International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 1072
ISSN 2229-5518
Recycling of Cement Kiln Dust in Red Clay Bricks and its Impact on their Physico- Mechanical Behaviors
Waleed A. Ogila
Abstract- Cement industry in Egypt produced large amounts of cement kiln dust (CKD) in the form of powder, and has to be discarded. The present work aims to study the effect of cement kiln dust on the physical and mechanical properties of red clay bricks. This could be cost effective by utilization of this material in a useful application and it can helpful in tempering the associated environmental problems. Brick samples of 70 mm, 35 mm and 20 mm dimensions were manufactured where the raw clay material was mixed with various proportions (2%, 4%, 8%, 10%, and 12%) of CKD. These mixtures
were prepared in batches which mixed with water to form plastic masses and sintered in an electric furnace (950oC – 1100oC) for three hours, raw clay bricks without additives (0% CKD) were used as control bricks. Chemical and mineralogical compositions of raw materials (Clay and CKD), physical,
mechanical and mineralogical composition of fired bricks were tested. The experimental results revealed that the technological properties of manufactured bricks are strongly influenced by the addition of CKD and firing temperature. The results revealed that the bricks of 2% CKD fired at
1100oC achieved the ASTM and Egyptian specifications for the low weathering and high resisting bricks, resulting great contributions to economy and
ecology of Egypt.
Index Terms- Cement kiln dust, Recycling of waste materials, Fired-clay bricks.
1 INTRODUCTION
owadays, the technological development and the increasing rate at which raw materials are continuously transformed into industrial products
result in environmental aggressions and waste generation affecting public health. Therefore, many countries have still been working on how to reuse the waste materials. So that it causes fewer hazards to the environment. Developed countries strictly follow some rules to protect the environment whereas many developing countries have almost no rules to protect the environment against wastes. Waste materials can be used to produce new products or can be used as admixtures, so that natural sources are used more efficiently and the environment is protected from waste deposits (1). Recycling of such wastes as building materials appears to be viable solution not only to such pollution problem but also to the problem of economical design of buildings. The increase in the popularity of using environmental friendly, low cost and durable construction materials in building industry have brought about the need to investigate how this can be achieved to benefit the environment as well as to maintain the material requirements affirmed in the standards (2).
Brick is one of the most important construction elements. The history of brick manufacturing goes back 8000 years when the fabrication of the earliest sun dried clay bricks was discovered. The use of waste materials as additives in the manufacture of masonry units has been attracting a growing interest of researchers in recent years and is becoming a common practice. With increasing demands of the construction industry, bricks quality and cost become more important day by day in Egypt.

• Waleed Abdelmoghny Metwaly Ogila, Lecturer of Engineering Geology, Geology Dep., Faculty of Science, Ain Shams University, Cairo, Egypt.
E-mail: walid_ogala@sci.asu.edu.eg walid_metwali@sci.asu.edu.eg
In Egypt, every year huge growing amounts of cement kiln dust (CKD) as a by-product are produced from cement industry during the manufacture of cement clinker by the dry process. The production of different types of cement reached nearly 30 million tons, with at least 3 million tons CKD/year (3). CKD is generally grayish in color and consists predominately of silt-sized, non-plastic particles representing a mixture of partially calcined and unreacted raw feed, clinker dust and fuel ash enriched with alkali sulfates and halides and other volatiles.
The properties of CKD depend on the kind of raw materials and the fuel used. The technical, economical, and environmental problems arise in the transportation of the dust from the plant to outside as well as the severe pollution to the surrounding environment. As indicated from the literature review that the most common applications for the CKD are in soil stabilization (4), hazard mineral removing (5), blended cements (6 and 7), manufacturing of vitrified sewer pipes (8), pavement works (9), ceramic mixtures (10), cement products (11), manufacturing in acid resistant masonry units (12 and 13), and waste water treatment (14).
In Tourah Portland Cement Factory, the production of by-pass kiln dust per day is about 5.3% of the total production of the rotary kiln which is about 9000 ton/day. So the amount of by-pass kiln dust is about 477 ton/day.
The present study aims to investigate the incorporation of cement kiln dust in manufacture of bricks, in order to obtain the suitable manufacturing condition, the proportion of cement kiln dust in the brick and the firing temperature that might affect the qualities of bricks were investigated, and its impact on the physico-mechanical characteristics of bricks for economical and environmental benefits. To achieve the aim of this investigation, physical, chemical and mechanical tests were conducted and assessed according to the American Society for Testing and Materials (ASTM) Specifications.
