO - Na2O - P2O5 has
the tendency to form the sodium calcium silicate crystalline phase
of the formula Na2Ca2Si3O9 as the main crystalline phase [36]. This tendency is confirmed in all the bioactive glasses (except bioactive glass 3 and7) where the Na2Ca2Si3O9 crystalline phase is detected by X-ray diffraction (XRD). In addition, there is another secondary crystalline phase (Na2CaSi3O8) [40]..The effect of introduction of 5
and 10 % B2O3 to the bioactive glass 45S5 (bioactive glass 6,7) is shown to lead to the formation of a new crystalline phase of calcium sodium borate of the formula (CaNa3 B5O10) [48]. The effect of introduction of ZnO and MgO to the bioactive glass 45S5 composition (bioactive glass 4 and 5) there is no additional crystalline phase occur.
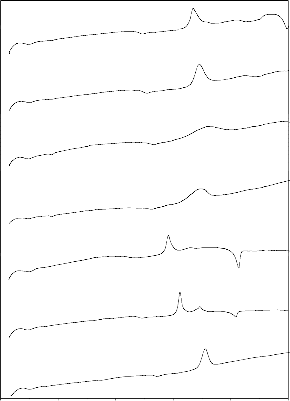
Figs. 6–7 show the corresponding FTIR transmittance spectra of
the bio glass and ceramic derivatives before and after treatment with SBF solution for different time period from 1 to 30 days. The
FTIR transmittance bands of bioglasses and their ceramic
derivatives were correlated before and after immersion in simulated body fluid (SBF) and were compared with vibrational mode
according to Kim et al. [49] and Filgueira et al.[50] .The functional group was shown in table 2. Fig .6 show the bioglass (G-2) FTIR transmittance spectra peaks were noticed before SBF treatment at
413, 453, 498, and 713 cm-1. After SBF treatment the FTIR
transmittance spectra peaks were noticed at 418, 432, 507, 526,
570, 686, 727, 1386, 1643 and 3454 cm-1. After SBF treatment new peaks were developed around at 507 and 526 cm-1 (P-O Bend- Crystalline), 570 (P-O Bend- Amorphous), 1643 (C-O
stretch) and 3454 cm-1 (O-H stretching). The hydroxy carbonated apatite layer (HCA) formed on the surface of the bioglass and there was a slight change in the peaks intensity as the soaking time increases. The stages of formation of hydroxyl carbonate apatite
were also mentioned earlier by Hench [1], ElBatal [46] and Oscar peitl [51]. Fig .7 show the bioglass-ceramic of GC-2 FTIR transmittance spectra peaks were noticed before SBF treatment at
410, 441, 534, 621, 692 and 731 cm-1. After SBF treatment the
FTIR transmittance spectra peaks were noticed around at 420, 526,
570, 619, 686, 727, 1386, 1643 and 3454 cm-1. After SBF treatment new peaks developed at 507, 526 (P-O Bend- Crystalline), 570 (P-O Bend- Amorphous), 1643 (C-O stretch)
and 3454 cm-1 (O-H stretching). The hydroxy carbonated apatite layer (HCA) formed on the surface of the bioglass-ceramic.
Fig.4 and Fig.5 show the change in pH with different time period for all the bioglasses and glass-ceramics. It was clearly observed from Figs 4 & 5 that the all samples the pH varies with change in compositions within 1 to 30 days compared to the initial pH of the solution (pH= 7.4) which is due to the fast release of Na+ and Ca2+ ions through exchange with H+ or H3O+ ions into the solution[21].
The H+ ions being replaced by cations, thereby increase in
hydroxyl concentration of the solution, which leads to attack in silica glass network and formation of silanols to decrease in pH.
The changes in pH are due to ion leaching i.e. chemical changes of
material surfaces at different time periods. The increase in pH shows that the reduction in the concentration of H+ ion due to the replacement of cation ions in the glass and subsequent production of OH- ions. It is also observed that the decrease in pH of the solution after 15days due to breaking of glass network. The reason may be considerable leaching of the glassy matrix from the surface. It can be understood that after 15 days the leached layer is removed and fresh layer is exposed, and therefore, demand for hydrogen ions is comparatively less. Similar change in pH, i.e. with decrease in pH to acidic region after 15 and 30 days respectively.
