sorption [46], and electrochemical precipitation [47] have been suggested for the removal of Cr (VI). Among
International Journal of Scientific & Engineering Research Volume 2, Issue 5, May-2011 1
ISSN 2229-5518
Sorption of Cr (VI) & As (V) on HDTMA –Modified
Zeolites
Vandana Swarnakar, Nishi Agrawal, Radha Tomar
Abstract-Sorption of Cr (VI) & As (V) on HDTMA modified zeolites were investigated by batch technique, X-ray diffraction, Fourier transform infrared analysis, Energy dispersive spectroscopy and Scanning electron microscopy. HDTMA was exchanged with extra structural cations of zeolite up to the external cation exhange capacity. The HDTMA modified surface was stable when exposed to extremes in pH, ionic strength and to oxoanions. The HDTMA modified zeolites showed significant sorption for chromate and arsenate ions in aqueous solution. Sorption data for each anion was well described by Freundlich isotherm equation. Increase in Cr (VI) & As (V) sorption on to modified surface occurred in neutral solution (pH7) and the amount of sorbed Cr (VI) & As (V) described rapidly with increasing pH since (OH-) concentration competes against Cr (VI) & As (V) for the sorption sites, thus, inhibiting formation of Cr (VI)- SMZ & As (V)-SMZ complex. FTIR analysis showed that sorbed SMZ forms an ad-micelle surfactant surface coverage, which is responsible for Cr (VI) and As (V) sorption.
Keyword: Sorption; Cr (VI); As (V); HexaDecylTriMethylAmmonium-Bromide (HDTMAB)
—————————— • ——————————
hromium is one of the most abundant inorganic groundwater contaminant at hazardous waste
sites. Compared to its trivalent counterpart, hexavalent
Cr forms chromate (CrO42-) or hydrogen chromate (HCrO4-) that is more toxic and more soluble at various pH. Because of the negative charges, chromate sorption on aquifer minerals is limited, making it more mobile in subsurface soils and aquifers. Conventional treatment of chromate-rich effluent is to reduce Cr (VI) to Cr (III) and precipitate Cr (III) as chromium hydroxide, or chromium iron hydroxide at high pH, followed by disposal of resulting dewatered sludge. Wastewater containing relatively low concentrations of Cr (VI) is usually treated with ion exchange resins to remove Cr (VI) [1]. These conventional methods for Cr (VI) removal are expensive and thus research to find inexpensive sorbent materials have been conducted recently [2-8].
These metals are found well above the tolerance limit many a times in the aquatic environment [9]. Chromium is widely used in electroplating, leather tanning, dye, cement and photography industries producing large quantities of effluent containing the
————————————————
• Vandana Swarnkar is currently pursuing Ph.D. freomSOS in
Chemistry Jiwaji University, Gwalior, India.
Mobile : +919425700609. E-mail: sonivandana20@gmail.com
• Nishi Agrawal is currently pursuingPh.D. . freomSOS in Chemistry
Jiwaji University, Gwalior, India.
• Radha Tomar, Professor, SOS in chemistry, Jiwaji University, Gwalior is
guide to help in pursuing Ph.D.
toxic metal [10]. Cr (VI) is of particular concern because of its toxicity [11]. The recommended limit of Cr (VI) in potable water is only 0.05 mgl-1 [12]. However, industrial and mining effluents contain much higher concentrations compared to the permissible limit. Arsenic in groundwater is largely due to minerals dissolving naturally from weathered rocks and soils. Also, it has many industrial applications and is also used extensively in the production of agricultural pesticides [13, 14]. Runoff from these uses and the leaching of arsenic from generated wastes has resulted in increased levels of various forms of soluble arsenic in water. Use of arsenic contaminated water may cause numerous diseases of the skin and internal organs [13–
17]. Consequently, extensive research to develop cost-
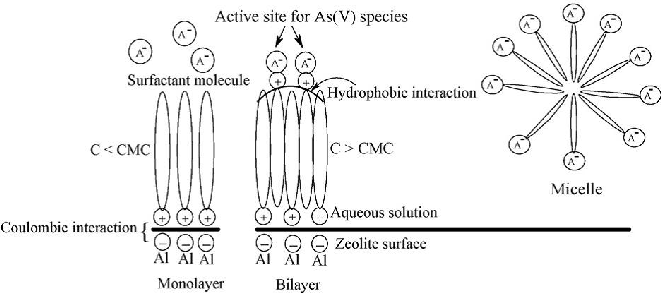
effective methods for arsenic removal has been carried out recently using various sorbents [17–21]. Sorption technique is generally considered to be promising method amongst the existing technologies due to easy separation of sorbent from aqueous media after treatment [22]. Naturally occurring zeolites are hydrated aluminosilicate materials with high cation exchange capacities [23–27]. Sorption of arsenic on natural zeolites has been studied extensively in recent years due to their low cost and availability in nature [28–42]. See Fig. 1.