IJSER © 2014 http://www.ijser.orgs
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 1073
ISSN 2229-5518
2 MATERIALS AND METHODS
In this study, the industrial raw clay material for the experimental studies was obtained from a local brick manufacturing plant. Upon collection, it was ground with a crushing machine. The sample of cement kiln dust (CKD) collected from Tourah Portland Cement Factory. The mineralogical compositions of the raw materials (clay and CKD), and some selected fired bricks were characterized by powder X-ray diffraction (XRD). The used XRD apparatus was a BRUKER-D8 Advanced diffractometer with Cu Kα target available at the Central Metallurgical Research and Development Institute, Egypt. This diffractometer runs at
40.00KV and 40.00 mA. The scanning speed is 2θ/Min in the range from 2o to 70o. The chemical compositions of the raw materials were characterized as the following; SiO2 , Al2 O3 , Fe2 O3 , CaO, MgO, MnO, TiO2 , and P2 O5 were determined by using X-ray fluorescence (XRF) spectrometer (model; GTX-09) according to (15). Na2 O and K2 O were analyzed by flame photometric method by using Genway apparatus, SO3-- and Cl- were analyzed by gravimetric and volumetric methods, respectively, according to (16). Loss On Ignition (LOI) test was measured according to (17), pH value of extracted water was measured at 20ºC by using an electronic pH meter model 6072 according to test method reported in the (18). Total Dissolved Salts (TDS) was measured according to (16). The chemical analysis was carried out at the Raw Building Materials and Processing Technology Research Center, Egypt.
The grain size analysis, and atterberg limits of the clay material were measured according to (19) for grain size analysis, and (20) for atterberg limits.
In order to determine the effect of CKD on the physico- mechanical characteristics of red clay bricks; 2%, 4%, 8%,
10%, and 12% of CKD were added into the raw clay
material to form brick mixtures (CKD percentages added to
the total dry weight of the brick mixtures). Each brick
mixture was mixed in a porcelain ball mill in order to
ensure homogeneous mixing. The brick mixtures were
thoroughly mixed in a conventional kitchen mixer with
20% to 40% of water (based on dry weight of each brick
mixture) to form a uniform paste. The amount of water is
increasing with increasing the amount of CKD additives; to
easily mix the mixtures and to obtain the desired shapes.
Then, the bricks were formed into soft-mud rectangle-
shaped specimens with an internal dimension of 70 mm, 35 mm and 20 mm using brick specimens hand-molding. Specimens were air dried at room temperature for 7 days, and then oven dried at 110±5°C for another 24 hours to
remove water content. All green brick samples were fired at optimized 1000oC in an electrical furnace under heating rate of 8.7oC/min from room temperature to 250oC, 500oC, and
750oC; respectively, soaking 30 minutes in each temperature degree, and adjusted rate of 6.25°C/min until the maximum temperature, followed by 3 hours soaking. The specimens were naturally cooled down to room temperature in the furnace. The raw clay bricks were used as control bricks (0% of CKD). The optimum composition of Clay-CKD bricks was tested to reveal the effect of firing temperature (from 950oC to 1100oC), with the same
sequence of firing, on quality of manufactured bricks.
The surface appearance of produced fired bricks was
observed via visual inspection to reveal the presence of
possible cracks or micro-cracks. Color of the fired bricks
was determined using Munsell's geological rock-color
chart. The produced bricks then received a series of tests,
including firing linear shrinkage (21), bulk density (22), firing weight loss, water absorption, saturation coefficient, initial rate of absorption, efflorescence, and compressive strength (23). The optimum composition of Clay-CKD fired
bricks at different firing temperatures and control clay fired bricks at 1000oC were characterized by XRD analysis. These properties used to determine the suitable conditions for producing qualified bricks.
3 RAW MATERIALS CHARACTERISTICS
3.1 Chemical and mineralogical compositions
Table (1) gives the chemical composition of the raw clay and the CKD materials. The chemical composition of clay shows the expected typical composition: rich in silica, alumina and iron oxides (minor contents of Mg, Ti, Ca, K, P and Mn oxides), accompanied by a significant amount of sodium oxide. The considerable amount sodium oxide is due to present of Na-feldspars (albite), and halite as shown in XRD pattern (Fig., 1). The loss on ignition (7.49%) is within the usual range for clay deposits, and is most likely associated with volatile components, organic matter burn- off and/or carbonate decomposition (24). The CKD is constituted mainly by CaO then Al2 O3 , and SiO2 , accompanied by significant anions content of Cl- and SO3 -- (about 3 %). The present content of calcium oxide and anions are reflected on the high value of loss on ignition (14.19%) and will be responsible for the light coloring of the green specimens. K2 O, TiO2 , P2 O5 , and MnO2 are represented with minor amounts. TDS and pH are more significant in CKD than clay, this due to the presents of soluble salts such as halite and sylvite minerals as shown in XRD pattern (Fig., 1).