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 9, September-2011 8
ISSN 2229-5518
The formation of hydroxyl carbonate apatite on the surface was explained earlier by Hench [1], ElBatal [46] and Oscar peitl [51]. Fig .9show the reflectance spectra of bioglass samples then the reflectance bands were observed within the frequency range 442, 631, 1384 and 1470 cm-1 showing the presence of functional groups. These frequencies are due to various modes of vibrations 442 (Si-O-Si bend), 631 (P-O bend), 1384 (C-O-Stretch), and
1470 cm-1 (C-O-C Stretch). Bioglass treated with SBF solution with increasing duration of soaking time the reaction occurred as explained above and correlated with table 2 and 3. When the bioglass was converted to bioglass ceramic in fig 9, after then the FTIR reflectance spectra was recorded. The presence of various functional groups were identified with reference to corresponding frequencies 457 Si-O-Si (bend), 525, 613 (P-O Bend- Crystalline), 671 (P-O Bend), 722 (Si-O-Si -Tetrahedral),
921 (Si-O Stretch), 1031 ,1098 (Si-O-Si Stretch), 1384 cm-1 and 1469 cm-1 (C-O Stretch). Bioglass-ceramic treated with SBF.
The density and the compressive strength increases with increasing the concentration of Li2O and K2O in the place of Na2O in bio-glass composition No. (2) and (3) respectively. Similarly density and the compressive strength of the bio glass composition No.(4) and (5) increases with increasing ZnO and MgO in the place of CaO. Similar trend was observed while B2O3 is substituted for SiO2 in bio-glass composition No. (6) and (7) respectively.The differential thermal analysis (DTA) curves of bioactive glasses reveals that the effect of introduction of Al2O3 and B2O3 in place of SiO2 both nucleation and glass transition temperature increases ,an introduction ZnO and MgO in place of CaO respectively, in the bioactive glass 45S5 cause increase of both glass transition and crystallization temperatures, while the effect of introduction of K2O in place of Na2O, in the bioactive glass 45S5 cause decrease of both glass transition and crystallization temperatures. The X-ray diffraction (XRD) patterns of the bioactive glass-ceramics show the presence of two main crystalline phases of sodium calcium silicate
(Na2CaSi3O8, Na2CaSi3O9). The effect of introduction of B2O3 in place of SiO2, to the bioactive glass 45S5 is shown to lead to the formation of a new crystalline phase of calcium sodium borate of the formula (Na2CaB5O10) and the effect of introduction of ZnO and MgO in place of CaO, to the bioactive glass 45S5 is shown that no additional crystalline phase developed other than two main crystalline phases of sodium calcium silicate (Na2CaSi3O8, Na2CaSi3O9) . The pH of the solution has varies from different glass samples No.(1-7) with substitution of different oxides after immersed in SBF solution. The fourier transform infrared (FTIR) absorption spectroscopy reveals that the effect of introduction of B2O3 in place of SiO2, in the bioactive glass 45S5 has decreasing of bioactivity, a minor change of bioactivity in an introduction of Li2O and K2O in place of Na2O, while the effect of introduction of MgO and ZnO in place of CaO, in the bioactive glass 45S5 cause a decrease in bioactivity. The fourier transform infrared (FTIR) reflectance spectra of the bioglass and bioglass-ceramic samples have also revealed that an increase in the number of bonds formed with increasing soaking time in SBF. References:
1. L.L. Hench, R.J. Splinter W.C. Allen, T.K. Greenlee Jr. Bonding mechanism at the interface of ceramics prosthetic
materials. J Biomed Mater Res Symp 2(1971) 117–41.
2. S.F. Hulbert. The use of alumina and zirconia in surgical implants. In:Hench LL, Wilson J, editors. An introduction to bioceramics. Singapore: World Scientific; (1993) 25–40.
3. T.Kokubo , Shigematsu M, Nagashima Y, Tashiro M, Nakamura T,Yamamuro T, et al. Apatite- and wollastonite- containing glass ceramics for prosthetic application. Bull Inst Chem Res Kyoto Univ. 60( 1982) 260–68.
4. J. M.archo, J.L Kay, Gumaer RH, Drobeck HP. Tissue, cellular and subcellular events at bone-ceramic hydroxyapatite interface. J Bioeng 1(1977)79–92.
5. L.L. Hench Bioceramics: from concept to clinic. J Am Ceram
Soc 74(1991)1487–510.
