International Journal of Scientific & Engineering Research, Volume 2, Issue 12, December-2011 1
ISSN 2229-5518
Restore islet β-cells dysfunction and study the expression of PPARα and PPARγ in Stevia rebaudiana treated mice
Kosta Susmit, Tiwari Archana
Abstract - Background and aims: The down regulation of PPARα and PPARγ at m-RNAs expression level is responsible f or β-cell dysfunction and insulin secretion seen in diabetic mice. PPAR α is found primarily in the liver and regulates genes involved with fatty acid utilization . PPARγ is an important regulator of adipocyte differentiation and function. Expression of the insulin sensitive glucose transporter, GLUT4, and the UCPs are als o influenced by PPARγ. The current investigation focuses on expression of m-RNA at PPARα and PPARγ receptors and the islet β-cells dysfunction in Stevia rebaudiana (SR) treated diabetic mice.
Methods and results: SR has been used for the treatment of diabetes mellitus in the traditional medical system worldwide. Extract were orally administrated regularly for 30 days in STZ diabetic induced mice. There was a significant (P<0.05) decrease in blood glucose (110.73±58.32) and a comparative increase (P<0.01) in body weight in intermediated time respectively in experimental period. There was also an increase the m-RNAs for PPARα and PPARγ respectively. β-cell dysfunction were significantly decreased in STZ induced diabetic mice. This decrease was restored in SR treated mice partially but not completely.
Conclusion: The main conclusion drawn from the study was that the SR oral administration of aqueous extract regulated the blood glucose level and weight (which control the report of hypoglycemic actions). The expression of m-RNA of PPARα and PPARγ were also significantly increased and restore the islet β-cells structure in SR treated diabetic mice respectively.
Key word: Stevia rebaudiana (SR), Stevioside (SVS), Peroxisome proliferator-activated receptor alpha and Gamma (PPARα and PPARγ),
Streptozotocin (STZ).
—————————— ——————————
ype-2 diabetes is a polygenic disease exacerbated by environmental factors such as obesity and a sedentary life style. The earliest detected metabolic abnormality is insulin resistance. However, insulin resistance alone does not cause diabetes if it is compensated by increased insulin production. The pancreatic islet β-cells initially respond to insulin resistance by increasing their capacity for insulin biosynthesis and secretion (1). Type-2 diabetes may have as its underlying metabolic causes, the combined effects of impairment in the insulin mediated glucose disposal and defective secretion of insulin by the β-cells of the pancreas (2). Peroxisome proliferator-activated receptor a (PPARα and PPARγ), which is activated by specific agonists such as fibrates and fatty acid, forms heterodimers with the retinoid X receptor (3) and
associates with PPAR response elements
————————————————
Susmit Kosta: School of Biotechnology, Rajiv Gandhi Proudyogiki Vishwavidyalaya (State Technological University of Madhya Pradesh), Air Port By-Pass Road Bhopal, M.P., Pin-462033, India. susbiotech@gmail.com
Archana Tiwari: School of Biotechnology, Rajiv Gandhi Proudyogiki Vishwavidyalaya (State Technological University of Madhya Pradesh), Air Port By-Pass Road Bhopal, M.P., Pin-462033, India.
in the promoter region of target genes. PPARα and PPARγ play an important role in the liver by regulating the metabolism of lipoproteins and fatty acids (4). In addition, PPARα is widely expressed throughout the cardiovascular system in the heart, blood vessels (endothelial cells and smooth muscle cells) and monocyte/macrophage cells. The high-dose STZ-induced diabetes mice have been comprehensively used to demonstrate the function of transplanted islets (5,6). Those studies were based on the assumption that mice treated with high-dose STZ are unable to recover endogenous β-cells function and PPARα and PPARγ activity.