TABLE 1
CHEMICAL COMPOSITION OF RAW MATERIALS.
2
The XRD patterns for the starting raw materials are manifested in Figure (1). The clay is composed mainly of quartz (SiO2 ), albite (NaAlSi3 O8 ), montmorillonite [(Na,Ca)0.33 (Al,Fe,Mg)2 (Si4 O10 )(OH)2 ·nH 2 O], and kaolinite (Al2 Si2 O5 (OH)4 ). Hematite Fe2 O3 , calcite (CaCO3 ) and halite (NaCl) minerals are minor phases. The CKD pattern
IJSER © 2014 http://www.ijser.orgs
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 1074
ISSN 2229-5518
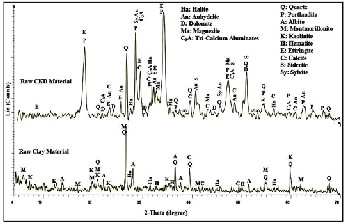
shows that the portlandite (Ca(OH)2 ), ettringite (Ca6 Al2 (SO4 )3 (OH)12.26 H2 O), calcite (CaCO3 ), and siderite (FeCO3 ) minerals are the major crystalline phases whereas quartz (SiO2 ), sylvite (KCl), halite (NaCl), anhydrite (Ca(SO4 ), dolomite (Ca,Mg(CO3 )2 ), magnesite (MgCO3 ), and tri-calcium aluminates (C3 A) minerals are minor phases.
Fig. 1. X-ray diffraction patterns of the raw materials.
3.2 Atterberg limits and grain size distribution of raw
clay material
Plasticity is an important parameter for the production of constructional clay bricks. Because, the brick industry mainly uses extrusion shaping and therefore insufficient plasticity and heterogeneities in clay body cause extrusion failures and low mechanical properties. The plastic limit (P.L) corresponds to the amount of water necessary for the material to reach the plastic consistency, which makes it possible to be formed by extrusion. The liquid limit (L.L) corresponds to the amount of water necessary for the material to loss its plastic consistency. The plasticity index (P.I) is associated with the range of plastic consistency. the non plastic soil has a plasticity index value ranging from
0% to 5%, low plasticity soil has a value ranging from 15%
to 30%, and high plasticity soil has a value greater than 35%
(25). If the plasticity index value is less than 10% leading to
risk of developing problems during the extrusion process.
These problems are related to a possible variation in the amount of extrusion water, causing in appropriate dimensional changes and even cracks in the pieces. The plasticity parameters of the clay material in terms of the atterberg limits are shown in Table (2). It can be observed that the values of L.L, P.L, and P.I of clay material are
37.9%, 18.8%, and 19.2%, respectively. These results are in
agreement with the range defined in the literature and
indicated as appropriate to the production of bricks by
extrusion (26). The grain size distribution of the raw clay
material indicated that is considered as sandy silt soil with
61% of silt fraction, 34% sand fraction, and 5% clay fraction
(Fig., 2).
4 FIRED BRICKS CHARACTERISTICS
4.1 Mineralogical composition
Figure (3) demonstrate the XRD patterns of the bricks that made from clay raw material at 1000oC, and 2% CKD at
950oC, 1000oC, 1050oC, and 1100oC. The figure represented that the main crystalline phases in the fired bricks are quartz (SiO2 ), and albite (NaAlSi3 O8 ), hematite (Fe2 O3 ), and microcline (KAlSi3 O8 ), with variable percentages. Calcite (CaCO3 ), and sylvite (KCl) represented only in one type of 2% CKD fired bricks at 1100oC.
TABLE 2
ATTERBERG LIMITS OF RAW CLAY MATERIAL.

Liquid Limit (L.L) % 37.9
Plastic Limit (P.L) % 18.8

Plasticity Index (P.I) % 19.2
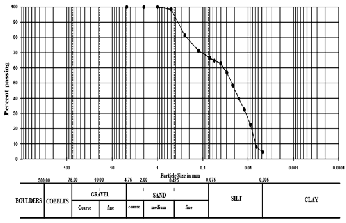
Fig.2. Grain size distribution curve of raw clay material.