Thus, treatment of effluent to reduce / remove the pollutant before discharging into the environment becomes inevitable. Different methods such as reduction and precipitation [43], ion exchange [44],
electrolysis, reverse osmosis, solvent extraction [45],
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 5, May-2011 2
ISSN 2229-5518

sorption [46], and electrochemical precipitation [47] have been suggested for the removal of Cr (VI). Among
Fig.1 - A model of modification of zeolite surface by surfactant and sorption of As (V) species.
all these, sorption is the most promising technique and a feasible alternative [48]. A variety of materials have been tried as sorbents for Cr (VI) and a number of studies have been reported using sorbents like granular activated carbon [49], soya cake [50], rubber tyres and sawdust [51], activated sludge [52], lingocellular substrate [53], fly ash [54], rice husk based activated carbon [55] etc.
The present work deals with sorption of Cr (VI) & As (V) on Surfactant Modified Zeolites and to determine their sorption capacity. The well known thermodynamic functions and isotherm studies have been reported to elucidate the equilibrium adsorption behavior at different temperatures. In addition to the effect of temperature, the effect of pH, adsorbent dose, time and concentration of sorbate on percentage sorption have been investigated. Thus, Zeolites Erionite, Cowlesite and Willhendersonite modified by cationic surfactant hexdecyltrimethylammonium bromide HDTMAB has been potentially used as sorbents to remove anionic contaminants such as Cr (VI) and As (V) from waste water.
2.1. Sample
Zeolites were synthesized by hydrothermal method and modified by hexadecyltrimethylammonium bromide (HDTMAB) followed by centrifugation. Scanning electron microscope (SEM) fig.2 and X-Ray diffraction
analysis (XRD) fig.3 confirmed that the sample was of
high purity. The zeolites were modified by HDTMAB (from HIMEDIA). In centrifugal tube, 5gm of raw zeolites were mixed with 2.5 gm of HDTMAB with
180ml of distilled water. The solutions were mixed on a
water bath shaker for 24 hrs. Zeolites were then separated from solution by centrifugation. After the supernatant solutions were decantated the zeolites were washed twice with deionized (DI) watered and air dried.
The surface morphology change after surfactant modification was similar to that of Ca-montmorillonite after hexadecyletrimethyl ammonium (HDTMA) modification [56]. Energy dispersion spectrum showed increase in carbon after surfactant modification.
2.2. X-Ray Diffraction Analysis
Powder X-ray diffraction analysis was performed on an X-ray diffractometer with CuKa radiation at 45 kV and 40mA. XRD patterns of randomly oriented samples were collected from 28 equal to 5o to 70o at the scanning rate of 2o/min using 1o divergent slit and scatter slit and
0.3 mm receiving slit, while those of oriented samples were collected from 28 equal to 5o to 50o.
2.3. Sorption of Cr (VI) and As (V)
Cr (VI) solution of desired concentration was made using K2CrO4 and As (V) solution was made using Na2HAsO4. The pH of Cr (VI) and As (V) solution was adjusted to 1, 3, 5, 7, 9 using HNO3 or NaOH. To each centrifugal tube 0.1 gm of surfactant modified Erionite
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 5, May-2011 3
ISSN 2229-5518
(E-SMZ), Cowlesite (C-SMZ) and Willhendersonite (W- SMZ) were added to 50ml Cr (VI) and As (V) solution at Cr (VI) and As (V) concentration 108.96 mg/L and 104.0 mg/L respectively. The highest Cr (VI) and As (V) sorption on E-SMZ, C-SMZ & W-SMZ were achieved at pH 7. Thus, all the solutions were adjusted to pH 7 in the subsequent sorption experiments. For Cr (VI) and As (V) sorption isotherm determination, different weights of E- SMZ, C-SMZ and W-SMZ were added to 50 ml metal oxoanions solution of initial concentration 108.96- 645.80 mg/L for Cr (VI) and 104.12-502.01mg/L for As (V) with
0.01N increment. To study the thermodynamic
parameters 0.1gm of E-SMZ, C-SMZ and W-SMZ were added to 50ml of 0.01N Cr (VI) and As (V) solutions at pH 7. Sorption was carried out at temperature 288, 298 and 308K in a constant temperature water bath shaker. The amount of Cr (VI) and As (V) sorbed on to E-SMZ, C-SMZ and W-SMZ were calculated from the difference between the initial and equilibrium concentrations of Cr (VI) and As (V).
