International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 129
ISSN 2229-5518
Y.Kiran Kumar1, Dr. Shashi.B.Mehta1, Dr. Manjunath Ramachandra1
1 Philips Electronics India Ltd
Keywords: Brain, AVM, Modeling, brain hemorrhage, Lumped Model, W indkessel, Simulation.

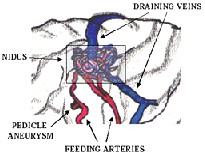
A Cerebral Arteriovenous Malformation (AVM) is an abnormal connection between the arteries and the veins in the brain. An Arteriovenous malformation is a tangled cluster of vessels, typically located in the supratentorial part of the brain, in which arteries connect directly to veins without any intervening capillary bed. The cause of cerebral arteriovenous malformation is unknown. Arteriovenous malformations vary in size and location in the brain. It is usually formed before birth. AVMs can occur anywhere in the body however Brain AVMs are of special concern because of the damage they cause when they bleed. They are very rare and occur in less than 1% of the general population. AVMs that occur in the coverings of the brain are called Dural AVMs.An AVM is not a cancer, and does not spread to other parts of the body. Dural AVM’s, in adults are an acquired disorder that can occur following an injury [1].
Figure 1: Arteriovenous Malformation (AVM)
It is accepted in the literature that Hemodynamics plays a major role in the process of AVM formation, progression and rupture. High-flow Arteriovenous malformations inducing a local increase of blood flow in the cerebral circulation can promote the disease. Furthermore, AVMs usually localize in sites of flow separation and elevated portions such as bifurcations. These conditions were found to be associated in animal models to fragmentation of the internal elastic lamina of blood vessels, Moreover, the literature survey shows that, experimental cerebral AVMs can be created in rats and primates through systemic hypertension and increased blood flow [2].
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 130
ISSN 2229-5518
Cerebral angiography is required to assess morphology and hemodynamic, which are essential for planning treatment. Important features include feeding arteries, venous drainage pattern, and arterial and venous aneurysms. Ten to fifty-eight percent of patients with AVM have aneurysms located in vessels remote from the AVM, in arteries feeding the AVM, or within the Nidus of the AVM itself. Intranidal aneurysms may have a higher risk of rupture than those outside the bounds of the AVM. Modalities used to diagnose AVM. : DSA, CTA, MRA, SPECT, PET. DSA is the gold standard dataset for the diagnosis and treatment of AVM [3].
Need for Modeling:
The physical principles governing fluid flows are similar to those governing the mechanics of solids: namely conservation of mass, momentum and energy. Fluids are distinguished from solids by the fact that they continuously deform under an applied shear stress. Flows can be divided generally into categories depending on whether or not they are compressible or incompressible; viscous or in viscid; and laminar or turbulent. Liquids are normally assumed to be incompressible, whereas gases are compressible. Models of cerebrovascular Hemodynamics have to address two main challenges: they must efficiently represent all major physiological mechanisms that take place in the cerebral vessels; and they should be patient-specific in terms of geometry and pathological conditions. [4].
Zero-dimensional (lumped parameter) and one dimensional model, based on simplified representations of the components of the cerebral vessels, can contribute strongly to our understanding of circulatory physiology. Zero-D models provide a concise way to evaluate the hemodynamic interactions among the cardiovascular organs, whilst one-D (distributed parameter) models add the facility to represent efficiently the effects of pulse wave transmission in the arterial network at greatly reduced computational expense compared to higher dimensional computational fluid dynamics studies. There is extensive literature on both types of models.
There are various models available in the literature, in this review paper, we have considered the review of Hydrodynamic, Mechanical and Electrical models for the Cerebral vessels pressure, intracranial pressure/volume relationship, AVM Modeling.
Hydrodynamic model:
This model is based on the
compartmental based modeling for the brain,
which is modeled by separating into a few
compartments that are connected by resistances
and capacitances to describe the overall hydrodynamic processes. In this work, we will use
6 compartments to describe the system: A=arterial, C=capillary, V=venous, S= sinus sagitalis, B=brain tissue, F=CSF. The first 4 compartments (A, C, V, S)
describe the blood circulation. The overall volume is constant due to the cranial bone. This assumption is also known as the doctrine of Monroe-Kellie. Putting together all the knowledge about the system we obtain our model (see Fig. 1). Since we are dealing with blood vessels, which can be treated as elastic materials, we will not only model the resistances between the compartments, but also the capacitances due to the elastic behavior of the blood vessels.
