INTERNATIONAL JOURNAL OF SCIENTIFIC & ENGINEERING RESEARCH, VOLUME 3, ISSUE 4, APRIL-2012
ISSN 2229-5518
Stimuli Sensitive Interpenetrating Network
Pham Trong Binh and Hsu, Hao-Chun and Zhao Zhenyu
Abstract- Hydrogels are three-dimensional, cross-linked polymer networks that can absorb a large amount of water or solvent without dissolution. Due to their high absorbency, soft consistency and other properties, such materials can be used in medic al industries to produce artificial organs, contact lenses, dressing for wound treatment and drug transporting devices. In this research poly(N-Vinylcaprolactam) (PVCL) hydrogels were synthesized with different cross-linkers. It was found that cross-linkers affected the stability of the hydrogel and those with high hydrophilicity enhanced its swelling capacity. Our experiments showed that ethylene glycol dimethylacrylate (EGDMA) was the ideal cross-linker. Hydrogels of an interpenetrating polymer network (IPN) composed of the thermo-sensitive PVCL and pH-sensitive poly(N-acryloyl-N′-ethylpiperazine) (PAcrNEP) were prepared via sequential polymerization. They were characterized for their thermo and pH -responsive behaviour by time dependence, equilibrium and oscillatory swelling studies. The results showed that these IPN successfully exhibited a combined p H and temperature-sensitivity at a temperature range of 20-35oC and a pH range of 3-6. This is a milestone in the study of hydrogels because most of them focused
mainly on gels sensitive to basic medium only. The network also exhibited superior swelling capacity compared to pure PVCL ev en at high pH. Oscillatory swelling study showed that the IPN was able to respond to pH pulses quickly and reversibly. Dye absorption
studies showed that IPN is a potential purifying agent. It was also found that the IPN is a good substrate for growing nanopa rticle. Further research may include permeation studies of the IPN to test its ability for drug delivery.
Index Terms- Dye Absorption, Hydrogels, Interpenetrating Network, Nanoparticles, Polymers, Stimuli Sensitive, Swelling Synthetic
Chemistry
—————————— ——————————
H ydrogels are three-dimensional, cross-linked polymer networks that can absorb a large amount of water or solvent without dissolution. Depending on the type of polymer that constitute the matrix, hydrogels can be responsive to various external stimuli such as pH, temperature, ionic strength, light, magnetic field, etc1. Those that experience changes in swelling in solutions are called stimuli- responsive materials. Due to their high absorbency, soft consistency and other properties, such materials can be used in medical industries to produce artificial organs, contact lenses, dressing for wound treatment and drug transporting devices2. In addition, hydrogels are found to be a very suitable material for stabilizing metallic nanoparticles in aqueous solutions by maintaining the nanostructure of the particles. Silver nanoparticles are nontoxic and exhibit good antimicrobial property3, but they have poor surface binding property, and this can be solved by growing hydrogel-embedded silver nanoparticles.
————————————————
![]() Hsu, Hao-Chun is currently pursuing a high school diploma at
Hsu, Hao-Chun is currently pursuing a high school diploma at
NUS High School of Math and Science, Singapore, (+65)
68970582, patrickhsu0905@hotmail.com
![]() Pham Trong Binh is currently pursuing a high school diploma at NUS High School of Math and Science, Singapore, (+65)
Pham Trong Binh is currently pursuing a high school diploma at NUS High School of Math and Science, Singapore, (+65)
65161709, ptrongbinh@gmail.com
![]() Zhao Zhenyu is currently pursuing a high school diploma at
Zhao Zhenyu is currently pursuing a high school diploma at
NUS High School of Math and Science, Singapore, (+65)
65161709, h0930054@nus.edu.sg
Furthermore, the dye absorption property derived from hydrogels can present an alternative way of treating industrial waste4.
Recently, work has been done to prepare hydrogels that are both pH and temperature sensitive by copolymerizing two monomers to form an interpenetrating polymer network (IPN)5,6. Since there is no chemical bond between the two component networks, each network can retain its own unique property. While substantial research has been done on IPNs that function in basic medium, not many looked into those that might function in acidic medium, even though many biological processes take place in acidic environment, such as digestion in the stomach.
Poly (N-acryloyl-N′-ethylpiperazine) (PAcrNEP) is an ionisable hydrophilic polymer. Cross-linked PAcrNEP is able to swell in water and its swelling behaviour is greatly pH-dependent due to the ionization/deionization of the amine groups. At a high pH, usually greater than 5.5, the amine groups are not ionized, which keeps the PAcrNEP in its collapsed state. At low pH, the amine groups are ionized and the charged amine groups repel each other, leading to the swelling of PAcrNEP7. Poly (N-Vinylcaprolactam) (PVCL) is a temperature sensitive IPN. Cross-linked PVCL exhibits drastic swelling transition at its lower critical solution temperature (LCST) of 35oC. At temperatures lower than 35oC the gels is swollen
INTERNATIONAL JOURNAL OF SCIENTIFIC & ENGINEERING RESEARCH, VOLUME 3, ISSUE 4, APRIL-2012
ISSN 2229-5518
whereas at temperatures higher than 35oC, the gel dehydrates to the collapsed state due to the breakdown of the delicate hydrophilic
/hydrophobic structure8.