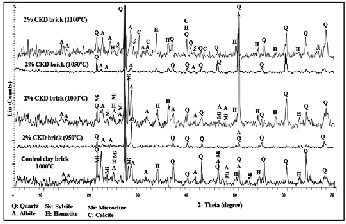
Fig. 3. X-ray diffraction patterns of the selected fired bricks.
4.2 Color and surface appearance
Color of the fired bricks according to Munsell's color system clarified that the fired bricks color became darker (very dark red, (5R 2/6)) with increasing of firing temperature and getting lighter (Moderate red (5YR 5/6)) with increasing the CKD content and decreasing the firing temperature (Fig., 4). Surface texture for all fired bricks shows a smooth touch, while for 2% CDK bricks at 1100oC
IJSER © 2014 http://www.ijser.orgs
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 1075
ISSN 2229-5518
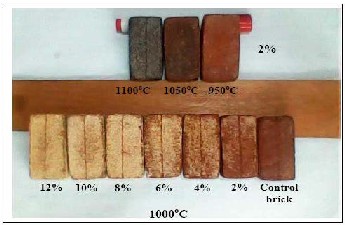
shows a glazed touch. The surface appearance of control clay bricks and 2% CKD bricks is mostly clear, while with increasing of CKD, the brick surface covered by yellowish white coating thin film instead of red (Fig., 4).
Fig.4. Color and surface appearance of fired bricks.
4.3 Bulk density
The measurements of bulk density for different proportions of CKD bricks fired at 1000oC are demonstrated in Figure (5) and Table (3a). As shown, the bulk density values of the bricks are inversely proportional to the quantity of CKD added in the mixtures with optimum decreasing at 8%, after that the bulk density increase gradually with amount of about 0.10 at 12% CKD. This decreasing in bulk density can be regarded as a result of the increase in porosity in the fired bodies, volume expansion or bloating of manufactured fired bodies (27). The firing temperature can also affect the bulk density of the fired bricks. The results show that increasing the firing temperature results in an increasing in bulk density (Fig., 6 and Tab., 3a). The increasing in the bulk density with firing temperature is in line with the formation of a more abundant liquid phase during firing that fills the open pores and reduces the open porosity.
4.4 Water absorption parameters
Water absorption is a key factor affecting the durability of brick. The less water infiltrates into brick, the more durability and resistance of brick to the natural environment are expected (28). Thus, the internal structure of the brick must be intensive enough to avoid the intrusion of water. Figures (7 and 8) and Tables (3a, and 3b) show the results of the water absorption tests for various fired bricks. As shown in Figures (7 and 8), the values of water absorption (5hrs. cooled, 24 hrs. cooled, and 5hrs. boiling) and initial rate of absorption (IRA) are directly proportional to the quantity of CKD added. This increasing in water absorption with increasing CKD content gives indication to the porosity increasing. This is confirmed with the result of bulk density. The bonding ability of the mixture is apparently related to the amount of CKD added to the mixture. When the mixture contains a rather high percentage of CKD; the adhesivity of the mixture decreases
and the internal pore of the brick increase. As a result, the quantity of absorbed water increases. The results of water absorption and initial rate of absorption (IRA) clarify a rapidly decreasing with increasing of firing temperature of
2% CKD bricks from 950oC to 1100oC (Figs., 9 and 10),
thereby increasing the weathering resistance. This is
essential due to the high amount of glassy phase formed
during firing, which decreases the open porosity by the
filling with the glassy phase causing densification of the fired body (29). Saturation coefficient values are ranging from 0.79 to 0.86 for all batches of fired bricks and with increasing of firing temperature, the saturation coefficient decrease to 0.68 for 2% CKD bricks fired at 1100oC (Tabs.,
3a, and 3b).
According to the criterion of water absorption of brick in (30), only the 2% CKD bricks fired at 1100oC achieved the ASTM specifications for the low weathering and high resisting bricks (water absorption value < 6%) and can be considered as the well sintered bricks. This means that the water absorption decreasing is associated with a more significant liquid phase formation. The liquid phase penetrates the open pores closing them and isolating neighboring pores.
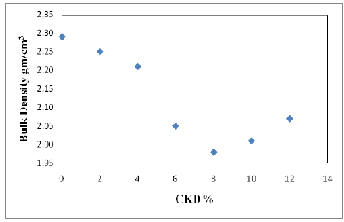
Fig. 5. Relationship between CKD% and bulk density of fired bricks.
