International Journal of Scientific & Engineering Research, Volume 4, Issue 2, February-2013
ISSN 2229-5518
Prognostic Significance of Microvessel Density in
Breast Cancer of Indian Population
Kamlesh Verma MS, Sandeep Kumar MS,FRCS,PhD, A N Srivastava MD.
ABSTRACT:
INTRODUCTION : Angiogenesis is of key importance in the process of tumor progression in a number of tumor types including breast cancer . In my study I have used anti CD34 antibody to assess intratumoral microvessel density (MVD) in 96 cases to estimate neo-angiogenesis and compared it in various prognostic groups .
OBJECTIVES OF STUDY :To study MVD in malignant breast lumps by immunohistochemistry with CD34 antibody . To compare MVD in different tumor stage , grade and age group. MATERIAL AND METHODS : Part of malignant breast lump removed for tissue biopsy or by mastectomy was used for detection of MVD using antibody against CD34. Counting the number and estimating the density of blood vessels has been used as a measure of angiogenesis . Tumor grading was done as per Nottingham histologic grade and staging was done according to AJCC sixth edition .
RESULTS : Distribution of Mean MVD in stage l ,ll,lll,lV was 20.92,22.37,26.82,29.50 respectively. Distribution of MVD in Nottingham histologic grade l,ll,lll was 20.80,
24.00, 26.71 respectively . Distribution of M.V.D. in pre and postmenopausal group was 27.51 and 21.71 respectively .
CONCLUSION : It may be concluded from this study that higher M.V.D. obtained by immunohistochemistry using anti CD34 antibody , are associated with higher tumor stage and grade and indirectly predict poor prognosis . However larger studies with bigger sample size are required to decide cutoff values between different tumor stages and grades.
KEY WORD : microvessel density ,breast cancer ,Indian population.
INTRODUCTION :
Angiogenesis is the term coined by Folkman [1] in
1971 to identify the complex process leading to the formation of new blood vessels from the pre- existing vascular network. Angiogenesis is a central part of many normal homeostatic processes and nonneoplastic diseases. Regarding malignant neoplasia, it is now evident that tumors have a very limited capacity to grow without vascular support; therefore, formation of blood vasculature is obligatory step to sustain the influx of essential nutrients to the cancer mass. Blood neovascularisation is a complex phenomenon that involves several molecular players and cells. Interaction between stromal and epithelial components is importantly enhanced, and most of the events observed in wound repair are
maintained [2]. We do not understand how the angiogenic activity is initiated by a given tumor, but it is clear that the switch to the angiogenic phenotype demarcates two stages in the development of a tumor — the prevascular phase and the vascular phase.[3,4,5,6]The prevascular phase, which has been elucidated in studies of carcinoma of the cervix, bladder, and breast, may persist for years and is usually associated with limited tumor growth (e.g., restricted thickness of melanoma and few or no metastases. The vascular phase is usually followed by rapid tumor growth, bleeding, and the potential for metastasis. The tumor growth dependency on angiogenesis [7] makes the hypothesis of angiogenesis as a prognosticator attractive .
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 2, February-2013
ISSN 2229-5518
A prognostic factor is defined as a measurement taken at the time of diagnosis or surgery that is associated with outcome ( eg. overall survival , disease free survival or local control ). Prognostic factor generally refer to a patient’s anticipated outcome at the time of diagnosis in the absence of systemic therapy ; however , they are sometimes useful to estimate outcome following a specific systemic therapy . Mathematically , a prognostic factor is demonstrated as a statistically (and clinically ) significant sepration of curves of outcome that are based on the presence or absence of the factor , in a cox proportional hazards regression.
The AJCC staging system is based on established clinical and pathologic prognostic factors and stage itself remains a major prognostic factor. The extent of axillary lymphnode involvement by breast cancer is the most established and reliable prognostic factor for subsequent metaststic disease and survival.Tumor size and histologic grading also have established prognostic significance . Tumor size is typically given as microscopic size invasive cancer
.Histologic grade is best determined by an established methodology , such as the Nottingham combined histologic grading system .Estrogen and Progesterone receptor expression are the most important and useful predictive factor currently available.