Indeed, there have been a few reports of functional β-cells regeneration in this high-dose model, despite of limited increases in the numbers of insulin-positive cells shortly after high-dose STZ treatment (7,8) .Several traditional plants have been used for hypoglycemic effects and the evidence from the literature suggests that some plants act like biguanides and do not affect the blood glucose basal state (9). The herbal hypoglycemic agents may also act by directly scavenging the reactive oxygen metabolites due to increasing the synthesis of anti-oxidant molecules. SR is a sweet herb its major component, stevioside (SVS), have been used as sugar substitute for medical treatment from centuries (10, 11). SVS was found to be a non-caloric sweetener and is used in several countries as a new non-caloric sweetener (12, 13). SR
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 2, Issue 12, December-2011 2
ISSN 2229-5518
has been used for the treatment of diabetes mellitus and
various other ailments in the traditional medical system world wide. It is probably the presence of the SVS themselves that has produced dozens of empirical and semi-controlled reports of hypoglycemic action. We have identified that the oral administration of aqueous extract of SR expresses the hypoglycemic as well weight reduction properties. It is reported that SR extract increases the m-RNA expression of both PPAR α and PPAR γ, it also restores the dysfunction of islet β-cells in STZ induce diabetic treated mice. The hypoglycemic activity may be because SR nourishes the pancreas and thereby helps to restore normal pancreatic function and the deterrence glycoside SVS stimulates lowers blood glucose (14).
2. MATERIALS AND METHODS
2.1 Plants and Preparation of plant extract
The SR leaves were collected from Agriculture University, Jabalpur. The leaves were identified taxonomically by department of Botany, Agriculture University, Jabalpur. The leaves were washed 3 times with fresh water, last wash was given by distilled water and air dried in shade at room temperature. The Air dried leaves were grounded into fine powder by an electrical mixture. The powdered leaves were kept in air tight container in deep freezer maintained at 4°C till further use. The drug solutions were prepared fresh each time and a known volume of the residual extract was suspended in distilled water and was orally administered to the animals during the experimental period.
Male Balb/Can.n (I.B.) mice, 7 weeks old and 25-30 gms in weight, received a single injection of STZ (CALBIOCHEM- Cat #572201, Lot#B69776), STZ dissolved in 0.1M sodium citrate buffer pH 6.5 at a dose of 65mg/kg body weight. Mice were allowed free access to drinking water and diet (Hindustan Lever food pellets) during the experiment. 18 mice, selected for study were divided into 03 groups, 1st group was control, 2nd group was STZ induced diabetic mice and 3rd group was diabetic mice treated with SR each consisting of six animals. 12 mice were made diabetic with a single dose of STZ by intra-peritoneal route. Diabetes was confirmed by the determination of fasting blood glucose concentration on the third day post administration of STZ (15). Body weight and fasting blood glucose level of all the mice were determined before the start of the experiment. The mice were sacrificed (see below) 30 days after the above treatment with SR in diabetic mice. All animal experiments were performed in accordance with the NIH guidelines. The experiments were designed and conducted in accordance with the ethical norms approved by Ministry of Social Justices
and Empowerment, Government of India and Institutional
Animal Ethics Committee guidelines. The approval for the work has been received from the Institution Animal Ethics Committee vides their letter No.379/01/ab/CPCSEA dated 22nd February, 2001.
Starting 7 weeks after the STZ injection, group-2 STZ-induced diabetic mice were given SR (8.6mg/kg, orally, regularly) for
30 days. After 30 days one mice was taken showing
prominent result, one normal and one untreated mice after
sacrificing by decapitation under diethyl ether anesthesia.
Collagenase Type-XI was purchased from Roche-applied- science, India. All other chemicals were of analytical grade and purchased from BD Bioscience. Fresh double distilled water was used to prepare all the solutions. All immunochemicals were purchased from Roche-applied- science Company.