TABLE 3a
BULK DENSITY AND WATER ABSORPTION PARAMETERS OF FIRED BRICKS.
IJSER © 2014 http://www.ijser.orgs
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 1076
ISSN 2229-5518
2 (1000oC) | 2.25 | 11.02 | 11.49 |
2 (1050oC) | 2.35 | 11.28 | 11.89 |
2 (1100oC) | 2.52 | 2.64 | 2.83 |
TABLE 3b
WATER ABSORPTION PARAMETERS OF FIRED BRICKS.
CKD % (Firing Temp.) | Water Absorption 5 hrs. (boiling) % | Saturation Coefficient % | Initial Rate of Absorption (IRA) gm/sec |
0 (1000oC) Control clay brick | 10.79 | 0.83 | 8.05 |
4 (1000oC) | 22.03 | 0.83 | 14.95 |
6 (1000oC) | 22.17 | 0.79 | 14.08 |
8 (1000oC) | 24.83 | 0.82 | 16.42 |
10 (1000oC) | 25.18 | 0.83 | 16.79 |
12 (1000oC) | 26.43 | 0.86 | 15.88 |
2 (950oC) | 15.18 | 0.85 | 10.28 |
2 (1000oC) | 13.99 | 0.82 | 10.27 |
2 (1050oC) | 14.40 | 0.83 | 9.51 |
2 (1100oC) | 4.52 | 0.68 | 1.76 |
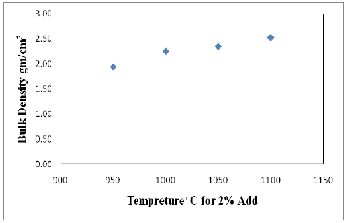
Fig. 6. Relationship between firing temperature and bulk density of 2% CKD fired bricks.
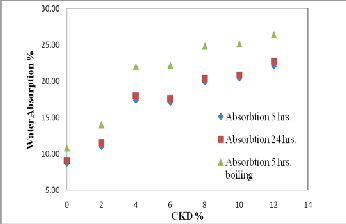
Fig. 7. Relationship between CKD% and water absorption of fired bricks.
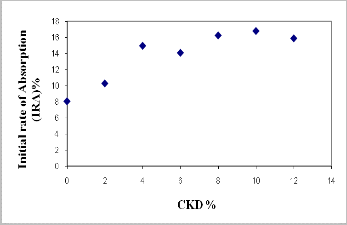
Fig. 8. Relationship between CKD% and initial rate of absorption of fired bricks.
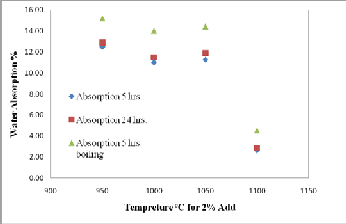
Fig. 9. Relationship between firing temperature and water absorption of
2% CKD fired bricks.
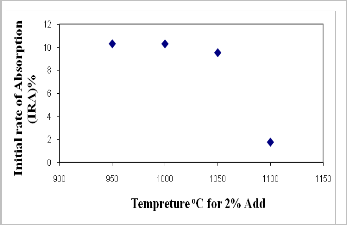
Figure (10): Relationship between firing temperature and initial rate of absorption of 2% CKD fired bricks.
4.5 Firing linear shrinkage
IJSER © 2014 http://www.ijser.orgs
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 1077
ISSN 2229-5518
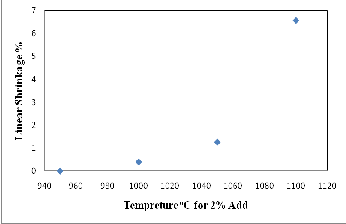
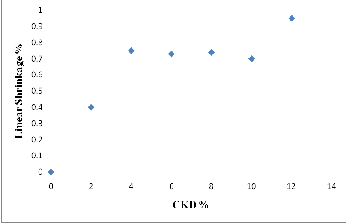
The quality of brick can be further assured according to the degree of firing linear shrinkage. Normally, a good quality of brick exhibits shrinkage below 8%. As shown in Figure (11) and Table (4), the firing linear shrinkage generally increase with increase the CKD contents in mixture and the percentage values of linear shrinkage are less than 1.00% for all Clay and Clay-CKD mixtures bricks. The firing temperature is another important factor affecting the degree of linear shrinkage. In general, the increasing of firing temperature results in an increase in linear shrinkage. As shown in Figure (12) and Table (4), the firing linear shrinkage values for 2% CKD bricks are 0.0%, 0.4%, 1.26 %, and 6.56% at firing temperatures of 950oC, 1000oC, 1050oC, and 1100oC, respectively. Shrinkage increasing with increasing firing temperature is a consequence of vitrification, which occurs during the sintering process helping to reduce porosity if the sintering temperature was enough and extended to a reasonable holding time. In this study, the brick shrinkage lies in the scope of the good- quality category. It is concluded that in order to yield a
good quality brick, the proportion of CKD in the mixture
and the firing temperature are the two key factors
controlling the brick shrinkage during the firing process.