Patient age has also consistently been shown to be a prognostic factor . Very young breast cancer patients (35 years or less ) have a poorer prognosis than old patients.The cancers in these patients tend to be higher grade , less often ER/PR positive, and more likely to have lymphovascular invasion than cancers in older patients (8).
The first study to examine intratumoral microvessel density (IMD) immunohistochemically was carried
out by Weidner and colleagues in 1991 using an
antibody against factor Vlll related antigen as an endothelial marker in a series of breast cancers. Since this initial work many antibodies including those against CD31 & CD34 has been used to assess MVD . In present study I have used anti CD34 antibody to study Microvessel Density and compared it in various clinical groups which are established prognostic factors to find out correlation between Microvessel Density and prognosis in carcinoma breast indirectly.
MATERIAL AND METHODS :
The study was based on the complete population of patients with the diagnosis of breast cancer from a certain geographical area . The region was the primary catchment area of King George Medical University, Lucknow, India , where the patients underwent biopsy or surgery from July 2008 to July
2009. The inclusion criteria were patients with a primary , unilateral and operable or nonoperable invasive breast carcinoma . The exclusion criteria were breast cancer patients who have received chemotherapy or radiotherapy , patients with any other co-existing malignancy in present/past , patients with any debilitating disease like AIDS , T.B. etc.,patients with a diagnosis of carcinoma in situ.
Proforma directed clinical history, demographic details and family history of breast cancer patients was taken .Physical findings including general examination and relevant systemic examination was noted. Routine haematological examination , LFT and KFT were done to rule out any systemic disease and get baseline value . Staging analysis
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 2, February-2013
ISSN 2229-5518
was done according to the 2002 TNM classification by American Joint Committee on cancer.
LAB METHODS
1.Cyto and histopathology : Routine
standardized methods were used . Histopathological Grading was done as per Nottingham Classification.
2. Immunohistochemistry: Counting the number and density of microscopic blood vessels in the section of breast cancer specimens has been used as a measure of angiogenesis using antibodies against endothelial markers such as CD34.
Secondary Antibody Kit (NovoLink
Polymer Detection System):
Reagents provided in the Kit include 3% Hydrogen Peroxide, Phosphate-buffered saline with stabilizers, Polymer penetration enhancer containing 10% (v/v) animal serum in tris buffered saline (0.09%), Anti-mouse/rabbit IgG-Poly-HRP (each at 8 µg/ml), DAB Chromogen 1.74% w/v 3,3’- diaminobenzidine, 0.02% Hematoxylin.
Reagents required but not supplied in secondary antibody Kit include Standard solvents used in immunohistochemistry, 50 mM tris buffered saline (TBS), Antigen retrieval solution, Enzyme retrieval solution, Antibody diluents, Primary Antibody,
Mounting medium.
Primary Antibody Kit:
CHAIN POLYMER CONJUGATED TECHNIQUE: Day 1 Procedure : Sections are cut from paraffin embedded tissue blocks. Blocks are fixed in egg albumin coated slides .Slides are kept in hot air oven overnight at 60°C .
Day 2 Procedure : Slides are deparaffinized with
four washes of xylene. Slides are rehydrated by propenol . Slides rinsed in running tap water . Slides were kept in citrate buffer , in the pressure cooker for antigen retrieval for 20-25 minutes. Slides were treated with Hydrogen Peroxide for 5-
10 minutes for elimination of endogenous peroxidase activity. Slides were rinsed in running tap water for 5-10 minutes . Slides were incubated with TRIS buffer saline for 5 minutes. 50 µl of NRS (1:10) was added to block non-specific binding sites and kept for 20 minutes. Primary antibody added with dilution and kept overnight at 4°C in refrigerator .