2.4. FTIR Analysis
FTIR analysis was carried out on a (NICOLET- 410) Fourier–transform infrared spectrometer. The spectra were recorded in the region 400-4000cm-1 with a spectral resolution of 2cm-1, using a pressed KBr pellet technique.
2.5. Quality Assurance
In order to ascertain reliability, accuracy and reproducibility of the assembled data, the batch equilibrium tests carried out for Cr (VI) and As (V) sorption were replicated twice and experimental blanks were run in parallel. All the glass wares were pre soaked before use in 5% HNO3 for about 24 h followed by washing with deionized water and drying in an oven. Sample blanks were analyzed for correction of background effect on instrument response.
3.1 XRD Analysis
X-ray diffractogram of synthetic gel has been recorded using Cu Ka radiation in a range 28 = 5o to 70o at a scanning speed of 1step/second. Powder X-ray diffraction pattern of hydrothermally synthesized material E-SMZ, C-SMZ and W-SMZ are represented in Fig 2a, Fig. 2b and Fig. 2c. In all cases medium was NaOH, the degree of crystallinity is very high as shown by the peak intensity for the major diffraction peak. These pattern shows maxima at 28 = 26.85o, 14.75o &
25.94o, therefore d spacing between the plane is found to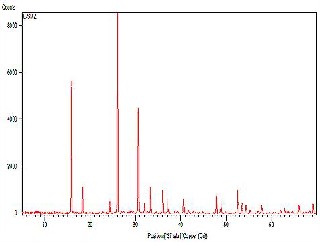
be d = 0.20Å, 0.20 Å &0.10 Å. Powder X-ray diffraction analysis shows that NaOH medium for E-SMZ, C-SMZ & W-SMZ type material synthesis resulted in crystalline products.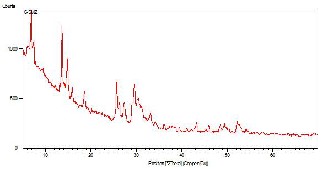
Fig. 2a - XRD diffractogram of hydrothermally synthesized E-SMZ
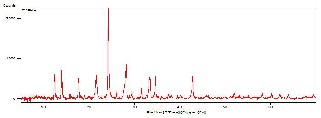
Fig. 2b - XRD diffractogram of hydrothermally synthesized C-SMZ
Fig. 2c - XRD diffractogram of hydrothermally synthesized W -SMZ
3.2. FTIR Spectroscopy
FTIR (NICOLET - 410 Spectrometer) spectra of E- SMZ, C-SMZ and W-SMZ are presented in Fig. 3. It is found in the range 950-1250 cm-1 & 420-500cm-1 strongest vibration at 950-1250 cm-1 is assigned to T-O stretching and the next strongest band at 420-500 cm-1 is assigned to T-O bending mode (T= Si or Al).
The Hydroxyl bond –OH stretch near 3550 cm-1 in
Spectra indicates the bimodal absorbance. The water
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 5, May-2011 4
ISSN 2229-5518

molecules attached to zeolite frame work shows strong characteristic structure sensitive bands due to water (H2O) bending vibration at 1630 cm-1. The peaks below
550 cm-1 indicates (O-T-O) bending of rotation mode. The
peaks between 700-850cm-1 and 1000-1150 cm-1 are assigned to symmetric and anti-symmetric T-O-T stretching vibration. Two bands around 3000-3500 cm-1 &
2800-2900 cm-1 appeared in the E-SMZ, C-SMZ and W- SMZ indicate asymmetric & symmetric stretching vibration of –CH2 of alkyl chain and band at about 1200 -
1600 cm-1 was assigned to vibration of trimethyl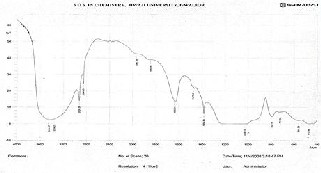
ammonium quaternary group CN (CH3)3+.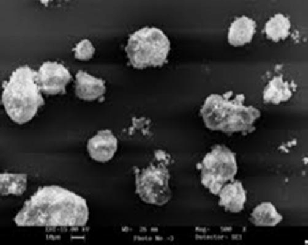
Fig. 3 - FTIR spectra of hydrothermaly synthesize E-SMZ
3.3. Scanning Electron Microscopy (SEM)

The SEM images Fig. 4 of synthetic modified Erionite, Cowlesite and Willhendersonite gel suggest the crystal size of the material to be in the range 1µm- 10µm show various morphology of the meso structured material depending on the crystallization conditions. Photomicrographs of the synthetic modified E-SMZ, C- SMZ & W-SMZ exhibited well defined narrow shape with excellent crystal edges. The aggregation of zeolites as HDTMAB is sorbed provides information about surfactant aggregation on the surface. These scanning electron microscopy images show the underlying crystalline structure of E-SMZ, C-SMZ and W- SMZ.