1. To synthesize pH- and temperature- sensitive interpenetrating networks based on PAcrNEP and PVCL
2. To investigate the swelling behaviour of IPNs with respect to pH, temperature, and ionic strength.
3. To study permeation behaviours of these
IPNs under various temperature and pH
condition.
4. To study heavy metal absorption
capability of these IPNs.
5. To study the ability of these IPNs to grow silver nanoparticles.
6. To study the dye absorption ability of
IPNs.
7. To characterize the prepared hydrogel- silver nanocomposites (HSNCs).
Hydrogels are made up of 3 components: the monomer, 2g N-vinylcaprolactam (VCL), the crosslinker, ethylene glycol dimethylacrylate (EGDMA) and the initiator, 4 wt% Azobis 4- cyanovaleric acid (ACA). The gels differed in their cross linker concentrations, which were set at 6, 9 and 12 wt%. The mixture was dissolved in 3.5mL ethanol and bubbled in nitrogen gas. The solution was heated in an oven set at 70 ⁰C until a gel was form. The gel was cut into disks of diameter 1cm and thickness 0.5cm and left to dry in a vacuum drier set at 70 ⁰C4.
To synthesize the IPN, a piece of hydrogel was soaked with an equal mass of AcrNEP and EGDMA, before adding the initiator (4wt% ACA) and dissolving it in 1mL ethanol. The concentration of EGDMA added was the same as the cross-linker concentration in the given gel. The mixture was heated in an oven set at 70⁰C overnight. The gel was washed with ethanol before being cut into disks of diameter 1cm and thickness
0.5cm and left to dry in a vacuum drier set at 70
⁰C4. Image 2 shows the completed product.
A piece of 2wt% non-IPN / 2wt% IPN gel was soaked in 10ml of 0.05M AgNO3 solution and left to equilibrate for 24 hours. After that, 10 ml of
0.05M NaBH4 was added and the solution was put in the freezer set at -20 ⁰C. This extracted the silver
ions from silver nitrate and embedded it into the gel.
To characterize the IPNs, swelling tests in de- ionized water at different temperatures and pH solution were first carried out. The swelling ratio was calculated using the following equation4.
SR=![]() .
.
IPN was soaked in water for 2 hours. Its weight was taken every 10 minutes. This was to investigate the swelling behaviour of the IPN with respect to time.
For the pH test, the IPN was soaked in solutions of different pH values ranging from 2 to 10. For the temperature test, the IPN was soaked in solutions at different temperatures ranging from 20⁰C to
50⁰C and left for 24 hours before their weights
were measured. This was to investigate the
swelling behaviour of the IPN with respect to pH
and temperature.
IPN was soaked in NaCl solutions with different molar concentrations ranging from 0.005 to 0.500
M and left for 24 hours before their weights were measured.
IPN was soaked in a pH 10 buffer solution for an hour, then transferred to a pH 3 buffer solution for the next hour, then finally put in the pH 10 solution for another hour. Weight of the IPN was measured every 10 minutes.
INTERNATIONAL JOURNAL OF SCIENTIFIC & ENGINEERING RESEARCH, VOLUME 3, ISSUE 4, APRIL-2012
ISSN 2229-5518
0.1 mM solutions of Congo red were prepared. The absorbance of the solutions was recorded on UV/Vis Spectrophotometer (Perking Elmer Lambda 25). A disk of IPN/PVCL of 1cm in diameter was soaked in each solution and left for
24 hours. The new absorbance was then recorded.
The samples were labelled as followed: dye
solution (D-P), dye solution with soaked PVCL (D- VCL), dye solution with soaked IPN (D-IPN).
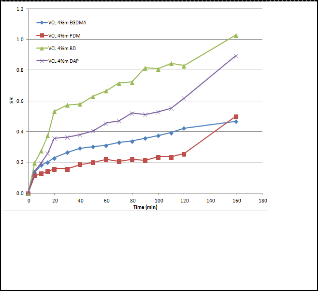
Hydrophilicity of the cross-linker played an important part in controlling the swelling of hydrogels. Figure 1 shows that an increase in hydrophilicity led to an increase in the hydrogel’s swelling capacity. Even though gels prepared with BD and DAP as cross-linkers had a high swelling ratio, they tend to break apart easily during swelling. Therefore, EGDMA was chosen as the cross-linker in subsequent experiments because the gel prepared with EGDMA had a moderate swelling capacity and great stability.