.
Fig. 11. Relationship between CKD% and firing linear shrinkage of fired bricks.
TABLE 4
FIRING LINEAR SHRINKAGE, FIRING W EIGHT LOSS AND COMPRESSIVE STRENGTH OF FIRED BRICKS.
Fig. 12. Relationship between firing temperature and firing linear shrinkage of 2% CKD fired bricks.
4.6 Firing weight loss
As shown in Figure (13) and Table (4), increasing the percent of CKD resulted in an increase in firing weight loss with range of about 2.00%. CaCO3 content in the Clay-CKD mixtures is responsible for increasing of weight loss, and decrease of bulk density of fired bricks. These results are confirmed with the chemical and mineralogical compositions of raw materials (Tab., 1, and Fig., 1). The firing temperature is another important factor affecting the firing weight loss. In general, the increasing of firing temperature results in an increase in firing weight loss (Fig.,
14). In this figure the firing weight loss increase with
optimum loss 8.35% at 1050oC and decreasing again at
1100oC, this may due to formation of new minerals such as

calcite and sylvite as shown in XRD pattern (Fig., 3).
10
9
8
7
6
5
4
3
2
1
0
0 2 4 6 8 10 12 14
CKD %
Fig. 13. Relationship between CKD% and firing weight loss of fired bricks.
IJSER © 2014 http://www.ijser.orgs
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 1078
ISSN 2229-5518
Fig. 15. Efflorescence of fired bricks.
9
8
7
6
5
4
3
2
1
0
900 950 1000 1050 1100 1150
Tempreture oC for 2% Add

Fig. 14. Relationship between firing temperature and firing weight loss of 2% CKD fired bricks.
4.7 Efflorescence

Brickwork sometimes develops an efflorescence of white salts brought to the surface by water and deposited by evaporation. These salts may have an external origin, like the water in soil in contact with the brickwork, or may derive from the mortar. However, the salts frequently originate in the bricks themselves. Visible efflorescence can be formed from very small amounts of salts. Efflorescence may be disfiguring but it is often harmless and disappears after a few seasons. However, efflorescent salts may contain a high proportion of sulfates and chlorides and may cause sulfate attack on the cement mortar joints. The efflorescence test is essentially a visual examination and detection of salt deposits, powdering or flaking on the exposed surface of the test specimen. The observation is described as nil, slight, moderate, heavy or serious. The efflorescence was of nil class for the 2% CKD fired bricks at 1050oC, and 1100oC, slight class for the control clay bricks and 2% CKD fired bricks at 950oC, and heavy class for other CKD percentage bricks (Fig., 15).
4.8 Compressive strength
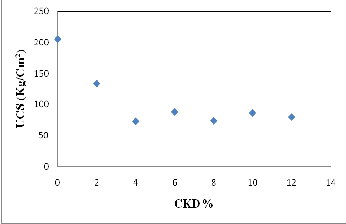
The compressing test is the most important test for assuring the engineering quality of building materials. The results of the compressive strength test on the bricks made from both clay and Clay-CKD mixtures are shown in Figure (16). As shown, up to 4% of CKD added to the bricks, the strength decrease with amount of about 35% for 2% CKD and 65% for 4% CKD, respectively, from total strength of control clay bricks, and the compressive strength values for other additive percentages are mostly constant with the same decreasing range of 4% CKD. These results indicate that the strength is greatly dependent on the amount of CKD in the bricks. The optimum amount of CKD that could be mixed with clay to produce good bonding bricks without bad surface appearance was 2% by weight. Figure (17) shows the relation between the firing temperature of
2% CKD bricks and the compressive strength, the results
indicated that the optimum firing temperature at which
maximum compressive strength occurred was 1100oC (459.43 kg/cm2). It is worth mentioning that the decrease in water absorption values and an increase in linear shrinkage with increasing firing temperature are due to effective formation of a liquid phase which hinders cracks formation and improves the mechanical strength. This is agreed with (31).