A well satisfied medical approximation is the assumption of only modeling the arteries and veins as capacitances, the capillary compartment and the sinus sagitalis can be assumed as hard walled. Since the production of CSF is working on capillary level, we have modeled the production as a resistance between C and F. The absorption process of CSF works in the same way between F and S. To put the model into equations, we can use two possible approaches: first using a hydrodynamic model, which is a “realistic” description, and second an electric circuit as an analogue to the hydrodynamic one, which is more “intuitive” for physicians [5].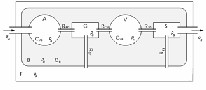
Figure 2: Hydrodynamic model for cerebral circulation
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 131
ISSN 2229-5518
Mechanical Modeling:
Hemodynamic factors such as the wall shear stress are believed to affect a number of cardiovascular diseases including atherosclerosis and aneurysm. Since resolving phenomena in a living human body is currently beyond the capabilities of in vivo measurement techniques, computer modeling is expected to play an important role in gaining a better understanding of the relationship between the cardiovascular diseases and the hemodynamic factors.
In the research study by Ryo [will add reference number at last], simulation tool is applied to a patient-specific model under Pulsatile blood flow conditions. The simulations helped to find the flow behavior with compliant arterial walls is different from what we see with rigid arterial walls. Consequently, the distribution of the wall shear stress on the compliant arterial walls is significantly different from that on the rigid arterial walls. The drawback of this study is that they have not considered the nonlinear effects into account by using a hyper-elastic model for the arterial wall.
In another study by Jonathan P. Mynard [will add reference number at last] , the researcher worked on the One-dimensional blood flow modeling is becoming a useful tool for investigating normal arterial hemodynamic and the dynamics associated with cardiovascular diseases. But this model does not account for the viscoelasticity of vessel. Thus, future studies could incorporate a more extensive geometric model as well as a more accurate representation of intramyocardial pressure distribution.
In another study by Dwyer [will add reference number at last], the researchers worked on the A three-dimensional and pulsatile blood flow in a human aortic arch and its three major branches has been studied numerically for a peak Reynolds number of 2500 and a frequency (or Womersley) parameter of 10. The simulation geometry was derived from the three-dimensional reconstruction of a series of two-dimensional slices obtained in
vivo using CAT scan imaging on a human aorta. The numerical simulations were obtained using a projection method, and a finite-volume formulation of the Navier-Stokes equations was used on a system of overset grids.The limitations of this paper is that computationally expensive parts of our work were the resolution of the time cycle, obtaining a periodic solution, and the convergence of the continuity based Poisson equation.
Mathematical Modeling:
Biomathematical models offer one way to understand theoretically AVM physiologic mechanisms under normal hemodynamic conditions and during simulated therapy, particularly in a region of an AVM (the Nidus) that is otherwise inaccessible to detailed investigations by any other current technique. The cerebral vessels (Elevated intracranial pressure (ICP)/ cerebral spinal fluid (CSF) drainage) can be regarded as a wide hydraulic network under the action of a pulsatile pump. Different behavior can be observed at various locations of the closed loop. For instance in the arterial tree, the wave propagation is of greater influence while in the capillary bed, the flow is almost steady, putting into evidence the lumped character of the system. On the other hand, local phenomena, like anastomosis for instance, create flow perturbations upwardly and downwardly. This shows the interdependency of different scales of the system, leading to a multiscale approach.
Variability in regional flow is likely to be related to multiple factors, including AVM size and flow rate, feeding artery pattern, availability of collaterals, venous pressure, metabolic demand, local anatomy, and others. AVM Hemodynamics is difficult to quantify, particularly within or in close proximity to the Nidus. Hence modeling using the electric circuit will help the radiologist for the treatment planning.