Whole pancreas from mice were removed under one hold anesthesia and fixed in 10% buffered formalin for 24 h. After dissection excised pancreas was rapidly immersed in ice cold Hank’s balanced salt solution (HBSS) (PH7.4) containing the enzyme collagenase was injected slowly into the pancreas. The pancreas were harvested and transferred to a 15 ml conical tube containing 5 ml cold Liberase Enzyme solution. This was Incubated for 20–35 minutes in a water bath at 37ºC (required time will vary inversely with enzyme concentration). The tube was shaken vigorously for 10 seconds. The tissue suspension was transferred to a 50 ml conical tube and 40 ml of cold quenching buffer was added. The tissue suspension was gently filtered through a 400- micron screen into a 50-ml conical tube and the screen rinsed with 10–50 ml quenching buffer. The tissue was washed twice in quenching buffer (spin, resuspend).For separation of islets, the filtrate was centrifuged at 700g for 15 sec. At the end of centrifugation the pancreatic digest was mixed in 5 ml of 31 per cent dextran solution. Four ml of 25, 23, 20 and 11 per cent dextran solutions were layered on top of each other. The tube was then centrifuged at 800Xg for 10 min. The islets were present at the 23-20 percent interface. Islets were easily identified by their higher opacity and pearly white appearance when viewed against a black background with side illumination under dissection microscope (16, 17).
Specific tests were used to identify mice islets after isolation. Dithizone stain was used to check the purity of the islets. Only the islets took up the stain and became crimson red colour. Group of islets were also subjected to histological
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 2, Issue 12, December-2011 3
ISSN 2229-5518
examination under light microscope and it was found that no acinar tissue was adherent to the islets.
RNA was isolated using the guanidium method (18). Aortae were carefully isolated and cleaned of adhering parenchyma and connective tissue and then homogenized in RNA buffer. The RNA was quantified by ultraviolet-absorbance spectrophotometry and an RNA level was performed by comparing the various products after Agrose gel electrophoresis. For the RT–PCR analysis, first-strand cDNA was synthesized from total RNA using Oligo (dT) and a cDNA Synthesis Kit (Life Sciences, Inc.). The oligonucleotides and PCR protocols are shown in table 1. The PCR products so obtained were analysed on ethidium-bromide-stained agarose (2.0%) gel.
DNA name | PCR primer sequences | PCR protocol |
PPARα | UP; 5’-GTTGCAAAGCCTGGGATAG-3’ DP; 5’-GGTAGGCTTCGTGGATTCTC-3’ | 95 °C min -1 59 °Cmin -1 72 °Cmin -1 (30 cycles) |
PPARγ | UP; 5’-AGCAGGTTGTCTTGGATGT-3’ DP; 5’-GACACCATACTTGAGCAGA-3’ | 95 °C min -1 57 °Cmin -1 72 °Cmin -1 (28 cycles) |
UP, upstream primer; DP, downstream primer |
The amount of each mRNA under study was measured using competitive PCR techniques, with a heterogeneous DNA fragment as internal standard (3). A part of the heterogeneous DNA fragment (300 or 500 bp) was amplified with a protruding 20 bp in the ends specific for PPARα and PPARγ primers, respectively. Composite primers were engineered to contain sequences that amplify the DNA fragment with gene-specific primer sequences flanking their
5’ ends. The DNA competitors were designed such that the PCR product from the cDNA could be separated from that of its competitor, and they were generated using reagents supplied in a commercial kit (Competitive DNA Construction Kit, Life Sciences, Inc.). Briefly, a 30-cycle PCR was carried out on the DNA using the relevant composite primers. A second PCR (PPARα and PPARγ: 30 and 28 cycles, respectively) was then carried out on 0.5 μl of the first amplicon using the corresponding primers for the target sequence. The concentrations of the DNA competitors were then measured by spectrophotometry (A260). A semiquantitative evaluation of mRNA levels was performed by comparing the various products after electrophoresis (19).