The compressive strength of the bricks made from Clay-
CKD mixtures all meets the ASTM standard for the brick
requirements: 207 kg/cm2 for a first-class brick, 172 kg/cm2
for a second-class brick, and 103kg/cm2 for a third-class brick. It is concluded that 2% CKD can be blended with clay to produce a good quality of brick under 1100oC firing temperature as a first class bricks, while the other firing temperatures bricks are considered as a third class bricks.
1100oC
2%
1050oC 950oC
12%
10%
6%
8% 1000oC%
2% Control brick
Fig. 16. Relationship between CKD% and compressive strength of fired bricks.
IJSER © 2014 http://www.ijser.orgs
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 1079
ISSN 2229-5518
5 CONCLUSIONS
This work has demonstrated a feasible way of using CKD as a clay substitute to produce quality bricks. Different measurements of Clay-CKD bricks were carried out to evaluate the factors that could affect brick quality, to obtain the suitable manufacturing condition, and for economical and environmental benefits. The results presented along this work enable to draw the following conclusions:
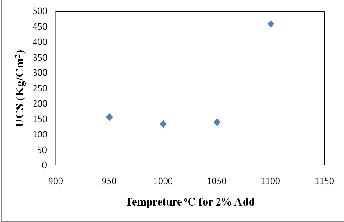
Fig. 17. Relationship between firing temperature and compressive strength of 2% fired bricks.
1. The bricks manufactured did not show any deformation or uneven surfaces occurring at all firing temperatures.
2. There are no new crystalline phases formed during
the sintering process.
3. Experimental results demonstrate clearly that the
brick properties are strongly dependent on both of
the added materials (CKD) and firing temperature.
4. Increasing of CKD in the investigated mixtures should be avoided hence it leads to deleterious in the technological properties of the clay bricks.
5. Increasing the firing temperature of the brick resulted in a decrease in water absorption
parameters, efflorescence, and increasing linear shrinkage, loss weight, bulk density, and compressive strength.
6. The fired bricks formulated from the mixture 2% CKD, fired at 1100oC through accelerated sintering
process can be utilized for making low weathering and high resisting bricks and suitable for civil constructions.
7. Based on this work an environmentally bricks, with good commercial characteristics was made
using recycling technology in a safe disposal manner for the studied pollutants.
8. In terms of the environmental performance, it is worth mentioning that it is recommended to establish an environmental profile analysis data for the waste materials.
6 REFERENCES
(1) Karasahin, M., and Terzi, S. (2007): Evaluation of marble waste dust Mixture of asphaltic concrete. Construction Building Material, 21: 616-620.
(2) Algin, H.M., and Turgut, P. (2008): Cotton and limestone powder wastes as brick material. Construct. Build. Mater., 22: 1074-1080.
(3) Abd El-Aleem, S., Abd El-Aziz, M ., Heikal, M., and
El-Didamony, H. (2005): Effect of cement kiln dust substitution on chemical and physical properties and compressive strength of Portland and slag cements. The Arabian J. Sci. and Engineering, 30 (2B): 362-273.
(4) Peethamparan, S., Olek, J., and Diamond, S. (2009):
Mechanism of stabilization of Na-montmorillonite clay
with cement kiln dust. Cement and Concrete Research,
39 (7): 580-589.
(5) Ali, O.I., Osman, H.H., Sayed, S.A., and Shalabi, M.E.
(2011): The removal of some rare earth elements from their aqueous solutions on by-pass cement dust. J. Hazardous Materials, 195: 62-67.
(6) Heikal, M., Aiad, I., and Helmy, I.M (2002): Portland cement clinker, granulated slag and by-pass cement kin
dust composites. Cement and Concrete Research, 32 (11): 1805-1812.
(7) El-Mahllawy, M. S. (2008): Characteristics of acid resisting bricks made from quarry residues and waste steel slag. Construction and Building Materials, 22:
1887-1896.
(8) El-Sherbiny, S.A., Youssef, N.F., Ibrahim, O.A., and
Abadir, M.F. (2004): Use of cement dust in the manufacture of vitrified sewer pipes. Waste Management, 24 (6): 597-602.
(9) Chen, L., and Lin, D. (2009): Stabilization treatment of soft subgrade soil by sewage sludge ash and cement. J. Hazardous Materials, 162 (1): 321-327.