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 2, February-2013
ISSN 2229-5518
Day 3 Procedure : Slides were incubated with TBS buffer for 5 minutes . After TBS wash, slides were incubated for 1 hour at room temperature . Slides were washed with TBS and incubated with secondary antibody (Polymer linked) for 1 hour. After TBS wash, slides were stained with 3’3’ Diaminobenzedene (DAB) for 2-3 minutes till brown colour developed . Slides were rinsed in running tap water and kept for 10-15 minutes . Slides were counterstained with haematoxylin and rinsed in tap water . Slides were diped in acid alcohol to remove excessive stain and rinsed in tap water. Kept in absolute alcohol followed by Xylene for 15 minutes . Slides were mounted with DPX . SCORING:
The areas of the most intense neovascularization are known as the neovascular hot spots . The assessment of angiogenesis is mainly based on this hot-spot approach, preferentially using the technique of counting microvessel profiles by all immunohistochemically stained distinct endothelial cells or cell clusters in a microscopic field . The three most vascular areas (hot spots) with the highest number of microvessel profiles were chosen subjectively from each tumor section by scanning a tumor section at low magnification (100X) .A higher magnification (400X) was then employed to count individual stained microvessels. Mean count of three areas was used as microvessel count in a tumor section.
The marker of angiogenesis, CD34 was studied
using the indirect histochemical approach . The clone and dilution of the antibodies used are mentioned below :
STATISTICAL ANALYSIS:
The data were statistically analysed using the SPSS statistical software . Two-tailed T test and Mann- Whitney Test were used to assess the association between two quantitative variables . Pearson’s correlation coefficient was used to measure linear association between two variables. Spearman correlation coefficient was used when relationship between two variables was nonlinear . The p value less than 0.05 were considered significant.
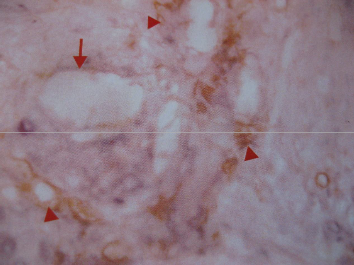
PHOTOMICROGRPH OF ANGIOGENIC PATTERN

Fig. 1: CD34 staining showing native larger vessel as well as tumor MVD. Arrow denotes native large vessel, Arrow head denotes tumor MVD; Hot spot.
Anti- body Used | Antigen Detecte -d | Clone | Dilutio -n | Company |
Anti- CD34 | CD34 | QBEnd/ 10 | 1:40 | Novocast ra |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 2, February-2013
ISSN 2229-5518
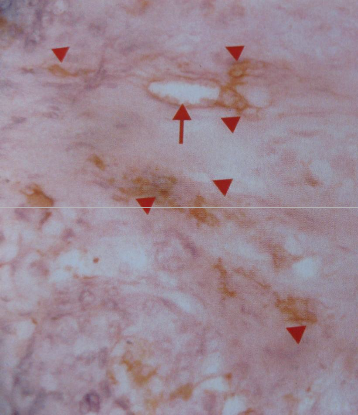
Fig. 2: MVD; Hot spot in case of carcinoma breast. Arrow denotes native large vessel, Arrow head denotes tumor MVD; Hot Spot.
OBSERVATION AND RESULTS:
Microvessel Density was studied in 96 carcinoma breast cases admitted in Department Of General Surgery King George Medical University Lucknow between July 2008
to July 2009 using CD34 antigen as marker of neoangiogenesis..