Fig.4 - SEM images showing high purity of Surfactant Modified E- SMZ, C-SMZ & W - SMZ.
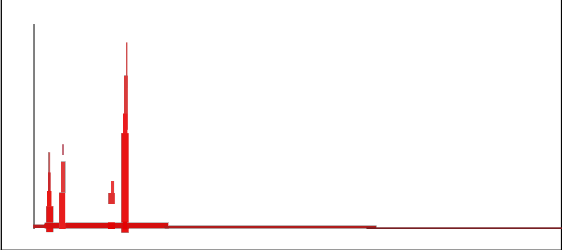
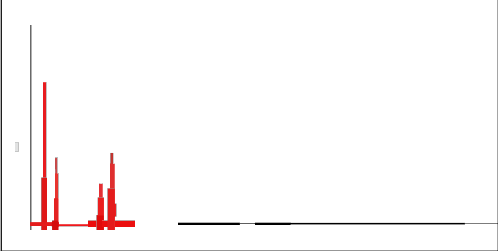
3.4. Energy dispersive spectrometry (EDS)
The chemical composition of the synthesized modified material was checked for metal ions Na, Ca, Al, and Si using energy dispersive spectrometry. A revealing feature in the synthesis of zeolite is the strong correlation between Si/Al ratio of the resulting crystals, the nature of the cation used and medium of synthesis. The flexibility in the synthesis to produce desired composition of zeolite represent a critical step in the improvement of the material for many environmental process in which Si/Al ratio is the key for maximizing performance. The chemical composition of the prepared material is given in Fig. 5 for E-SMZ, C-SMZ and W-SMZ. It is observed that composition of the prepared material is quite close
to modified zeolites E-SMZ, C-SMZ and W-SMZ.
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 5, :May-2011 5
ISSN 2229-5518
c:l.eda>c32"\ge<n.,..i$'1g)ennn<ap$..s;pC G'I- .Ap.--2CUO '1:2::49t:-
LSecs: 12
91115-
SIK
732-
$--
OK
31515-
All
1183-
0
.._ ..
01)
3,o.o
4..10:0 5..00 s..ea
7.GD 8CIDo
9c01> 1110..0
""'t
.....
KK
KIC
2.
Energy -lkeV
3'19-
2.3:9 -
"'JSV-
OII!C
.A.U
..... .JI&'
:a:.oo 4.1000 5.100 •.oo
la•«•QV - .._.....
.,_..
·- ·

c:'tedax:32lcle is'\genrn ps..spc 01-AprO'14<42:52
IL..Sec·s:: 12
57':91
4>63:
/ K
3:411''
23:1 I SiK
I < K
All<
115·
J.
Ca'K
caK
IKK
KK
0
"J..OO 2...0GI 1ee 4WOO 5.00 &..GO 7.•• 8 00 !I..GO "JO.G
Energy - keV
Fig.5- EDAX spectra showing high purity of Surfactant Modified E-SMZ, C-SMZ & W- SMZ
IJSER© 2011 http!!Www.iiser.oru
International Journal of Scientific & Engineering Research Volume 2, Issue 5, May-2011 6
ISSN 2229-5518
3.5. Thermodynamics of Sorption
The effect of temperature on the sorption of oxoanions on E-SMZ, C-SMZ & W-SMZ was also checked using the optimized conditions. The temperature was varied from 288 to 308K. The amounts of metal oxoanions sorbed at various temperature which reveals that the uptake of Cr (VI) and As (V) increase with increase in temperature, indicating better sorption at higher temperature. The enhancement amount of oxoanions sorbed at equilibrium with the rise in temperature may be either due to creation of some new active sites on the sorbent surface.
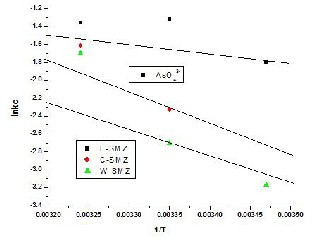
The amounts of oxoanions sorbed at equilibrium at different temperatures have been utilized to evaluate the thermodynamically parameters for the sorption system. The van't Hoff plot of lnkc V/s 1/T was a straight line Fig.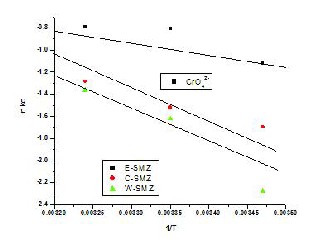
6(a) & Fig. 6(b).
Fig. 6(a) - Vant's Hoff Plot of lnkc Vs 1/T for Cr (VI) sorption on
Zeolites.
Fig. 6(b) - Vant's Hoff Plot of lnkc Vs 1/T for As (V) sorption on modified zeolites.