In the research study on the Hemodynamic Simulation Study of Cerebral Arteriovenous Malformations by Shiro Nagasawa.etal, 1994 [6], the limitations of their work are cerebral swelling and hemorrhage following hyper perfusion could not be simulated in their model.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 132
ISSN 2229-5518
In another study on the Cerebral AVM by Tarik F. Massoud .etal, 2000 [7], the limitation of their work is that the model is subjective, Models represent isolated systems, whereas the real systems being modeled are rarely isolated.
In an another work by Tarik F. Massoud.etal, 2000 [8], on the theoretical modeling of AVM, the limitation is that assumption is not correct for the nidal vessel occlusion by stepwise / random and limited by the lack of biological and biovariability traits. An AVM model based on electrical network analysis was used to investigate theoretically the potential role of hemodynamic perturbations for elevating the risk of Nidus vessel rupture (erupt) after simulated AVM embolotherapy, and to assess the potential benefit. In this work, the author had proposed the five separate hypothetical mechanisms for Nidus hemorrhage were studied: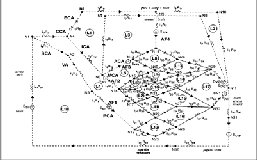
1) intranidal rerouting of blood pressure; 2) extranidal rerouting of blood pressure; 3) occlusion of draining veins with glue; 4) delayed thrombosis of draining veins; and 5) excessively high injection pressures proximal to the Nidus. Simulated occlusion of vessels or elevated injection pressures were implemented into the AVM model, and electrical circuit analysis using Nodal based analysis was studied and revealed the consequent changes in intranidal flow, pressure. The limitation of the model is that it is subjective, models represent the isolated system, and Minimal- parameter models may not be explanatory to one’s satisfaction because they may neglect properties that are emphasized in more traditional physiologic work.
Figure 3: Node based Electric Model of AVM
In the research work by Spiegel.etal, 2011 [9], the limitations is the assumption used for their modeling and which causes the variation in results with the in vivo measurements
Advantage of Electrical Model over the Mechanical
Model:
In the research work by Vuk[], analyzed and investigated the electrical modeling advantages, the main advantages are as follows:
1. lumped models is that they are easy to solve since they give rise to simple ordinary differential equations.
2. one dimensional models of the human arterial system yield partial differential equations expressing the conservation of mass and momentum for inviscid flow.
3. It has been shown numerically that the linearization of the one dimensional model around a constant state matches in a very suitable manner the nonlinear system itself, even for very realistic test cases.
4. The transmission line can be represented to any desired accuracy by a lumped section.
Windkessel Model:
The research work in the modeling using Lumped circuits in the Analysis of the lumped parameters for the blood flow in the cardiac and Brain by Vuk [], where the author provides new results of consistence and convergence of the lumped parameters (ODE models) toward one-dimensional (hyperbolic or parabolic) models for blood flow. Lumped parameter models (exploiting the electric circuit analogy for the circulatory system) are shown to discretize continuous 1D models at first order in space. We derive the complete set of equations useful for the blood flow networks, new schemes for electric circuit analogy, the stability criteria that guarantee the convergence, and the energy estimates of the limit 1D equations. The drawback is that it drawbacks: stability of solutions under a parabolic condition.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 133
ISSN 2229-5518
Mathematical modeling has become a prerequisite for hemodynamic analysis, in tandem with the experimental approach. The whole arterial network is described by a single combination resistor-inductor-capacitor (RLC). Hales in 1733 [14] was the first to put forward such model, named Windkessel, suggesting that the pressure variations are related to the large arteries elasticity. This theory was quantitatively formulated later by Frank in 1899 [15], who de-scribed the hemodynamic of arterial network in terms of compliance and resistance.
The 0D models (named lumped parameter models) consider as uniform the distribution of fundamental variables (pressure, volume and flow rate) in every single compartment (organ, blood vessel, etc.) of the model, in every moment of time. The models of higher dimensions account for the spatial variation of these parameters, as illustrated in Figure 4.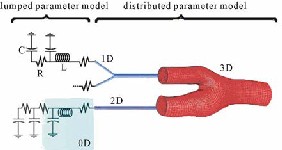
Figure 4: Lumped Parameter Model
The 0D models that represent the main components of the system, (vasculature compartments and the heart with its valves), are appropriate for the study of global distribution of the pressure, flow rate and blood volume, for specific physiological conditions. The spatial distribution of the parameters can be approximated by setting up the so called multi- compartments models, every compartment of which is supposed to be homogenous and described by a lumped parameter model.