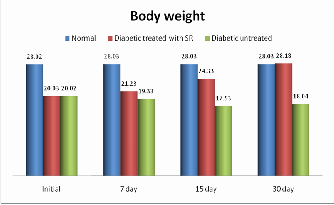
The body weight was changed in the experimental groups of mice after the induction of STZ. The mice showed loss in body weight (28.02±1.0 to 20.03±11.00) which was reversed by oral administration of crude aqueous extracts of SR. The body weight of the normal mice did not show any significant change in 30-days and the diabetic untreated showed a randomly decrease in their body weight (19.33±1.29) after 7- days. A dose-dependent body weight improvement was observed starting from 7-days (21.23±2.4) in diabetic mice treated with crude aqueous of SR at 8.6 mg/kg doses respectively. The body weight was increased (28.18±0.31) during the experimental period in 30-days treatment with the aqueous extract of SR respectively as shown in table 2. Literature survey has shown that SR leaves powder shows hypoglycemic activity and control the body weight in STZ induced rat (20). By correlating it our finding suggests that the mode of action of the active constituent (s) of SR aqueous extract was significantly (P<0.01) increased in the body weight of the mice after the oral administration in 30 day shown in figure no.1
Mice | Body weight (in grm. ± S.D.) | |
Mice | Initial 7 day 15 day | 30 day 28.03±1.0 28.18±0.31 18.04±1.20 |
Normal 28.02±1.00 28.03±1.5 28.03±1.0 Diabetic treated 20.03±11.00 21.23±2.4 24.33±12.82 with SR Diabetic 20.02±11.04 19.33±1.29 17.53±2.07 untreated | 30 day 28.03±1.0 28.18±0.31 18.04±1.20 |

The table 2 represents body weight in normal, treated diabetic and untreated diabetic STZ induced mice the study period.
Fig. 1. Body weight screening of normal, STZ-induced and SR STZ- diabetic mice during the study period. The graph values are expressed in gram and represent significantly increase in (p < 0.01) body weight.
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 2, Issue 12, December-2011 4
ISSN 2229-5518
Table 3 depicts blood glucose levels in untreated and treated
diabetic mice with SR respectively. After intra peritoneal injection of STZ induced diabetic the blood glucose levels were increased after 24 to 48h (389.52±33.6). A significant reduction (p < 0.05) in blood glucose (223.40±45.6) was observed on the 7th day with the aqueous extract form of SR at 8.6 mg/kg respectively, 172.20±64.23 was observed on the
15th day and 110.73±58.32 was observed on the 30th day with
the aqueous extract of SR respectively, while untreated animals exhibited sustained hyperglycemia. A gradual decrease in random blood glucose was observed in the SR treated group that approached normal levels at the end of the study period. There was no reduction in the blood glucose levels of normal mice. The present study is direct evidence of the aqueous extract of SR a significant decrease in random blood glucose level (figure no.2) respectively.
Mice | Blood glucose (in mg/dl ± S.D.) | |
Mice | Initial 7 day 15 day | 30 day 88.78±0.30 110.73±58.32 398.18±51.19 |
Normal 90.42±11.3 99.43±0.4 100.23±0.47 Diabetic 389.52±33.6 223.40±45.6 172.20±64.23 treated with SR Diabetic 393.40±43.5 395.43±.50 395.30±67.3 untreated | 30 day 88.78±0.30 110.73±58.32 398.18±51.19 |
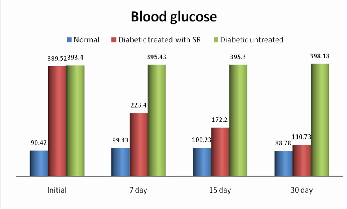
The table 3 represents non-fasting blood glucose levels in normal, treated diabetic and untreated diabetic STZ induced mice during the study period.
Fig.2. Blood glucose screening of normal, STZ-induced and SR STZ- diabetic mice during the study period. The graph values are expressed in mg/dl and represent significant reduction (p < 0.05) in blood glucose.