3.6. Sorption Isotherm
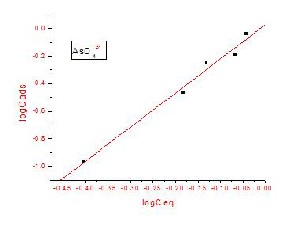
Analysis of the isotherm data is necessary in order to develop an equation that can accurately represent the results and could be used for design purposes. The data obtained from the sorption isotherm studies were fitted to the Freundlich Isotherms.
The Freundlich isotherm shown in above equation assumes that the uptake of metal ions occurs on a heterogeneous surface by multi-layer sorption and the amount of sorbate adsorbed increases with increasing concentration. The K and 1/n are the constants of the Freundlich isotherm that corresponds to the sorption capacity and intensity respectively. The parameter Ceq correspondence to the remaining concentration of the sorbate in the solution and Cads is the amount sorbed at equilibrium.
The constants of the isotherm equations can be computed from the intercept and slope of the linearized plot of the experimental:
[Cads = K Ceq 1/n ] (1)
The isotherm constants were calculated from the slope and intercept, which is shown in Fig. 7(a) & Fig.
7(b). The values of correlation coefficient R2 for CrO42- &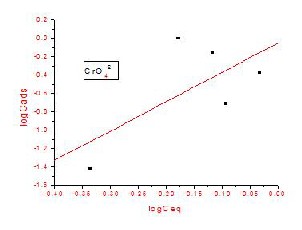
AsO43- are higher in the Freundlich isotherm which indicates that the sorption process is well represented by the Freundlich equation.
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 5, May-2011 7
ISSN 2229-5518
modification with HDTMAB. The drop of final pH is due to OH- consumption via deprotonation of surface AlOH2+ groups giving back terminal aluminol group, AlOH (Eq.
(4)):
Fig .7(a) & (b) - Freundlich sorption isotherm of CrO42- & AsO 3- on
AlOH2+ � AlOH + H+ (4) In case of Cr (VI) ion maximum sorption occure at
pH 7 chromium is present in the form of CrO42- which is more stable form of Cr (VI). However, at lower pH dichromate ions are formed which has larger particle size as compared to chromate ions. The pore size of zeolite is suitable for the sorption of CrO42- ions rather than the Cr2O72- ions.
E-SMZ at 25 0C
HCrO4 -
� CrO42-
+ H+
(5)
3.7. Effect of pH on Cr (VI) And As (V) Sorption:
The results of experiments carried out in order to evaluate the efficiency of E- SMZ, C-SMZ & W-SMZ sorbent in removing As (V) over a range of pH 1-9. The sorbent removed arsenic effectively over the initial pH range 6–10. The predominant forms of arsenate in this pH range are H2AsO4- and HAsO42- [14]. The surface anion exchange between these two arsenate forms and counter ion bromide of surfactant-modified zeolites (SMZ) can be presented conceptually by Eq. (2) and (3) which were well verified and discussed by previous workers [21]. It is evident from the figure that both of these forms can be effectively sorbed by E-SMZ, C-SMZ
& W-SMZ. Compared to the previous works related to As (V) sorption by surfactant-modified zeolites, where optimum pH range were reported to be 7.2–7.5 [21] and
7.4 [37], the sorption for As (V) by investigated surfactant-modified zeolites is of a wide optimum pH range, which should be of significant importance for practical operation. At initial pH <1, As (V) in solution exist in neutral form H3AsO4 [14], no ion exchange took place with bromide and observed As (V) sorption is only for physical sorption. Therefore, As (V) sorption efficiency is remarkably low at pH~1.
SMZ–Br + H2AsO4- = SMZ–H2AsO4- +Br- (2)
2SMZ–Br + HAsO42- = SMZ2–HAsO4 +2Br- (3) The change of final pH as a function of initial pH
range 1-9. The sorbent follow the same trend of pH change indicating homogeneous nature of the surfaces in respect of existing ions. In the pH range 6–10, the pH of solution shifted towards acidic region. This may be due to the fact that zeolite surface could still generate
protonated AlOH2+ groups in solution even after
H2CrO4 �HCrO4- + H+ (6)
Cr2O7 + H2O � HCrO4- (7) In case of basic solution when pH is greater than 7
the chromate ions are converted into Cr(OH)3 species, it
has less oxidizing ability. In the case of Cr, +6 oxidation states are more stable as compared to +3 oxidation state therefore chromium shows maximum sorption at pH 7.