The 1D model, where the wave transport effect is easily represented, describes the variation of the
velocity of flow through the length of the blood
with axial symmetry are requested. The use of 3D models is needed for the complex description of the blood flow in bifurcations of the vessels, through heart valves, inside ventricles, etc. The 1D,
2D and 3D (distributed parameter models) and
lumped models of the venous system are not investigated in this review.
Table 1. Comparison of modeling techniques for the study of Cerebrovascular Hemodynamics | |
Distributed parameters | 3D - Study of the local flow in 3-dimensional areas. |
Distributed parameters | 2D - Study of local flow in vessels with axial symmetry. |
Distributed parameters | 1D - Pulsed wave reflection effect in systemic circulation. |
Lumped parameters | 0D - Cerebrovascular Hemodynamics Pressure & flow changes in local areas of circulation. |
The flow of blood is described by the equation of continuity for the mass conservation, by the Poiseulle’s law for the steady flow and by the Navier-Stokes equation for the non-steady state. Ana-logically, the current in a circuit is described by Kirchhoff’s laws, Ohm’s law and the line transmission equation for the voltage-current relation for high frequencies. Hence, as shown in Table 2, the friction due to viscosity, the inertia of the flow, the capacitance of the vessel, the blood pressure and the flow rate, can be respectively described by the resistance R, inductance L, capacitance C, voltage and the current in an electric circuit. As consequence the methods used for the study of these circuits can be used to analyze the cardiovascular dynamics too. Quarteroni [12] proved that 0D models describe the vessel with a set of two ordinary differential equations (Eq.1) for each compartment, (which represent the mass and momentum conservation), added by one algebraic equilibrium equation (which relates the volume of the compartment with the pressure in it).![]()
Table 2: Relationship Table between Physiological parameter with Electrical/Mechanical.
vessel. The 2D models are appropriate to use when the radial changes of the flow velocity in a tube
Fluid
Dynamics
Physiological
Variables
Electrical
Analogue
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 134
ISSN 2229-5518
Pressure P[Pa = J/m3] | Blood press. [mmHg] | Voltage U [V = J/C] |
Flow rate Q[m3/s] | Blood flow rate [L/s] /Velocity | Current I [A = C/s] |
Volume V[m3] | Blood volume [L] | Charge q [Columbs] |
Viscosity η | Blood resistance | Electrical resistance R |
Elastic coefficient | Vessel’s wall compliance | Capacitor’s - . C |
Inertance | Blood inertia | Inductor’s inertance L |
Fank’s model (Figure 5(a)) comprises a capacitor C (which describes the depository properties of large arteries) connected in parallel with a resistor (which describes the dissipative nature of peripheral vessels). This 2-element WK, which describes the pressure decay in aorta during diastole, is still in use today to evaluate the total arterial capacity when the aortic pressure and peripheral resistance are known. This model ignores the veins, which are described as zero pressure far fields.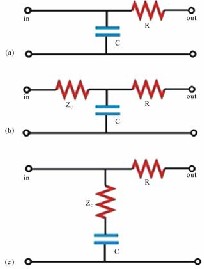
Figure 5 . (a) 2-element WK model [15]; (b) 3- element WK by Westerhof et al. [18]; (c) 3-element
WK by Buratini et al. [22].
In order to increase the model’s accuracy in describing the impedance of the blood vessels and decrease the errors in the low frequencies range, Stergiopulos [23] added an inductive element L (Figure 6(a)) which describes the inertial effect of the blood flow, introducing the 4-element WK. This fourth element represents the total arterial inertance (the sum of all inertances in the arterial segments) [23]. The use of total arterial inertance corrects the behavior of the input impedance in low frequencies where the 3-element WK is inaccurate. Another configuration (Figure 6(b)) with the inductive element is studied by Grant in [24], Burattini in [25, 26], or others [27,28]. This series inertances affects the arterial input impedance only at high frequencies and not at low frequencies.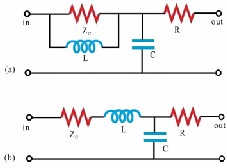
Figure 6. (a) 4-element WK by Stergiopulos et al. [23]; (b) 4-element WK by Grant et al. [24].