6. M. Neo, T .Nakamura, C. Ohtsuki,T. Kokubo ,T.Yamamuro . Apatite formation on three kinds of bioactive material at an early stage in vivo: a comparative study by transmission electron
microscopy. JBiomed Mater Res 27(1993) 999–1006.
7. T. Kokubo A/W glass-ceramic: processing and properties. In: Hench LL, Wilson J, editors. An introduction to bioceramics. Singapore:World Scientific; (1993) 75–88.
8. S.D. Stookey, Ind. Eng. Chem. 51 (1959) 805.
9. W. Vogel, Chemistry of Glass, American Ceramic Society, Columbus, OH,(1985)
10. P.F. James, in: M.H. Lewis (Ed.), Glasses and Glass–
Ceramics, Chapman and Hall,London, (1989) 59.
11. A.A. Ahmed, H.A. ElBatal, N.A. Ghoneim, F.A. Khalifa, J. Non-Cryst. Solids 41(1980) 57
12. M.Yamaguchi,H Oishi , Y Suketa . Stimulatory effect of zinc
on bone formation in tissue culture. Biochem Pharmacol
36(1987)4007–12.
13. A Ito, K.Ojima , H.Naito , N Ichinose , T .Tateishi . Preparation,solubility and
cytocompatibility of zinc-releasing calcium phosphate ceramics. J Biomed Mater Res 50(2000)178–83.
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 9, September-2011 9
ISSN 2229-5518
14.Y. Sogo ,T. Sakurai , K. Onuma ,A. Ito . The most appropriate (Ca + Zn)/P molar ratio to minimize the zinc content of ZnTCP/HAP ceramic used in the promotion of bone formation. J Biomed Mater Res .62 (2002) 457–63.
15. L.L. Hench, R.J. Splinter, W.C. Allen, T.K. Greenlee Jr., J. Biomed. Mater. Res. Symp. 2 (1971) 117–141.
16.L.Larry . Hench & June Wilson, “An Introduction to
Bioceramics”, World Scientific
Publishing Company: (1993).
17.N. Mohan Kumar, L. Szpiro et al., “Bioceramics: Properties, Characterizations and Applications”, Springer: (2008).
18.L.Larry . Hench, “Bioceramics: From concept to clinic”,
Journal of American Ceramic Society: (1991) 1487 – 1510.
19.J.J.Vidueau ,V. Dupuis ., “Phosphates and biomaterials”,
European Journal of Solid State Inorganic Chemistry 28 (1991)303 – 343
20.L. L. Hench, “Bioceramics: Research and development opportunities”, Brazilian Journal of Physics 22 (1992) 70 - 84
21.J.P.Zhong .,G.P. La Torre et al., “The kinetics of bioactive
ceramics part VII: Binding of collagen to hydroxyapatite and
bioactive glass”, In Bioceramics: 7 (1994) 61 – 66.
22D.C..Greenspan .,J.P. Zhong et al., “Effect of surface area to volume ratio on in vitro surface reactions of bioactive glass
particulates”, In Bioceramics: 7(1994)28 – 33.
23.J.J.Mazer , J.V.Walther ., “Dissolution kinetics of silica glass as a function of pH between 40 and 85C”, J Non-Crystalline
Solids: 170 (1994) 32 – 45.
24.M.M.Pereira , A.E Clark. et al., “Effect of texture on the rate of hydroxyapatite formation on gel-silica surface”, J American Ceramic Society: 78 (1995) 2463 – 2468.
25. O.P. Filho, G.P. La Torre, L.L. Hench, J. Biomed. Mater. Res. 30(1996) 509–514.
26.Zhong J.P., Greenspan D.C., “Bioglass surface reactivity:
From in-vitro to in-vivo”, In
Biomaterials: 10 (1997)391 – 394.
27.M. Brink, Tia Turunen et al., “Compositional dependence of bioactivity of glasses in the system Na2O-K2O-MgO-CaO- B2O3-P2O5-SiO2”, J Biomed Mater Res: 37 (1997) 114 –121.
28.D.C.Greenspa., J.P.Zhong . et al., “The evaluation of degradability of melt and sol–gel derived Bioglass in vitro”, In Bioceramics: 10 (1997)391 – 394.
29.D.C.Greenspan , J.P.Zhong et al., “Bioactivity and
biodegradability: Melt vs. sol-gel derived bioglass in vitro and in
vivo”, In Bioceramics: 11 (1998)345 – 348.