CrO4- + 4H2O + 3e- ![]() Cr(OH)3 + 5OH- (8) The sorption behavior of CrO4-2 & AsO4-3 on E-SMZ,
Cr(OH)3 + 5OH- (8) The sorption behavior of CrO4-2 & AsO4-3 on E-SMZ,
C-SMZ and W-SMZ were checked at different pH of the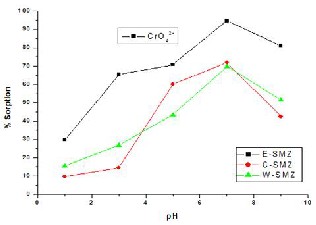
solution viz. pH 1, 3, 5, 7, 9. Fig. 8 (a) & Fig. 8 (b) show the effect of pH on sorption of CrO4-2 & AsO4-3 on E- SMZ, C-SMZ and W-SMZ. The data shows maximum sorption at pH 7 for E–SMZ. The results show that the sorption percentage increases up to pH 7 with increase in pH of the solution and thereafter sorption percentage decreases.
Fig. 8(a) - Sorption Cr (VI) on surfactant modified zeolites as a function of solution pH
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 5, May-2011 8
ISSN 2229-5518
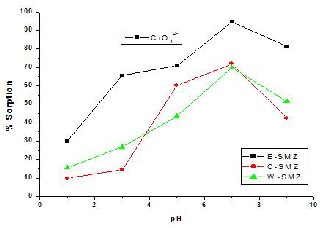
Fig. 8(b) - Sorption As (V) on surfactant modified zeolites as a function of solution pH
Surfactant modified- Erionite, Cowlesite and Willhendersonite sorbs Cr (VI) and As (V) effectively in neutral solutions and sorption of Cr (VI) and As (V) is strongly pH dependent. Cr (VI) sorption capacity reached 21 kg, and As (V) sorption capacity reached
19g/kg the sorption process is well described by
freundlich equation. The enthalpy change (l'lH) of the sorption is 0.1258 kj/mol and 0.9188 kj/mol indicating sorption of Cr (VI) and As (V) on to surfactant modified Erionite . FTIR analysis shows that HDTMA cations in the interlayer region caused polarization of adsorbed H2O molecules. The uptake of negatively charged Cr (VI) and As (V) ions in the interlayer regions of surfactant modified – Erionite, Cowlesite and Willhendersonite are probably due to electrostatic force derived from the polarized water molecules and positively charged groups of the HDTMA cations.
The authors acknowledge to Head IIT, IIT Roorkee providing necessary instrumental facilities for XRD, EDS and SEM analysis.
[1] D. Kratochvil, P. Pimentel and B.Volesky, “Removal of trivalent and hexavalent chromium by seaweed biosorbent.”, Environ. Sci. Technol. 32, 2693–2698, 1998.
[2] J.M. Zachara, C.E. Cowman, R.L. Schmidt and C.C.
Ainsworth, “Chromate adsorption by kaolin”, Clays Clay
Miner. 36, 317–326, 1988.
[3] Z. Li and R.S. Bowman, “Counterion effects on the sorption of cationic surfactant and chromate on natural clinoptilolite”, Environ. Sci. Technol. 31, 2407–2412, 1997.
[4] Z. Li, and R.S. Bowman, “Sorption of chromate and PCE by
surfactant modified clay minerals” Environ. Eng. Sci. 15, 237–
245, 1998.
[5] Z. Li, “Sorption of oxyanions and surface anion exchange by surfactant modified clay minerals”, J. Environ. Qual. 28, 1457–
1463, 1999.
[6] Z. Li, D. Alessi, P. Zhang, R.S. Bowman, “Organo-illite as a low permeability sorbent to retard migration of anionic contaminants”, J. Environ. Eng. 128, 583–587, 2002.
[7] B.S. Krishna, D.S.R. Murty, B.S. Jai Prakash,
“Thermodynamics of chromium (VI) anionic species sorption onto surfactant-modified montmorillonite”, clay. J. Colloid Interface Sci. 229, 230–236, 2000.
[8] B. S. Krishna, D. S. R. Murty and B. S. Jai Prakash, “Surfactant-modified clay as adsorbent for chromate”, Appl. Clay Sci. 20, 65–71, 2001.
[9] N. Tewaria, P. Vasudevana and B.K. Guhab, “Study on biosorption of Cr (VI) by Mucor hiemalis, Biochem”, Eng. J. 23,
185–192, 2005.
[10] C. Raji and T.S. Anirudhan, Water Re, 32, 3772, 1998.
[11] E. Oguz, “Adsorption characteristics and the kinetics of the Cr (VI) on the Thuja oriantalis”, Colloids Surf. A: Physicochem. Eng. Aspects 252, 121–128, 2005.
[12] K. Slvaraj, S. Manonmani and S. Pattabhi, “Bioresour”.
Technol, 89, 207–211, 2003.