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 135
ISSN 2229-5518
The multi-compartments models are developed to correct the inability of the single-compartment models to calculate the flow and the pressure in different segments of the vessel since they treat the systemic tree as a single block. In the multi- compartments models, the systemic circulation is partitioned in a number of segments (compartments), each described by a combination RLC according to the local characteristics of the vessel. These compartments are combined to create the whole model of the whole arterial network (e.g. Figure 7).
Figure 7: Whole model for complete vessel
In another research work by Wayne[], where the intracranial pressure dynamics, where the author reviewed the literature regarding the development, testing, and application of physiology-based computer simulation models of intracranial pressure dynamics. In another research by Marmarou [], where the investigator had performed the simulation model of CSF dynamics (hydrocephalus focus.). Studies Pressure – Volume relationship and introduces concept of PVI 1ml in cats) as a measure of lumped cranial compliance, and also defines infusion test to measure PVI and CSF uptake resistance. In the research by E.Gao [], describes the Models vasculature more fully than other models, with a focus on AVM shunts and associated surgical procedures.
Feasibility Model for Vessel Modeling:
We have investigated as the cerebral vessel analysis as the basic pipe flow modeling as the feasibility study. We have implemented as the electrical model for the vessel model, with known cerebral parameters and converted to RLC using mechanical to electrical and cerebral parameters to electrical analogy. In our research work, we have investigated the vessel modeling using electrical
and mechanical for each branch of vessel
considering the pressure, flow rate and other clinical parameters.
Electrical Model for the single vessel flow:
The feasibility study is performed for a single arterial vessel of cerebral region with known radius, thickness and pressure values, is shown in figure 8.0. With the known input pressure, we can find the pressure value s at various nodes of the circuits, the nodes denotes the various locations of the vessels, which are based on the vessel radius. The value of R, L, C is calculated using the following equations:![]()
![]()
![]()
Figure 8.0 Electrical Model of Vessel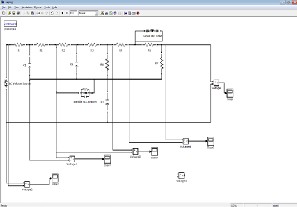
The research results that the pressure outputs are matching with voltage results .The results of pressure outputs for each segment of the vessels
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 136
ISSN 2229-5518
using electrical and mechanical simulation is shown in the below table 3:
Table 3: Quantification results
Input Node: 0.3 Volts
Node s | Vess el Radi us | Electri cal Circuit | Electrical (volts) | Mechanical (bar-g) |
Node 1 | < 1cm | R LC paralle l | Node 1 = 6 x 10-14 | 0.0008barg= 0.000613333 volts |
Node 2 | 1-1.5 cm | RL Paralle l | Node 2 = 1.7x10-4 | 0.177525 V(1.7x 10-4) |
Node 3 | 1.5-3 cm | RC paralle l. | N0de3=0. 3x10-4 | 0.0003 volts |
Conclusion:
This paper works on the review of the clinical part of AVM, Modeling of the AVM, Mechanical and Electrical Modeling of the AVM and also the segmentation of the brain vessels. In this paper we have provided a rigorous approach for electric circuits whose physical constants (R, L and C), are parameterized on the standard length of the vessel using normals in order to recover correct values for pressure or flow-rate. For large and medium sized vessels we have shown the results mapping of the lumped parameter model toward the solution of a
1D linear system that is shown itself to be a
linearization of the widely used 1D nonlinear
system. Our result can therefore be regarded as a unifying approach for the three hierarchical models based on electric circuits, linear 1D hyperbolic systems and the (more complete) nonlinear 1D hyperbolic system.