Characterstics | Number of patients (%) | MVD (Mean±SD) |
Age(years) ::45 >45 Menopausal Status Premenopausal Postmenopausal Tumor Staging Stage l Stage ll Stage lll Stage lV Tumor Grading Grade l Grade ll Grade lll Axillary Lymph Node Status Lymph Node positive Lymph Node negative ER/PR Recepter status Positive Negative | 36(37.50%) 60(62.50%) 41(42.70%) 55(57.29%) 26(27.08%) 32(33.33%) 22(22.92%) 16(16.67%) 30(31.25%) 24(25.00%) 42(43.75%) 56(58.33%) 40(41.67%) 78(81.25%) 18(18.75%) | 28.0±2.619 21.9±3.139 27.51±2.785 21.71±3.213 20.92±2.952 22.37±3.066 26.82±1.435 29.50±2.828 20.80±2.074 24.00±5.779 26.71±1.929 27.45±2.407 21.86±3.575 18.56±2.036 25.49±3.391 |
Table 1: Tumor Characteristics of 96 patients
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 2, February-2013
ISSN 2229-5518
The characterstics and clinicopathological, factors of 96 patients are shown in Table 1. 37.5% patients were in less than 45 year age group whereas
62.50% patients were in more than 45 year age group with mean age of 50.66 years. 42.70% patients were premenopausal and 57.29% patients were postmenopausal.Most of them were operable breast cancer 27.08% stage l and 33.33% stage ll.
22.92% were stage lll (large operable and locally
advanced breast cancer). 16.67% were metastatic breast cancer.31.2% breast cancer were low grade,
25% were intermediate grade, 43.8% were high grade. Axillary Lymph node involvement was present in 41.7% and absent in 58.3% which was determined on the basis of pathological examination of modified radical mastectomy specimen in operable tumor and by clinical examination in locally advanced and metastatic tumor. Estrogen and Progesterone Receptor were positive in 18.8% and negative in 81.2%.
Microvessel count was done in 96 invasive breast cancer cases. In carcinoma breast cases it was in the range of 16 to 32 with a Mean±SD= 24.19±4.178 and median of 24.0. Higher mean microvessel count was found in patients of carcinoma breast with younger age (S 45 yrs.) and in premenopausal age group. The Mean±SD of microvessel density in S45 yrs. age group was 28.0±2.619 whereas it was 21.90±3.139 in ›45 yrs. age group. This difference was statistically significant with p value of 0.0028. The Mean±SD of microvessel density in premenopausal age group was28.0±2.619 whereas it was
21.90±3.139 in ›45 yrs. age group. This difference was statistically significant ( p value = 0.000).
Higher mean microvessel count was found in patients of carcinoma breast with increase in TNM stage of tumor. Comparing MVD in various stage
groups , difference in MVD in stage l and stage lll
& lV was statistically significant (p value =0.000) . In the same way difference between stage ll and stage lll& lV was also significant ( p value=0.000). Similarly difference between MVD of stage lll and lV was significant ( p value =.020). But difference between MVD of stage l & ll was not significant ( p value= 0.269).
Poorly differentiated and undifferentiated tumors were associated with higher microvessel density in comparison to well differentiated tumor. On multiple comparison difference between MVD in Grade l, ll and lll tumor was statistically significant ( p valueS .05).
Mean ± SD of lymphnode positive and negative tumor was 27.45± 2.407 and 21.86± 3.575 respectively. Difference of which was statistically significant on 2 tailed T test and Mann Whitney test (p value=0.000).
Mean ± SD of ER/PR positive and negative tumor was18.56± 2.036 and 25.49± 3.391 respectively. Difference of which was statistically significant on
2 tailed T test and Mann Whitney test (p value of
0.000).