[13] R. Menhage-Bena, H. Kazemian, M. Ghazi-Khansari, M.
Hosseini and S.J. Shahtaheri, “Evaluation of some natural zeolites and their relevant synthetic types as sorbents for removal of arsenic from drinking water,” Iran. J. Public Health
33, 36–44, 2004.
[14] S. Shevade and R.G. Ford, “Use of synthetic zeolites for arsenate removal from pollutant water”, Water Res. 38, 3197–
3204, 2004.
[15] C.J. Wyatt, V.L. Quiroga, R.T.O. Acosta and R.O. Mendez, “Excretion of arsenic (As) in urine of children”, 7–11 years, exposed to elevated levels of As in the city water supply in Hermosillo, Sonora, Mexico, Environ. Res. 78, 19–24, 1998.
[16] M.P. Elizalde-Gonzalez, J. Mattusch and R. Wennrich,
“Application of natural zeolites for preconcentration of arsenic species in water samples”, J. Environ. Monit. 3, 22–26,
2001.
[17] S.W. Al Rmalli, C.F. Harrington, M. Ayub and P.I. Haris, “A biomaterial based approach for arsenic removal from water”, J. Environ. Monit. 7, 279–282, 2005.
[18] D. Mohan, C.U. Jr. Pittman, M. Bricka, F. Smith, B. Yancey, J.
Mohammad, P.H. Steele, M.F. Alexandre-Franco, V. Gomez- Serrano and H. Gong, “Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production”, J. Colloid Interface Sci. 310, 57–73,
2007.
[19] D. Mohan and Jr,C.U. Pittman, “Arsenic removal from water/wastewater using adsorbents—a critical review”, J. Hazard. Mater. 142, 1–53, 2007.
[20] D. Borah, S. Satokawa, S. Kato, T. Kojima, “Surface-modified carbon black for As (V) sorption”, J. Colloid Interface Sci. 319,
53–62, 2008.
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 5, May-2011 9
ISSN 2229-5518
[21] Z. Li, R. Beachner, Z. McManama and H. Hanlie, “Sorption of arsenic by surfactantmodified zeolite and kaolinite,” Micropor. Mesopor. Mater. 105, 291–297, 2007.
[22] Y. H. Xu, T. Nakajima and A. Ohki, “Adsorption and removal
of arsenic(V) from drinking water by aluminum-loaded shirasu-zeolite,” J. Hazard. Mater. B 92, 275–287, 2002.
[23] A. Kuleyin, “Removal of phenol and 4-chlorophenol by surfactant-modi.ed natural zeolite”, J. Hazard. Mater. 144, 307–
315, 2007.
[24] E. Erdem, N. Karapinar and R. Donat, “The removal of heavy metal cations by natural zeolites”, J. Colloid Interface Sci. 280,
309–314, 2004.
[25] E. Chmielewska, “Adsorption of arsenate and chromate from waters on hydrophobized zeolite media”, Turk. J. Chem. 27,
639–648, 2003.
[26] M. Ghiaci, R. Kia, A. Abbaspur and F. Seyedeyn - Azad, “Adsorption of chromate by surfactant-modi.ed zeolites and MCM-41 molecular sieve”, Sep. Purif. Technol. 40, 285–295,
2000.
[27] G.M. Haggerty and R.S. Bowman, “Sorption of chromate and other inorganic anions by organo-zeolite”, Environ. Sci. Technol. 28, 452–458, 1994.
[28] B. Dousova, T. Grygar, A. Martaus, L. Fuitova, D. Kolousek, V. Machovic, “Sorption of As V on aluminosilicates treated with FeII nanoparticles”, J. Colloid Interface Sci. 302, 424–431,
2006.
[29] K.B. Payne and T.M. Abdel – Fattah “Adsorption of arsenate and arsenite by iron treated activated carbon and zeolites: effects of pH, temperature, and ionic strength”, J. Environ. Sci.
40, 723–749, 2005.
[30] Y. Xu, A. Ohki and S. Maeda, “Adsorption of arsenic (V) by use of aluminium-loaded shirasu-zeolites”, Chem. Lett. 27,
1015–1016, 1998.
[31] M. P. Elizalde - Gonzalez, J. Mattusch, W. Einicke and D. R.
Wennrich, “Sorption on natural solids for arsenic removal”,
Chem. Eng. J. 81, 187–195, 2001.
[32] H. Genc-Fuhrman, P.S. Mikkelsen and A. Ledin, “Simultaneous removal of As, Cd, Cr, Cu, Ni and Zn from stormwater: experimental comparison of 11 different sorbents”, Water Res. 41, 591–602, 2007.