Many researchers model the systemic vasculature in segments which represent the various blood vessels (capillaries and veins, arterioles, arteries and aorta), (e.g. [33-36]). Since the flow in aorta and in the arteries (which are elastic) is pulsatile, it should be considered the combination RLC of the three elements. As in arterioles and capillaries (with relatively rigid walls) the flow is steady and the friction is dominant, a resistive element is enough. The inertial effect is ignored in veins (because they have great capacity and the flow is relatively steady), by making so the RC combination good enough to represent the flow characteristics. Some multi-compartments models, such as [6, 9, 37-43], describe the distribution of the pressure and the flow characteristics in each bifurcation of the vessels. Models of this complexity, like [32] and [39, 40], even though describe accurately the system; introduce practical difficulties in defining the exact values for most of the parameters.
In this review the main aspects and use of the WK are discussed. This lumped parameter model can be used for the systemic arterial system and the pulmonary arterial bed of all mammals [44]. As limitations of WK model of the arterial system we mention its incapability to study the wave transmission phenomena, the changes in the blood flow distributions, the effects of local vascular changes, etc. [44].
Future work:
The extension of this work is for the live scenarios and live cases. The pressure value and voltage value are simulated using the measured pressured values from the live cases. The feasibility results are validated with mechanical results as well as with the invasive cerebral pressure measurements.
References:
1. Guyton, A.C. and Hall, J.E. (2006) Textbook of medical physiology. 11th Edition. Elsevier Saunders, Philadelphia.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 137
ISSN 2229-5518
2. Guyton, A.C., Coleman, T.G. and Granger, H.J. (1972) Circulation: Overall regulation. Annual Review of Physiology, 34, 13-44. doi:10.1146/annurev.ph.34.030172.000305
3. Modeling cerebral Hemodynamics: a
move towards predictive surgery, Edlong
,March 9, 2007,Thesis Report.
4. Coleman, T.G. and Randall, J.E. (1983)
HUMAN: A comprehensive physiological model. Physiologist, 26, 15- 21.
5. Theoretical modeling of Arteriovenous malformation rupture risk: a feasibility and validation study,.Erzhen Gao a, William L. Young ,a Department of
Electrical Engineering, Columbia University, New York, NY 10027, USA ,b Department of Anesthesiology, College of Physicians and Surgeons of Columbia University, New York, NY 10032, USA, IPEM, 1998.
6. Can Induction of Systemic Hypotension
Help Prevent Nidus Rupture
Complicating Arteriovenous
Malformation Embolization?: Analysis of
Underlying Mechanisms Achieved Using a Theoretical Model, Tarik F. Massoud, George J. Hademenos, AJNR Am J Neuroradiol 21:1255–1267, August 2000.
7. Experimental model of intracranial avm in the acute stage , Shinichi, Department of
Neurology, Medical university, Fukushima, Neurological Medical Chir (Tokyo), 288-293, 2005.
8. Wu, X.M. (1994) Modeling and simulation of cardiovascular circulation system:
Status and prospect. Science- paper
Online, 2, 112-116.
9. Cellier, F.E. and Kofman, E. (2006)
Continuous system simulation. Springer-
Science, New York.
10. Werner, J., Böhringer, D. and Hexamer, M. (2002) Simulation and Prediction of cardiotherapeutical phenomena from a pulsatile model coupled to the guyton circulation model. IEEE Transaction on Biomedical Engineering, 49, 430-439. doi:10.1109/10.995681
11. Simanski, O., Kähler, R., Schubert, A.,
Janda, M., Bajorat, J., Hofmockel, R. and
Lampe, B.P. (2008) Proceedings of the 17th
IFAC World Congress. In: Chung, M.J.,
Misra, P. and Shim, H., Eds, The International Federation of Automatic Control, Seoul, 9601-9606.
12. Lu, K., Clark, J.W., Ghorbel, F.H., Ware, D.L. and Bidani, A. (2001) A human cardiopulmonary system model applied to the analysis of the Valsalva maneuver. American Journal of Physiology—Heart and Circulatory Physiology, 281, 2661-
2679.