Using RT–PCR and competitive PCR method on the total RNA were isolated from the aortae of age-matched controls, untreated diabetic and SR treated diabetic mice. Each total RNA preparation (2.0 mg) was reverse-transcribed and half of the cDNA product was PCR-amplified using the appropriate
primers, 30 cycles (PPARα), 28 cycles (PPAR γ) being
employed. A portion of the PCR reaction product was electrophoresed on a 2.0% agarose gel containing ethidium bromide.We found that the expressions of PPARα and PPARγ mRNAs were each significantly lower in STZ induced diabetic mice than in normal mice (figure 3). The lowered PPARγ level was modestly but significantly raised in SR treated diabetic mice. The expression of the mRNA for PPAR α was markedly and significantly higher following orally administration of SR than in untreated diabetic mice.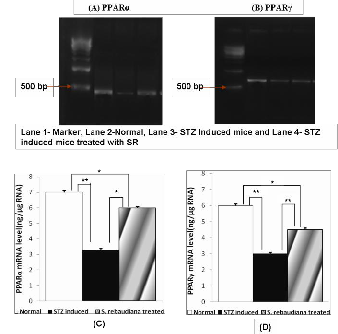
Fig. 3. RT–PCR assay of the expressions of the mRNAs for PPARα and PPARγ in aortae from Normal, STZ-induced and S. rebaudiana STZ- diabetic mice. (A,B) Expression of PPAR α and PPAR γ mRNAs assayed by RT–PCR. (C, D) Quantitative analysis of the expressions of PPAR mRNAs (by scanning densitometry) (PPARα and PPARγ). Normal mice (white column); STZ-induced diabetic mice (black column); STZ induced mice treated with SR (shaded column). Each column represents the mean±s.e.m. of five determinations (PPAR *P <
0.05, **P < 0.01).
Examination of the cellular content of islets 30 days after STZ treatment using standard immunohistochemical approaches indicated a reduction in the proportion of insulin-positive ß- cells structure and an increase in glucagon-positive function β-cells structure in SR treated mice as compared with normal controls. Under light microscopy the haematoxylin and eosin stained sections of isolated mice islets appeared intact and completely free of acinar cells. The islets were round or elogated in shape (figure 4). Morphometric analysis of the mice islets revealed that the mean diameters of the native and isolated islets were 198.5±6.63 μm and 190.23±7.11 μm respectively, our observations are consistent reports indicated
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 2, Issue 12, December-2011 5
ISSN 2229-5518
the ß-cell destruction in STZ induced mice and restored ß-cell
function and structure statistically significant in SR treated mice in 30 days, the proportion of insulin-positive cells in the islets was increased.
Fig. 4. Light micrograph of an islet isolated β-cell stained with haematoxylin eosin at 400 xs:
(A) Normal, (B) STZ Induced and (C) SR treated
The conclusion to be drawn from the present study was that the oral administration of aqueous extract of SR express the hypoglycemic as well weight reduction activities. It was reported that down-regulations of PPARα and PPARγ may lead to an increased expression of mRNA and this increment may trigger endothelial islet β-cells structure dysfunction in STZ induce diabetic mice.
In the present study, we found that the SR oral administrations of aqueous extract regulating the blood glucose levels and weight, which control the report of hypoglycemic action in STZ diabetic mice and that the expression level was modestly but significantly restored by the oral administration of SR. Current pharmacologic approaches are unsatisfactory in improving such consequences of insulin resistance as hyperglycemia, diabetic dyslipidemia, abnormal coagulation and fibrinolysis, and hypertension each of which may require the use of at least one medication. SR extract is helpful for hypoglycemia and diabetes because it nourishes the pancreas and thereby helps to restore normal pancreatic function in semi-controlled clinical reports (21).