30.L.L..Hench “Biomaterials: A forecast for the future”,
Biomaterials: 19(1998)1419 –1423.
31.I. Rehman, J. C. Knowles et al., “Analysis of in vitro reaction layers formed on bioglass using thin-film X-ray diffraction and
ATR-FTIR microspectroscopy”, J Biomed Mater
Res: 41(1998) 162 – 166.
32.A. Stoch, W. Jastrzebski et al., “FTIR monitoring of the
growth of the carbonate containing apatite layers from simulated and natural body fluids”, Journal of Molecular Structure: 511 (1999) 287 – 294.
33. J.D. Rinehart Taylor T.D. et al., “Real-time dissolution
measurement of sized and unsized calcium phosphate glass
fibres”, J Biomedical Materials Research: 48 (1999) 833 – 840.
34.Oscar Peitl, Edgar Dutra Zanotto, et al., “Highly bioactive P2O5-Na2O-CaO-SiO2 glass- ceramics”, Journal of Non- Crystalline Solids: 292(2001) 115 - 126.
35.Mathai Mathew and Shozo Takagi, “Structures of Biological Minerals in Dental Research”, Journal of Research of the National Institute of Standards and Technology: 106,(2001)1035
– 1044.
36.P. Sepulveda, J.R. Jones et al., “In vitro dissolution of melt- derived 45S5 and sol-gel derived 58S bioactive glasses”, J Biomed Mater Res: 61 (2002)301 – 311.
37.E. Kontonasaki, T. Zorba et al., “Hydroxy carbonate apatite formation on particulate bioglass in vitro as a function of time”, Crystal Research and Technology: 37 (2002) 1165 – 1171.
38.H.A. ElBatal, M.A. Azooz et al., “Characterization of some
bioglass–ceramics”, Materials Chemistry and Physics: 80
(2003)599 – 609.
39.R. Xin, Yang Leng et al., “A comparative study of calcium phosphate formation on bioceramics in vitro and in vivo” Biomaterials: 26 (2005) 6477 - 6486.
40.Irene Barrios de Arenas, Carol Schattner et al., “Bioactivity
and mechanical properties of Na2O-CaO-SiO2-P2O5 modified
glasses”, Ceramics International: 32 (2006) 515 – 520.
41.Qizhi Z. Chen, Ian D. et al., “45S5 Bioglasss-derived glass-
ceramic scaffolds for bone tissue engineering”, Biomaterials
27(2006)2414-2425.
42.Aldo R. Boccaccini, Qizhi Chen et al., “Sintering, crystallization and biodegradation behaviour of bioglass- derived glass–ceramics”, The Royal Society of Chemistry: 136
(2007) 27 – 44.
43.G.A. Stanciu, I. Sandulescu et al., “Investigation of the hydroxyapatite growth on bioactive glass surface”, Journal of
Biomedical & Pharmaceutical Engineering: 1 (2007) 34 - 39.
44. O.P. Filho, G.P. La Torre, L.L. Hench, J. Biomed. Mater. Res. 30 (1996) 509–514
45.S.M. Best, A.E. Porter et al., “Bioceramics: Past, present and for the future”, Journal of the European Ceramic Society: 28 (2008)1319–1327.
46. H. ElBatal, Amany ElKheshen, “Preparation and
characterization of some substituted bioglasses and their ceramic derivatives from the system SiO2–Na2O–CaO– P2O5 and effect of gamma irradiation”, Materials Chemistry and Physics: 110, (2008), 352 – 362.
47M.H. Fathi, A. Doost Mohammadi, “Preparation and
characterization of sol–gel bioactive glass coating for improvement of biocompatibility of human body implant”, Materials Science and Engineering: 474 (2008) 128–133.
48. Hench L.L., Splinter R.J. , Allen W.C., Greenlee Jr T.K.., J.
Biomed.Mater. Res. Symp. 2 (1971) 117–141.
49. C.Y. Kim, A.E. Clark, L.L. Hench, J. Non-Cryst. Solids 113 (1989)195–202.
50. Filgueiras M.R.T., La Torre G.P., Hench, L.L. J. Biomed.
Mater. Res. 27 (1993) 1485– 1493.
51. Oscar peitl, E.D.Zanotoo, L.LHench : “ Highly bioactive P2O5-Na2O-CaO-SiO2 glass- ceramics”, Journal of non- crystalline solids 292(2001)115-126.
IJSER © 2011 http://www.ijser.org