13. Magosso, E., Biavati, V. and Ursino, M.
(2001) Role of the baroreflex in cardiovascular instability: A modeling study. Cardiovascular Engineering, 1, 101-
115. doi:10.1023/A:1012574513589
14. Abram, S.R., Hodnett, B.L., Summers, R.L.,
Coleman, T.G. and Hester, R.L. (2007)
Quantitative circulatory physiology: An integrative mathematical model of human physiology for medical education. Advances in Physiol-ogy Education, 31,
202-210. doi:10.1152/advan.00114.2006
15. Quarteroni, A. (2004) Analysis of lumped parameter models for blood flow simulations and their relation with 1D models. Mathematical Modeling and Numerical Analysis, 38, 613-632. doi:10.1051/m2an:2004036
16. Grodins, F.S. (1959) Integrative
cardiovascular physiology: A
mathematical synthesis of cardiac and
blood vessel hemodynamic. Quarterly
Review of Biology, 34, 93- 116.
doi:10.1086/402631
17. Hales, S. (1733) Statistical essays:
Containing haemostaticks, or an account
of some hydraulick and hydrostatical
experiments on the blood and blood-
vessels of animals, etc. 2, Innys & Manby, London.
18. Frank, O. (1899) Die grundform des arterielen pulses erste abhandlung: Mathematische analyse. Zeitschrift für
Biologie, 37, 483-526.
19. Wetterer, E. (1954) Flow and pressure in
the arterial sys-tem, their hemodynamic
relationship, and the principles of their
measurement. Minnesota Medicine, 37, 77-
86.
20. Westerhof, N., Stergiopulos, N. and
Noble, I.M. (2010) Snapshots of
hemodynamics an aid for clinical research
and graduate education. 2nd Edition,
Springer, New York. doi:10.1007/978-1-
4419-6363-5.
21. Westerhof, N., Bosman, F., DeVries, C.J.
and Noorder-graaf, A. (1969) Analogue
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 138
ISSN 2229-5518
studies of the human systemic arterial tree. Journal of Biomechanics, 2, 121-208. doi:10.1016/0021-9290(69)90024-4 .
22. Westerhof, N., Elzinga, G. and Sipkema, P. (1971) An artificial arterial system for pumping hearts. Journal of Applied Physiology, 31, 776-778.
23. Wang, J.J., O’Brien, A.B., Shrive, N.G., Parker, K.H. and Tyberg, J.V. (2003) Time- domain representation of ventricular- arterial coupling as a Windkessel and wave sys-tem. American Journal of Physiology—Heart and Circulatory Physiology, 284, 1358-1368.
24. Burkhoff, D., Alexander, J. and Schipke, J. (1988) Assessment of windkessel as a model of aortic input impedance. American Journal of Physiology—Heart and Circulatory Physiology, 255, 742-753.
25. Burattini, R. and Natalucci, S. (1998) Complex and frequency-dependent compliance of viscoelastic Windkessel resolves contradictions in elastic Windkessels. Medical Engineering & Physics, 20, 502-514. doi:10.1016/S1350-
4533(98)00055-1
26. Stergiopulos, N., Westerhof, B.E. and
Westerhof, N. (1992) Total arterial
inertance as the fourth element of the
Windkessel model. American Journal of
Physiology— Heart and Circulatory
Physiology, 276, 81-88.
27. Grant, B.J.B. and Paradowski, L.J. (1987)
Characterization of pulmonary arterial
input impedance with lumped parameter
models. American Journal of Physiology— Heart and Circulatory Physiology, 252,
585-593.
28. Burattini, R. and Gnudi, R. (1982)
Computer identification of models for the
arterial tree input impedance: Comparison between two new simple models and first experimental results. Medical and Biological Engineering and Computing,
20, 134-144. doi:10.1007/BF02441348
29. Burattini, R. and Di Salvia, P.O. (2007) Development of systemic arterial mechanical properties from infancy to adulthood interpreted by four-element Windkessel models. Journal of Applied Physiology, 103, 66-79. doi:10.1152/japplphysiol.00664.2006
30. Kolh, P., D’Orio, V., Bernard, L., Gerard,
P., Gommes, C. and Limet, R. (2000)
Increased aortic compliance maintains left ventricular performance at lower energetic cost. European Journal Cardio-Thoracic Surgery, 17, 272-278. doi:10.1016/S1010-
7940(00)00341-9
31. Sharp, K.M., Pantalos, G.M., Minich, L.,
Tani, L.Y., McGough, E.C. and Hawkins,
J.A. (2000) Aortic input impedance in
infants and children. Journal of Applied
Physiology, 88, 2227-2239.
