International Journal of Scientific & Engineering Research Volume 4, Issue3, March-2013 1
ISSN 2229-5518
Rapid Analytical and Standardization method de- velopment for the Proprietary Ayurvedic and Siddha drug formulations
Dr.F.Emerson Solomon * and Dr.Kathir.Viswalingam1
Abstract - The present investigation is aimed to forward a new analytical tool for the analysis Ayurvedic and Siddha formulation ( which prepared as per the respective Formulary) to study the quick review of the quality as well as assessment of the efficacy by analyzing the property of the different Polar, Medium Polar and Non Polar constituents which will facilitate to identify the efficacy of the formulation using HPTLC-FTIR / HPTLC –MS chro- matographic finger prints as a tool to standardize the individual spots in three polar solvents. This will be sufficient to identify a characteristic substance or mixture of substances present in chromatographic fingerprint to ensure consistent quality of the product.
Index Terms – Polar solvent, Medium Polar solvent, Non polar solvent, Chromatography, Finger prints, Ayurveda, Siddha
—————————— ——————————
1 INTRODUCTION
Introduction:
Most of the Proprietary Ayurvedic and Siddha formulation is the comprises of not less ten herbal ingredients, that the assessing the quality and quantity of each ingredients is very difficult as like allopathic drugs since the complex nature of the herbal ingre- dients. Hence, we have developed a method for the possibility to assure the quality and quantity of the formulation by the compo- nents as per their polarities by different elution solvents. And by analyzing with different mobile phases can conclude the efficacy of the drug as per the polarities.
Although there are traditional methods of identification of medic- inal plants like organoleptic, microscopic and physical, none of them gives an authentic identification, as given by a fingerprint of the plant material, as far as the chemical profile is concerned.
HPTLC is a powerful analytical method due to its reliability, sim- plicity, reproducibility and speed. Additionally, the method is economical as it utilizes smaller amounts of solvents with mini- mum sample clean up. Many samples can be simultaneously ana- lyzed in a short duration. HPTLC has no limitation on the choice of the mobile phase and the possibility of direct application of suspensions or turbid samples. Furthermore, it permits a simulta- neous assay of several components in multi-component formula- tions or herbal extracts.
Hence, it is proposed that the Chromatographic Fingerprint is much useful for quality control of medicinal plants instead of other organoleptic and microscopic studies. Since, ultimately it is the chemical constituents that are largely going to participate in the therapeutic efficacy of the medicine, along with other proper- ties of the herbal medicines; the analytical data of the chemical constituents should be able to provide the authentic efficacy of the medicine. It is like fingerprint of an individual gives the identity.
The elution of the samples will be done from high polarity mo- bile phase to low polarity mobile phase. Thus in the finger prints the constituents present in the High Polar solvent (Chloroform) will be of high polar in nature. The same pattern applies to the other medium polar constituents eluted in the Medium Polar solvent (Ethyle acetate) and the low or non-polar constituents eluted in the Non-polar solvent (Hexane).
This is compared that in Ayurveda, the intestinal part of the hu- man body is classified as Pitta zone, where the High Polar mole- cules are playing a major role. It indirectly indicates the molecules of high reactive, the high polar molecules. After the absorption, the blood with all the absorbed constituents will carry them to
heart and the parts related to it. Then the blood will be sent to
different parts of the body.

*Professor, Department of Bio Medical Engineering,
1Dean- Research, BIST, Bharath University,
173, Agharam Road, Selaiyur- 600073, Tamil Nadu, India.
The upper portion of the human body is defined as the Kapha zone, where the cold mechanism will be playing an important role. Thus, the molecules of medium polar molecules will play an important role in the mechanisms related to Kapha zone.
The low polar and non-polar constituents will be able to enter to the human body only through blood transfer, Thus the body organs where the mechanism of availability of the chemical con- stituents is only by blood, will be coming in the last category of
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue3, March-2013 2
ISSN 2229-5518
the polarity. The non-polar oils, fats and other such molecules and mechanisms in the human body are classified as Vata disor- ders and all such disorders are cure using the same type of mate- rials. The low and non-polar constituents will be eluting in the last zone of the fingerprint is considered as Vata zone.
Therefore, the basic humors of the molecules can be identified based on their polarity, which facilitates to know on what disor- der (dosha) it is going to act upon. The present method is useful for the therapeutic standardization of the medicines.