It was found that the cross-linker concentration also had a significant impact on the swelling capacity of the hydrogel. Figure 2 shows that a higher cross-linker concentration led to a lower swelling capacity, because a higher amount of cross-linker rigidified the polymer network which then exhibited poor elastic response. Such a structure would hinder the diffusion of solvent into the network. The same can apply to IPN, as seen from the trend in figure 3.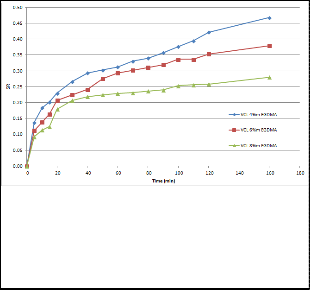
Fig 1. Swelling behavior as a function of time at 26o C in water of VCL gels prepared with different cross-linkers
(160’= infinity)
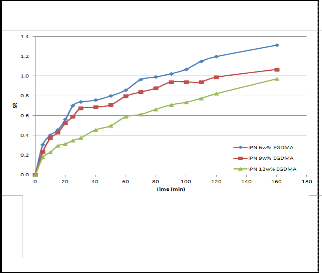
Fig 3. Swelling behaviour as a function of time at 26oC in water of IPNs prepared with different concentration of EGDMA (160’= infinity)
Fig 2. Swelling behaviour as a function of time at 26oC in water of hydrogels prepared with different concentration of EGDMA (160’= infinity)
Equilibrium swelling studies indicated that the IPN samples were capable of responding to external stimuli: pH, temperature and ionic strength.
INTERNATIONAL JOURNAL OF SCIENTIFIC & ENGINEERING RESEARCH, VOLUME 3, ISSUE 4, APRIL-2012
ISSN 2229-5518
Fig 4. Equilibrium swelling behaviour as a function of pH
of 6w% IPN and VCL hydrogels at 25oC.
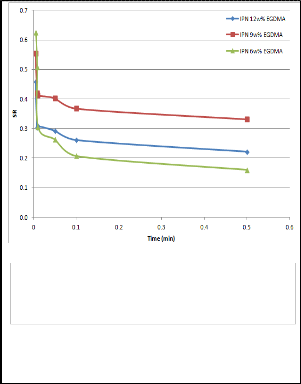
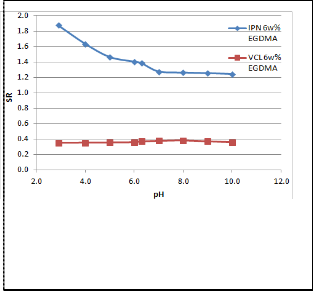
Figure 4 shows the influence of pH on the swelling capacity of the IPN and pure VCL hydrogels from pH 3 to pH 10. The swelling curve of the IPN exhibited significant transition from pH 3 to pH 6: the swelling ratio decreased from 1.9 to 1.4. This transition was a result of the nature of the IPN network. In a buffer solution of low pH, the amino nitrogen in the AcrNEP component of the network was ionized, leading to the formation of like charges within the gel network. The repulsion of these charges resulted in the swelling of the IPN, which increaseed the water uptake capacity. The pKa of AcrNEP was 4.58; hence excess ionization was expected to occur around a pH of 3–4.5, which corresponded to the steep portion of the curve. In contrast, pure VCL gel exhibited little change in swelling capacity since they could not be ionized. It was also noted that IPN had better swelling capacity compared to PVCL gels even at high pH.
Figure 5 shows the effect of temperature on the swelling behaviour of IPN and VCL hydrogels. Deswelling transition in the temperature range of
20oC to 35oC was observed for both samples and
the transition curves were rather similar since the
VCL component in the IPN was responsible for the
thermo-sensitivity. However the transition of the VCL gel was sharper, with its swelling ratio almost halved from 20oC to 25oC. At a low temperature the gels were able to swell, leading to a high swelling ratio. As temperature increased, the delicate hydrophilic/hydrophobic balance in the VCL network was broken, leading to dehydration. In the IPN, the existence of AcrNEP network might have interfered with the hydrophilic/hydrophobic balance of the VCL network, so dehydration might not have taken place at the same time, resulting in a gradual deswelling transition.
Figure 6 shows the influence of ionic strength on swelling behaviour. As the concentration of NaCl increased, the swelling ratio dropped drastically. This was due to the presence of counterions at high ionic strength, which hindered ionization of the amino nitrogen.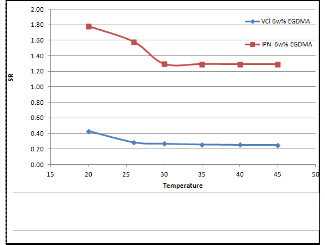
Fig 6. Equilibrium swelling behaviour of IPN and
VCL hydrogels in water at 25oC.