[33] R. Menhaje - Bena, H. Kazemian, S. Shahtaheri, M. Ghazi -
Khansari and M. Hosseini, “Evaluation of iron modi.ed zeolites for removal of arsenic from drinkingwater”, Stud. Surf. Sci. Catal. 154, 1892–1899, 2004.
[34] M. Habuda-Stanic, M. Kules, B. Kalajdzic and Z. Romic, “Quality of groundwater in eastern Croatia. The problem of arsenic pollution, Desalination,” 210, 157–162, 2007
[35] V. Campos and P. M. Buchler, “Anionic sorption onto modified natural zeolites using chemical activation”, Environ. Geol. 52, 1187–1192, 2007.
[36] E.J. Sullivan, R.S. Bowman and I.A. Legiee “Sorption of arsenic from soil washing leachate by surfactant-modified zeolite”, J. Environ. Qual. 32, 2387–2391, 2003.
[37] P. Misaelides, V.A. Nikashina, A. Godelitsas, P.A. Gembitskii,
E.M. Kats, “Sorption of As (V) from aqueous solutions by organo-modified natural zeolitic materials”, J. Radioanal. Nucl. Chem. 227 183–186, 1998.
[38] Z. Li and R.S. Bowman, Regeneration of surfactant-modified zeolite after saturation with chromate and perchloroethylene,
Water Res. 35, 322–326, 2001.
[39] Z. Li, “Sorption kinetics of hexadecyltrimethylammonium on natural clinoptilolite”, Langmuir, 15, 6438–6445, 1999.
[40] A. I. P. Cordoves, M. G. Valdes, J. C. T. Fernandez, G. P. Luis, J. A., Garcia-Galzon, M. E. D. Garcia, “Characterization of the binding site affinity distribution of a surfactant-modified clinoptilolite”, Micropor. Mesopor. Mater. 109, 38–48, 2008.
[41] H. Faghihian and R.S. Bowman, “Adsorption of chromate by
clinoptilolite exchanged with various metal cations”, Water
Res. 39, 1099–1104, 2005.
[42] Md. J., Haron, S.A., Masdan, M.Z., Hussein, Z., Zainal, Kassim, “Kinetics and thermodynamic for sorption of arsenate by lanthanum-exchanged zeolite, Malays”, J. Anal. Sci. 11, 219–228, 2007.
[43] J. M. Philipot, F. Chaffange and J. Sibony, Water Sci. Technol.
17,1121–1132, 1984.
[44] S. E. Jorgensen, Ind. Wastewater Manage. 7, 81–92, 1979.
[45] J. W. Patterson, “Water Treatment Technology, 3rd ed., Ann
Arbor Science, Ann Arbor Michigan, MI”, 1978.
[46] N. Kongsricharoern and C. Polprasert, Water Sci. Technol. 31,
109–117,1995.
[47] C. P. Huang and M. M. Wu, J. Water Pollut. Control Fed. 47,
2437–2446, 1975.
[48] D. D. Das, R. Mahapatra, J. Pradhan S. N. Das and R. S.
Thakur, “Removal of Cr (VI) from aqueous solution using activated cow dung carbon”, J. Colloid Interf. Sci. 232, 235–240,
2000.
[49] D. Agrawal, M. Goyal, and R. C. Bansal, “Adsorption of chromium by activated carbon from aqueous solution, Carbon”, 37, 1989–1997, 1999.
[50] N. Daneshvar, D. Salari and S. Aber, “Chromium adsorption and Cr (VI) reduction to trivalent chromium in aqueous solutions by soya cake”, J. Hazard. Mater. 94, 49–61, 2002.
[51] N. K. Hamadi, X.D. Chen, M. M. Farid, M.G.Q. Lu,
“Adsorption kinetics for the removal of chromium(VI) from aqueous solution by adsorbents derived from used tyres and sawdust”, Chem. Eng. J. 84, 95–105, 2001.
[52] S. E. Lee, H. S. Shin and B. C. Paik, “Treatment of Cr (VI) -
containing waste water by addition of powdered activated carbon to the activated sludge process”, Water Res. 23, 67–72,
1989.
[53] T. Aoki and M. Munemori, “Recovery of chromium (VI) from wastewaters with iron(III) hydroxide-I. Adsorption mechanism of chromium (VI) on iron (III) hydroxide”, Water Res. 16,793–796, 1982.
[54] D. D., X. Meng, “Utilization of fly ash for stabilization
/solidification of heavy metal contaminated soils”, Eng. Geol.
70, 377–394, 2003.
[55] Y. Guo, J. Qi, S. Yang, K. Yu, Z. Wang and H. Xu, “Adsorption of Cr (VI) on micro- and mesoporous rice husk- based active carbon”, Mater. Chem. Phys. 78. 132–137, 2003.
IJSER © 2011 http://www.ijser.org