32. Rager, G.N., Westerhof, N. and
Noordergraaf, A. (1965) Oscillatory flow
impedance in electrical analog of arterial
system: Representation of sleeve effect
and non-newto- nian properties of blood. Circulation Research, 16, 121- 133. doi:10.1161/01.RES.16.2.121
33. Frasch, H.F., Kresh, Y.J. and Noordergraaf, A. (1996) Two-port analysis of
microcirculation: An extension of windkessel. American Journal of Physiology—Heart and Circulatory Physiology, 270, 376-385.
34. Formaggia, L. and Veneziani, A. (2003)
Reduced and multiscale models for the human CVS. Technical Report, PoliMI, Milan.
35. Avolio, A.P. (1980) Multi-branched model of the human arterial system. Medical and Biological Engineering and Computing,
18, 709-718. doi:10.1007/BF02441895
36. Beyar, R., Hausknecht, M.J., Halperin,
H.R., Yin, F.C. and Weisfeldt, M.L. (1987)
Interaction between cardiac chambers and
thoracic pressure in intact circulation.
American Journal of Physiology—Heart and Circulatory Physiology, 253, 1240-
1252.
37. Santamore, W.P. and Burkhoff, D. (1991)
Hemodynamic consequences of
ventricular interaction as assessed by model analysis. American Journal of Physiology—Heart and Circulatory Physiology, 260, 146-157.
38. Ursino, M. (1998) Interaction between
carotid baroregulation and the pulsating heart: A mathematical model. American Journal of Physiology—Heart and Circulatory Physiology, 275, 1733-1747.
39. Žáček, M. and Krause, E. (1996) Numerical simulation of the blood flow in the human
CVS. Journal of Biomechanics, 29, 13-20. doi:10.1016/0021-9290(95)00027-5
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 139
ISSN 2229-5518
40. Chen, S., Zhang, S., Gong, Y., Dai, K., Sui, M., Yu, Y. and Ning, G. (2008) The role of the autonomic nervous system in hypertension: A bond graph model study. Physiological Measurement, 29, 473-495. doi:10.1088/0967-3334/29/4/005
41. Heldt, T., Shim, E.B., Kamm, R.D. and
Mark, R.G. (2002) Computational
modeling of cardiovascular response to orthostatic stress. Journal of Applied Physiology, 92, 1239- 1254.
42. Noordergraaf, A., Verdouw, P.D. and Boom, H.B.K. (1963) The use of an analog computer in a circulation model. Progress
in Cardiovascular Diseases, 5, 419-439. doi:10.1016/S0033-0620(63)80009-2
43. O’Rourke, M.F. and Avolio, A.P. (1980) Pulsatile flow and pressure in human systemic arteries. Studies in man and in a
multibranched model of the human systemic ar- terial tree. Circulation Research, 46, 363-372. doi:10.1161/01.RES.46.3.363
44. Olansen, J.B., Clark, J.W., Khoury, D.,
Ghorbel, F.H. and Bidani, A. (2000) A closed-loop model of the canina cvs that includes ventricular interaction. Computer and Bio-medical Research, 33, 260-295. doi:10.1006/cbmr.2000.1543
45. Ursino, M. and Magosso, E. (2003) Role of
short-term cardiovascular regulation in heart period variability: A model study. American Journal of Physiology—Heart and Circulatory Physiology, 284, 1479-
1493.
46. Ursino, M. (1999) A mathematical model of the carotid baroregulation in pulsating conditions. IEEE Transactions on Biomedical Engineering, 46, 382-392. doi:10.1109/10.752935
47. Westerhof, N., Lankhaar, J.W. and Westerhof, B.E. (2009) The arterial Windkessel. Medical and Biological Engineering and Computing, 47, 131-141. doi:10.1007/s11517-008-0359-2
IJSER © 2013 http://www.ijser.org