Oviedo et.al, (22) reported a 35.2% fall in normal blood sugar levels 6-8 hours following the ingestion of a SR leaf extract. Other workers have reported similar trends in humans and experimental animals. A good deal of experimental work has been done on the effects of SR and SVS on cardiovascular functioning in man and animals. Turan Karaca et.al,(23) demonstrate very good protective effects of ginseng and green tea+ginseng against STZ-induced pancreatic β-cell damage, which is probably due at least partly to antioxidative properties in scavenging STZ-associated free radicals. Some of this work was simply looking for possible toxicity, while some was investigating possible therapeutic action. In neither case have significant properties been found. The most curious finding is a dose dependent action on heartbeat, with a slight increase appearing at lower doses, changing to a mild
decrease at higher doses. In both instance is the result
remarkable, and it is extremely doubtful that humans would experience any effect at normal doses. The long-term use of SR would probably have a cardiotonic action, that is, would produce a mild strengthening of the heart and vascular system. The study reports the antidiabetic activity of crude extracts of SR grown in different species of induced diabetic mice. A preliminary toxicity study of SR crude extracts was done using Swiss albino mice in oral doses of 1000, 2000 and
5000 mg/kg body weight. The result showed that the medium
lethal dose (LD50) of the extracts is higher than 5000 mg/kg
body weight and hence, in a single dose administration, the plant extracts had no adverse effect (24). The results obtained for preliminary phytochemical screening indicated the presence of stevioside and alkaloids in the leaves of SR and the absence of anthraquinones. These results are in close proximity with past work (25, 26).
In the present study the oral administration of SR normalized both the expressions of the mRNAs for PPARα and PPARγ in aortic segments showed down regulation expression of the mRNA and the blood glucose level. Furthermore, we also significantly increase and restore the islet β-cells structure dysfunction in STZ-induced diabetic mice (9). Thus, the development of drugs targeted to reverse insulin resistance is important. The insulin-sensitizing SVS which are selective ligands of the nuclear transcription factor peroxisome- proliferator-activated receptor (PPAR) (27). Lehmann et al., (28) reported that thiazolidinediones unlike metformin or sulfonylureas were the first drugs to address the basic problem of insulin resistance in patients with type 2 diabetes which decreases hepatic fat content and increase insulin sensitivity in muscle. These drugs particularly useful in patients with insulin-resistant type-2 diabetes, but no data is currently available to help identify the patients who would respond best to these drugs. Although thiazolidinediones lower glucose concentrations and increase insulin sensitivity, their nonglycemic effects on body weight, lipids, and blood pressure have been a disappointment, implying that this class of medications will not reduce the need to treat dyslipidemia and hypertension with separate therapies. Before the advent of insulin injection and other pharmaceutical preparations, healers relied heavily upon herbs to treat diabetes (29).
Recent studies have underscored the importance of the expressions of the mRNAs for PPARα and PPARγ, those were decreased in STZ diabetic mice and that the expression level was modestly but significantly increased by the oral administration of SR and how ß-cell regeneration is normally regulated could lead to the identification of new approaches for enhancing ß-cell regeneration. To this end we tested the effect of the spleen in the restoration of ß-cell function in this model. A role for the spleen in ß-cell regeneration was
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 2, Issue 12, December-2011 6
ISSN 2229-5518
suggested by clinical data showing that the incidence of diabetes was significantly higher in patients undergoing partial pancreatectomy and splenectomy than in those undergoing pancreatectomy alone (6). Further evidence for a role of the spleen in ß-cell regeneration came from the NOD mouse model of autoimmune diabetes. Kodama et al. (30) reported that stem cells residing in the spleen of NOD mice were capable of differentiating into ß-cells, thereby restoring ß-cell mass and curing diabetes. We here demonstrated that the removal of the spleen resulted in significantly reduced restoration of ß-cell function in balb mice, consistent with the clinical data. We also confirmed that the oral administration of SR doses partially restored the ability to recover ß-cell function in the STZ induced mice.
In conclusion, we found that oral administration of SR, which normalize the blood glucose levels and decreased the weight in STZ induce diabetic mice, which control the report of hypoglycemic actions respectively. These effect of SR may, exerts an improvement effect on the endothelial dysfunction seen in the aorta in mice with established STZ-induced diabetes. The expressions of the mRNAs for PPARα and PPARγ were significantly decreased in STZ induced diabetic mice (compared with the controls) and this decrease was restored partially, but not completely, by the oral administration of SR. We had also observed significant restoration in the islet β-cells dysfunction structure in SR treated diabetic mice respectively.