Fig 5. Equilibrium swelling behaviour as a function of temperature of 6w% IPN and VCL hydrogels in water.
INTERNATIONAL JOURNAL OF SCIENTIFIC & ENGINEERING RESEARCH, VOLUME 3, ISSUE 4, APRIL-2012
ISSN 2229-5518
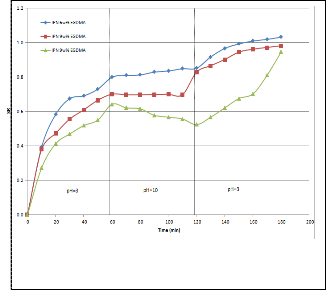
Fig 7. Oscillatory swelling behavior of IPN hydrogels at 25oC.
Temperature-sensitive PVCL and pH-sensitive PAcrNEP interpenetrating networks (IPN) were synthesized by sequential polymerization. The most suitable cross-linker used to synthesize the IPN was determined to be EGDMA, as it gives the IPN moderate swelling capacity and good stability. The IPN was shown to have superior swelling capacity compared to PVCL. It was also determined that high cross-linker concentration will lead to low swelling ratio of the gel. The IPN was shown to exhibit swelling transition at a temperature range of 20-35oC and pH range of 3-6. This indicated that each network is able to retain its properties in the IPN. Presence of ion salt was also found to hinder the IPN ‘s swelling capacity.
Oscillatory swelling study was carried out to investigate the ability of the IPN to respond to pH pulses. Fig 7 shows that the IPN gels were able to respond to changes in pH and the responses were quick and reversible.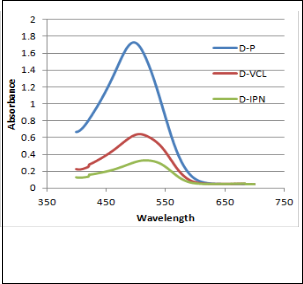
Fig 8. Absorbance of dye solutions
Since Congo red dye exists as anions in solution, it is strongly attracted to the positive charged amine groups in the IPN. The change in absorbance (fig 8) verified the ability of the IPN to act as a potential purifying agent for waste contaminated with dye. The IPN was also found to be a superior agent compared to PVCL.
INTERNATIONAL JOURNAL OF SCIENTIFIC & ENGINEERING RESEARCH, VOLUME 3, ISSUE 4, APRIL-2012
ISSN 2229-5518

Image 1: Dry PVCL hydrogel

Image 2 Dry IPN
Image 3 Swollen IPN in pH 3
The authors express gratitude for guidance and equipment from Associate Professor Dr.Roshan Deen of National Institute of Education, Singapore.
[1] Xiaohu Xia, B.E., Fabrication and light scattering study of multi-responsive nanostructured hydrogels and water soluble polymers, University of North Texas, 2003
[2] Zbigniew Ruszczak, Robert A Schwartz, Ewa Joss-Wichman, Surgical Dressings, Medscape Reference (2011)
[3] Sangphil Park, P.S. Keshava Murthy, Saemi Park, Y. Murali Mohan, Won-Gun Koh, Preparation of silver nanoparticle-containing semi- interpenetrating network hydrogels composed of pluronic and
Preparation of silver nanoparticle-containing semi-
interpenetrating network hydrogels composed of pluronic and pol (acrylamide) with antibacterial property, Journal of Industrial and Engineering Chemistry (2011) 293-297
[4] S.J. Allen, B. Koumanova, Decolourisation of water/wastewater using adsorption (review),
Journal of the University of Chemical Technology and Metallurgy 40-3 (2005) 175-192
[5] Y. Murali Mohan, Kurt E. Geckeler, Polyampholytic hydrogels: Poly (N- isopropylacrylamide)-based stimuli responsive networks with Poly (ethyleneimine), Reactive and Functional Polymers 67 (2007) 144-155
[6] Jing Zhang, Nicholas A. Peppas, Synthesis and Characterization of pH- and Temperature- Sensitive Poly (methacrylic)/Poly (N- isopropylacrylamide) Interpenetrating Polymeric Networks, Macromolecules 33 (2000) 102-107
[7] G. Roshan Deen, Swelling Behavior and Metal- Ion Uptake Capacity of pH-Responsive Hydrogels of Poly (N-acryloyl-N’-ethylpiperazine), Journal of Dispersion Science and Technology 31 (2010) 1673-
1678
[8] Volodymyr B. Boyko, N-Vinylcaprolactam
based bulk and microgels: synthesis, structural formation and characterization by dynamic light scattering, Doctoral dissertation, Dresden University of Technology (2004)