2. Objective:
a) Develop authenticated standard products
b) Develop the method to separate the molecule in 3 stage elu- tions
c) Characterization of the TLC separated compounds with IR, UV and MS
d) Generate the data base for chromatographic finger printings for the Few important Ayurvedic and Siddha products on their basis of three humors Pitta, Kapha and Vata
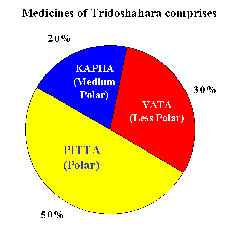
Tridosh a | Linked Dis- ease/Disorder | Product Type | No . of ingre- dients | Polarity |
Pitta | Digestive, Enzy- matic, Endocrine | Syrup, Tablet, Capsule | 25-40 | Polar com- ponent |
Kapha | Mucus secretions, Lubrication, Body Fluid | Syrup, Capsule, Syrup | 20 – 35 | Mid Polar component |
Vata | Nervous disorder, blood circulation | Oil, Balm, Ointment | 20 -30 | Less Polar component |
3. Present knowledge and relevant bibliography including full titles of articles relating to the subject
The finished product should comply with general requirements for particular dosage forms. Many herbal remedies consist of a combination of several active ingredients, and as experience of the use of traditional remedies is often based on combination prod- ucts, assessment should differentiate between old and new com- bination products.
Identical require-
ments for the assess- ment of old and new combinations would result in inappropri- ate assessment of certain traditional medicines.
A method of identifi- cation and where possible, quantifica- tion of the plant ma- terial in the finished
product should also be defined If the identification of an active
principle is not possible, it should be sufficient to identify a char- acteristic substance or mixture of substances to ensure consistent quality of the product. The finished product should comply with general requirements for particular dosage forms. The effective regulation of the quality of herbal medicines moving in interna- tional commerce also requires close liaison between national insti- tutions that are able to keep under regular review all aspects of production and use of herbal medicines.
The therapeutic property of any food or drug will depend on its chemical and physical status. Thus, understanding the chemical constituents using their physico-chemical properties will help to understand the therapeutic efficacy of the medicine.
The physico chemical properties of the medicines play a major role on the therapeutic activity of the medicine. These properties of molecules can be studied using two parameters, the polarity and conjugative properties.
Polarity is a resultant electrochemical property due to different electron donating (nucleophilic) and electron accepting (electro- philic) moieties attached to the molecules along with the unsatu- rated double and triple bonds present in it. They will influence the rate of activity or reactivity of a molecule in chemical and bio- chemical reactions. A thorough estimation of the total polarity of the molecule will give the efficacy of a single or group of mole- cules as to how active they are chemically and therapeutically. Hence, any standardization, which assesses the above properties, will be useful to know their activity.
In general, the constituent molecules present in the drugs and foods can be broadly classified in to three categories as polar, me- dium polar and the non-polar molecules. The total polarity of the molecule depends on the total Electrophlic and Nucleophilic moi-
eties attached to the molecule along with the unsaturation of the molecules by their conjugation.
The elution of the samples will be done from high polarity mo- bile phase to low polarity mobile phase. Thus in the finger prints the constituents present in the High Polar solvent (Chloroform) will be of high polar in nature. The same pattern applies to the other medium polar constituents eluted in the Medium Polar solvent (Ethyle acetate) and the low or non-polar constituents eluted in the Non-polar solvent (Hexane).
This is compared that in Ayurveda, the intestinal part of the hu- man body is classified as Pitta zone, where the High Polar mole- cules are playing a major role. It indirectly indicates the molecules of high reactive, the high polar molecules. After the absorption, the blood with all the absorbed constituents will carry them to heart and the parts related to it. Then the blood will be sent to
different parts of the body.
The upper portion of the human body is defined as the Kapha zone, where the cold mechanism will be playing an important
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue3, March-2013 3
ISSN 2229-5518
role. Thus, the molecules of medium polar molecules will play an important role in the mechanisms related to Kapha zone.
The low polar and non-polar constituents will be able to enter to the human body only through blood transfer, Thus the body organs where the mechanism of availability of the chemical con- stituents is only by blood, will be coming in the last category of the polarity. The non-polar oils, fats and other such molecules and mechanisms in the human body are classified as Vata disor- ders and all such disorders are cure using the same type of mate- rials. The low and non-polar constituents will be eluting in the
last zone of the fingerprint is considered as Vata zone.
The basic humors of the molecules can be identified based on their polarity, which facilitates to know on what disorder (dosha) it is going to act upon. The present method is useful for the thera- peutic standardization of the medicines.