The authors would like to thank the Indian Council of Medical Research, New Delhi for financial support. I am grateful to School of Biotechnology Rajiv Gandhi Proudyogiki Vishwavidyalaya, Bhopal for providing the lab facilities.
1. Unwin, N. and Marlin 2004 A Diabetes Action Now: WHO and IDF
working together to raise awareness worldwide. 49(2): 27-31.
2. Unger, RH, Grundy S 1985 Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes. Diabetologia 28:119-121.
3. Noriyasu Kanie, Takayuki Matsumoto, Tsuneo Kobayashi & Katsuo Kamata 2003 Relationship between peroxisome proliferator- activated receptors (PPARα and PPARγ) and endothelium- dependent relaxation in streptozotocin-induced diabetic rats British Journal of Pharmacology 140: 23–32.
4. Inoue, i., noji, s., awata, t., takahashi, k., nakajima, t., sonoda, m., komada, t. & katayama 1998 S .Bezafibrate has an antioxidant effect: peroxisome proliferator-activated receptor a is associated with Cu2+, Zn2+-superoxide dismutase in the liver. Life Sci 63:135 – 144.
5. Dengping Yin, Jing Tao, David D. Lee, Jikun Shen, Manami Hara, James Lopez, Andrey Kuznetsov, Louis H. Philipson and Anita S. Chong 2006 Recovery of Islet ß-Cell Function in Streptozotocin- Induced Diabetic Mice, Diabetes 55:3256-3263.
6. Ar’Rajab A, Dawidson IJ, Harris RB 1994 Sentementes JT: Immune privilege of the testis for islet xenotransplantation (rat to mouse). Cell Transplant 3:493–498.
7. Fernandes A, King LC, Guz Y, Stein R, Wright CV, Teitelman G 1997
Differentiation of new insulin-producing cells is induced by injury in adult pancreatic islets. Endocrinology 138:1750–1762.
8. Teitelman G, Guz Y, Ivkovic S, Ehrlich M 1998 Islet injury induces neurotrophin expression in pancreatic cells and reactive gliosis of peri-islet Schwann cells. J Neurobiol 34:304–318.
9. Kazi Rafiq, Shamshad J. Sherajee, Akira Nishiyama, M. A. Suf iun and Mahbub Mostofa 2009 Effects of indigenous medicinal plants of Bangladesh on blood glucose level and neuropathic pain in streptozotocin-induced diabetic rats. African Journal of Pharmacy and Pharmacology 3(12):636-642.
10. Hermann LS, Schersten B, Bitzen PO, Kjellstrom T, Lindgarde F, Melander A 1994 Therapeutic comparison of metformin and sulfonylurea, alone and in various combinations. A double-blind controlled study. Diab. Care 17: 1100-1109.
11. Chen, J., et al. 2006 "Stevioside counteracts the glyburide-induced desensitization of the pancreatic beta-cell function in mice: studies in vitro." Metabolism 55(12): 1674-80.
12. Ferreira, E. B., et al. 2006 "Comparative effects of Stevia rebaudiana leaves and stevioside on glycaemia and hepatic gluconeogenesis." Planta Med 72(8): 691-6.
13. Chang, J. C., et al. 2005 “Increase of insulin sensitivity by stevioside
in fructose-rich chow-fed rats.” Horm. Metab. Res 37(10): 610-6.
14. Chen, T. H., et al. 2005 “Mechanism of the hypoglycemic effect of
stevioside, a glycoside of Stevia rebaudiana.” Planta Med 71(2): 108-13.
15. Junod A, Lambert AE, Staufacher W, Renold AE 1969 Diabetogenic action of Streptozoticin; relationship of does to metabolic response. J Clin Invest 48:21-29.