(10) Youssef, N., El-Sokkary, T., and El-Mahllawy, M. (2004): Simultaneous utilization of cement kiln dust and blast furnace slag in ceramic floor tile mixtures. Int. Conf. Future Vision and Challenges for Urban Development. 20-22 Dec., Cairo, Egypt.
(11) Maslehuddin, M., Al-Amoudi, O., Shameem, M.,
Rehman, M., and Ibrahim, M. (2008): Usage of cement kiln dust in cement products-research review and preliminary investigations. Construction and Building Materials, 22 (12): 2369-2375.
(12) El-Mahllawy, M., and El-Sokkary, T. (2006): Recycling of cement kiln dust and water-glass sludge in the manufacture of acid resistant masonry units. J. Housing and Building National Research Center (HBRC), 2 (1): 23-35.
(13) El-Mahllawy, M. (2013): An Investigation on the Effect of Cement Kiln Dust and Glauconite on the Properties of Acid Resisting Brick. Int. J. of Science and Technology. UK. 2 (1): 30-43.
(14) Waly, T., Dakroury, A., El-Sayed, G., and El-Salam, S.
(2010): Assessment removal of heavy metals ions from
IJSER © 2014 http://www.ijser.orgs
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 1080
ISSN 2229-5518
wastewater by cement kiln dust (CKD). J. American
Science, 27: 1715-1720.
(15) ASTM C114-04 (2004): American Society for Testing
and Materials "Standard test methods for chemical analysis of hydraulic cement". USA, V. 04.01.
(16) British Standards (B.S.)1377:3 (1988): Methods of test for soils for civil engineering purposes. Part (3). Chemical and electro-chemical tests.
(17) ASTM D7348 (2008): American Society for Testing and Materials "Standard test methods for loss on ignition (LOI) of solid combustion residues. ignition (LOI) of solid combustion residues". USA. V. 05.06.
(18) ASTM D4972 (2007): American Society for Testing and
Materials "Standard test method for pH of soils". USA,
V. 04.08.
(19) ASTM D421, and D422 (1994): American Society for
Testing and Materials "Standard test methods for
laboratory determination of particle size analysis of
soil". USA, V. 04.08.
(20) ASTM D4318 (1994): American Society for Testing and Materials "Standard test methods for laboratory determination of liquid limit, plastic limit and plasticity index of soil". USA, V. 04.08.
(21) ASTM C326-03 (2003): American Society for Testing
and Materials "Test Method for Drying and Firing
Shrinkages of Ceramic Whiteware Clays". USA. V.
15.02 .
(22) ASTM C373-88 (2006): American Society for Testing and Materials "Standard Test Method for Water Absorption, Bulk Density, Apparent Porosity, and Apparent Specific Gravity of Fired Whiteware Products". USA. V. 15.02 .
(23) ASTM C67-03 (2003): American Society for Testing
and Materials "Standard test methods for sampling and
testing brick and structural clay tile". USA, V. 04.05.
(24) Santos, P.S. (1997): Ciência e Tecnologia de Argilas
(Science and Technology of Clays), V.1, 3rd ed. Edgard
Blücher Ltd., S. Paulo (in Portuguese).
(25) Wray, W. K. (1986): Measuring soil properties, Prentice-Hall publishing Co., Inc., Englewood Cliffs, NJ.
(26) Menezes, R.R., Ferreira, H.S., Neves, G.A., Lira H de,
L., and Ferreira, H.C. (2005): Use of granite sawing wastes in the production of ceramic bricks and tiles. J. Europ. Ceramic Soc., 25: 1149-1158.
(27) Souza, A. J., Pinheiro, B. C., and Holanda, J. N. (2010): Recycling of gneiss rock waste in the manufacture of vitrified floor tiles. J. Env. Management, 91: 685- 689.
(28) Lin, K.L. (2006): Feasibility study of using brick made
from municipal solid waste incinerator fly ash slag. J. Hazardous Mater., B137: 1810-1816.
(29) Moreira, J. M., Manhaes, J. P., and Holanda, J. N. (2008): Processing of red ceramic using ornamental rock powder waste. J. Matr. Proc. Technology, 196: 88-
93.
(30) ASTM C62-04 (2004): American Society for Testing and Materials "Standard test methods for building
brick (solid masonry units made from clay or shale)". USA, V. 04.05.
(31) Monteiro, S. N., and Vieira, C. M. (2004): Influence of firing temperature on the ceramic properties of clays from Campos dos Goytacazes, Brazil. Appl. Clay Science, 27: 229-234.
IJSER © 2014 http://www.ijser.orgs
