Tridos ha | Linked Dis- ease/Disorder | Product Type | No . of ingre- dients | Polarity |
Pitta | Digestive, Enzy- matic, Endocrine | Syrup, Tablet, Capsule | 25 - 40 | Polar com- ponent |
Kapha | Mucus secretions, Lubrication, Body Fluid | Syrup, Capsule, Syrup | 20 - 35 | Mid Polar component |
Vata | Nervous disorder, blood circulation | Oil, Balm, Ointment | 20 - 30 | Less Polar component |
Thus this method can be used as a Herbal Formulation pharma- copoeia for the Proprietary Ayurveda and Siddha medicines which is prepared as per the respective formulary and the thera- peutic efficacy is standardized quantitatively. This can contribute the insight purview of the formulation for increase or decrease of any one or two of the other doshas is done by formulating medi- cine by adding other ingredients and prepare a suitable formula- tion needed to cure a specific individual.
The present investigation is aimed to forward a new analytical tool for the analysis Ayurvedic and Siddha formulation to study the quick review of the quality as well as assessment of the effi- cacy by analyzing the property of the different polar, medium polar and non polar constituents which will through the efficacy
of the formulation. The analyzing method with HPTLC-FT-IR /
HPTLC–MS as a tool to standardize the individual spots after three stage polar solvent elution. This will be the adequate method to identify a characteristic substance or mixture of sub- stances present in chromatographic fingerprint to ensure con- sistent the qualitative and quantitative the product.
Detailed Research Plan
A. Collection and authentication of the plants
Authenticated Herbal Raw materials and Herbal extracts will be procured for the preparation herbal formulation a per the Ayur- veda and Siddha formulary.
B. Selection formulation as per TRI DHOSA viz., PITTA, KAPHA AND VATA
Various dosage forms (Tablets, Capsule and Syrup, Oil, Oint- ment) of Proprietary Ayurvedic and Siddha drugs will be select- ed as per the basic disorders like Pitta, Kapha and Vata. The drugs will be procured from the well reputed Indian Drug Manu- facturer companies like M/s.Himalaya Drugs, M/s.Charak, M/s.Zandu and M/s.Maharishi for the studies. Details of the selection as follows :
C. Preparation of Extracts
The Ayurvedic and Siddha drugs will be prepared as per the giv- en dosage in the relevant formulary as per the extract solvent. The shed dried coarsely powdered plant material will be extract- ed respective solvent by maceration / boiling / soaking method and followed by evaporation of the solvents using rotary vacuum evaporator. The concentrated extract will be stored in vacuum desiccators until the analysis.
D. Fractionation and Qualitative chemical test of the Extract
The concentrated extracts will be subject to fractionation using Hexane, Ethylacetate, and Chloroform. All the fractions are sub- jected to preliminary phytochemcial examination as per standard protocol.
E. Isolation, characterization and finger print analysis using
HPTLC-IR / HPTLC-MS
High-performance TLC makes use of silica gel of a very uniform and small particle size, permitting excellent separations with comparatively short elution times. Separation of fraction with Three developments with three different mobile phase. HP-TLC plates will be utilized for first development for 1/3rd of the way in a polar solvent to separate polar materials, and then second development for 2/3rd length of the same plate with a medium polar mobile phase and full length of the same plate with less polar mobile phase in order to resolve different polar components present in the extraction in a single HPTLC plate. In routine ana- lytical work, ten or more samples can be applied to a 20 x 20 cm plate and then quantified by HPTLC densitometry.
After separation of the molecules the each spot in the three stage eluted plate will be scanned for the HPTLC scanner with different wavelength using Deuterium Lamp (D2) [190–450 nm], Tungsten lamp (W) [400-800 nm] and Mercury Lamp (Hg) [220- 650 nm]. Thus the prepared standard chromatographic finger prints will be examined for the Market samples for their efficacy.
After scanning once again all the spots in TLC plate will be exam- ined directly with HPTLC-FTIR and HPTLC- MS for the prepara- tion of Library for the Standard Spectrum of Herbal formulations
for the future analysis. This can be used as a Herbal Formulation
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue3, March-2013 4
ISSN 2229-5518
pharmacopoeia for the Proprietary Ayurveda and Siddha medi- cines which is prepared as per the respective formulary.
References :
[1] Ahirwal B, Ahirwal D and Ram A. 2006. Evaluation of standards and quality control parameters of herbal drugs, Souvenir, recent trends in herbal therapy, 25-29.
[2] Bilia AR, Bergonzi MC, Lazari D, Vincieri FF. 2002. Characterization of commercial Kavakava herbal drug & herbal drug preparation by means of Nuclear Magnetic Resonance Spectroscopy, J. Agric. Food Chem., 50, 5016.