16. Elayat AA, Mostafa El-Naggar M, Tahir M 1995 An immunocytochemical and morphometric study of the rat pancreatic islets. J Anat. 186: 629-37.
17. S. Rebecca Sujatha, Ansu Pulimood & S. Gunasekaran 2004
Comparative immunocytochemistry of isolated rat & monkey pancreatic islet cell types Indian J Med Res 119: 38-44.
18. Chomczynski, p. & sacchi, n. 1987 Single-step method of RNA isolation by acid guanidinium thiocyanate – phenol – chloroform extraction. Anal. Biochem 162: 156 – 159.
19. Nitenberg, a., valensi, p., sachs, r., dali, m., aptecar, e. & attali, j.
1993 Impairment of coronary vascular reserve and ACh-induced coronary vasodilation in diabetic patients with angiographically normal coronary arteries and normal left ventricular systolic function. Diabetes 42: 1017 – 102.
20. Cho S.Y., J.Y. Park, E.M. Park, M.S. Choi, M.Y.Lee, S.M. Joen, M.K.
Jang, M.J. Kim and Y.B.Park 2002 Alternation of hepatic antioxidant enzyme activities and lipid profile in streptozotocin –induced diabetic rats by supplementation of dandelion water extract, Clin. Chem. Acta 317: 109-117.
21. Jeppesen, P. B., et al. 2000 “Stevioside acts directly on pancreatic beta cells to secrete insulin: actions independent of cyclic adenosine monophosphate and adenosine triphosphate-sensitive K+-channel activity.” Metabolism 49(2): 208–14.
22. Oviedo, C.A 1971"Accion hipoglicemiante de la stevia rebaudiana
Bertoni (Kaa-he-e)." Excerpta Medica 208: 92-93.
23. Turan Karaca, Mecit Yoruk, Ibrahim H. Yoruk and Sema Uslu 2010
Effects of Extract of Green Tea and Ginseng on Pancreatic Beta Cells and Levels of Serum Glucose, Insulin, Cholesterol and Triglycerides in Rats with Experimentally Streptozotocin-Induced Diabetes: A Histochemical and Immunohistochemical Study, Journal of Animal and Veterinary Advances 9 (1) :102-107.
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 2, Issue 12, December-2011 7
ISSN 2229-5518
24. Tadesse Bekele , Ariaya Hymete ,Mekuria Tadesse , Yalemtsehay Mekonnen 2008 Antidiabetic activity and phytochemical screening of crude extracts of Stevia rebaudiana Bertoni and Ajuga remota Benth grown in Ethiopia on alloxan-induced diabetic mice, Department of Pharmaceutical Chemistry, School of Pharmacy, Addis Ababa University April.
25. Geuns, J. M. C. 2003 Molecules of Interest Stevioside. Phytochemistry.
64: 913–921.
26. Anonymous-a 1999. Opinion on Stevia rebaudiana bertoni plants and leaves http://ec.europa.eu/food/fs/sc/scf/out36_en.pdf (Accessed on
23/04/07).
27. Parton LE, Diraison F, Neill SE, Ghosh SK, Rubino MA, Bisi JE, Briscoe CP, and Rutter GA 2004 Impact of PPAR γ {gamma} overexpression and activation on pancreatic islet gene expression profile analyzed with oligonucleotide microarrays. Am J Physiol Endocrinol Metab 287: E390–E404,
28. Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, and Kliewer SA 1995 An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ). J Biol Chem 270: 12953–12956.
29. Marles RJ, Farnsworth N 1996 Antidiabetic Plants and their Active
Constituents: An update. Prot J Bot Med 1:85-135.
30. Kodama S, Kuhtreiber W, Fujimura S, Dale EA, Faustman DL 2003
Islet regeneration during the reversal of autoimmune diabetes in
NOD mice. Science 302:1223–122.
IJSER © 2011 http://www.ijser.org