[3] Blumenthal M, Brusse WR, Goldberg A, Gruenwald J, Hall T, Riggins CW, Rister RS. 1998. The Complete German Commission E Monographs. Therapeutic Guide to Herbal Medicines, The American Botanical Coun- cil, Austin, TX.
[4] Brain KR and Turner TD. 1975. Practical Evaluation of phytopharmaceu- ticals. Wright Scientechnica Bristol.
[5] Bylund D, Danielsson R, Malmquist G, Markides KE. 2002. Chroma- tographic alignment by warping & dynamic programming as a pre- processing tool for PARAFAc modeling of liquid chromatography- mass spectrometry data, J. Chromatogr.A , 961, 237-244.
[6] Caoa Y, Wang L, Yu X, Ye J. 2006. Development of the chromato- graphic fingerprint of herbal preparations Shuang–Huang–Lian oral liquid, Journal of Pharmaceutical and Biomedical Analysis, 41, 845–856.
[7] Chaudhury RR. 1999. Herbal medicine for human health. World Health Organization Geneva,CBS publishers and distributors LTD, New Delhi,
[8] Cheng Y. 2003. An approach to comparative analysis of chromato- graphic fingerprints for assuring the quality of botanical drugs. J. Chem. Inf. Comput. Sci, 43, 1068-1076.
[9] Cheng YY, Chen MJ, Tong WD. 2003. An approach to comparative analysis of chromatographic fingerprints for assuring the quality of botanical drugs, Chin. J. Chem. Inf. Comput. Sci., 43(3): 1068-1070.
[10] Choi DW, Kim JH, Cho SY, Kim DH, Chang SY. 2002. Regulation and quality control of herbal drugs in Korea, Toxicology, 181/182, 581-586.
[11] Chuang, W.C., Wu, S.K., Sheu, S.J., Chiou, S.H., Chang, H.C. and Chen, Y.P. (1995). A comparative study on commercial samples of ginseng radix, Planta Medica, 61, 459–465.
[12] Collantes ER, Duta R, Welsh WJ, Zielinski WL, Brower J. 1997. Pre- processing of HPLC trace impurity patterns by wavelet packets for pharmaceutical fingerprinting using artificial neural networks, Anal Chem, 69,1392–1397.
[13] Gong F, Liang Yi-Z, Xie Pei-S and Chau FT. 2003. Information theory applied to chromatographic fingerprint of herbal medicine for quali- ty control, Journal of Chromatography A, 1002, 25 – 30.
[14] Gong F, Liang YZ, Xie PS, Sung AJ. 2003. Information theory applied to chromatographic fingerprint of herbal medicine for quality con- trol, J. Chromatogr. A, 1002, 1-2, 25-40.
[15] Gupta MK and Sharma PK. 2007. Test Book of Pharmacognosy, Ayur- vedic formulations, Pragati Prakashan Meerut Vol II, Ist edition.
[16] WHO. 2005. General Guidelines for Methodologies on Research and
Evaluation of Traditional Medicines, p. 1.
[17] WHO. 2004. Guidelines on Good Agricultural and Collection Practices
(GACP) for Medicinal Plants. World Health Organization, Geneva.
[18] Williamson E, Okpako DT, Evans F J. 1996. Pharmacological Methods in Phytotherapy Research, Vol. 1. Selection, Preparation and Pharmaco- logical Evaluation of Plant Material. John Wiley and Sons, Chiches- ter.
[19] Windig W. 1997. Chem. Intell. Lab. Syst. 36, 3. W. Windig, Spectral Data Files for Self Modeling Curve Resolution with Examples Using the SIMPLISMA Approach,Chemometrics and Intelligent Laboratory Systems, 36, 1997, 3-16.
[20] Wolfender JL, Maillard MP, Hostettmann K. 1993. Liquid chromato- graphic thermospray mass spectrometric analysis of crude plant ex- tracts containing phenolic and terpene glycosides, J. Chromatogr., 647,
183-190.
[21] Xie PS. 2001. A feasible strategy for applying chromatography fin- gerprint to assess quality of Chinese herbal medicine. Tradit. Chin. Drug Res. Clin. Pharm. 2001, 12 (3), 141-169.
[22] Yan XJ, Zhou JJ, Xie GR, Milne GWA. 1999. Traditional Chinese Med- icines: Molecular Structures, Natural Sources and Applications, Al- dershot, Ashgate.
IJSER © 2013 http://www.ijser.org