DISCUSSION:
Different median values of MVD have been reported in different studies on breast cancer. In an overall analysis of published data, the highest concentration of about 100 (Weidner et al.,
1992;Vartanian and Weidner, 1994; Morphopouloset al.,1996) or more (Costello et al.,1995) microvessels per mm 2 was reported when relatively large areas of tumor were scored, and very low values were recorded when small areas were scored (Foxet al.,1994; Hallet al.,1992). Intermediate values were observed regardless of the area extension analyzed. This indicates how difficult it is to make MVD evaluation reproducible. Various endothelial markers has been used for immunohistochemical staining. In his first study (1992) relating MVD to survival, Weiner et al. used an antibody against factor Vlll related antigen , also termed von willebrand’s factor, staining mainly
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 2, February-2013
ISSN 2229-5518
mature vessels. Some studies used antibodies directed against platelet endothelial cell adhesion molecule (also known as CD31) or CD34. JC-70, a monoclonal antibody against CD31, has the advantage over F.Vlll Ag of being present also on immature blood vessels. Consequently, counts using this marker are 30% higher than those using F. Vlll Ag. However, CD31 can react mildly with fibroblasts and some plasma cells, and staining failure can reach 20% in routinely fixed breast specimens.CD34 has the same characterstics as CD31, but without high rate of staining failure (9).The optimal marker has not been identified yet. A study by Martin L. compared F. Vlll Ag , CD31, and CD34, and was positive only for CD34 (10).
A large number of studies have examined the
prognostic utility of MVD counts in a variety of human tumors, including breast cancer. In general, an inverse relationship has been observed between MVD counts in primary breast cancer and disease free survival and overall survival. However, not all studies have demonstrated this association. Uzzan et al. performed a meta-analysis of all 87 published studies linking intratumoral microvessel density (MVD), reflecting angiogenesis, to relapse free survival (RFS) and overall survival (OS). High MVD significantly predicted poor survival [ RR =
1.54 for RFS and OS with the same 95% confidence
interval (CI) , 1.29 -1.84]. Twenty-two studies (4779 patients) found an inverse relationship between survival and MVD ( so called positive studies), whereas 21 studies (4157 patients) found no relation ( negative studies). Immunostaining was obtained by use of antibodies directed against F. Vlll Ag in 27 studies, CD31in 10 studies and CD34 in 8 studies. When pooling the results from CD31 and CD34, the proportion of positive studies was what higher compared with that of F. Vlll Ag(11).
In the current study , the authers have examined angiogenesis using MVD in patients who had invasive breast cancer using antibodies directed against CD34. MVD correlated significantly with established prognostic facturs like age, axillary lymphnode status, histopathological grade, Estrogen Progesterone receptor status. This study was done exclusively in women of Indian origin.
Thus it may be concluded from the present study that higher microvessel count obtained by immunohistochemistry using antibody against CD34 , are associated with higher tumor stage and grade ,and indirectly predict poor prognosis in Indian Population. However, larger studies with bigger sample size are required to decide cut off values between different tumor stages and grades.
In the present study it has been tried to estimate prognosis indirectly by analyzing microvessel density in different established prognostic markers. For establishing microvessel density as prognostic marker, long term followup studies and calculation of overall survival and relapse free survival is needed.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 2, February-2013
ISSN 2229-5518
REFERENCE :
1. . Folkman J: Tumor angiogenesis:
therapeutic implications. N Engl J Med
285:1182-1186, 1971
![]()
2. R. S. Kerbel, “Tumor angiogenesis,” The New England Journal of Medicine, vol. 358, no. 19, pp. 2039–2049, 2008 3
3 Folkman J. Angiogenesis. In: Verstraete M, Vermylen J, Lijnan R, Arnout J, eds. Thrombosis and haemostasis. Leuven,

1987:583—96
4 Sillman F, Boyce J, Fruchter R. The significance of atypical vessels and neovascularization in cervical neoplasia
. Am J Obstet Gynecol 1981; 139: 154–9
5 Chodak GW, Haudenschild C, Gittes RF, Folkman J. Angiogenic activity as a marker of neoplastic and of preneoplastic lesions of the human bladder . Ann Surg 1980; 192:762–71
7. Folkman, J. Tumor angiogenesis. In: J. Mendelsohn, P. M. Howley,
M. A. Israel, and L. A. Liotta (eds.), The
Molecular Basis of Cancer, pp.
206–232. Philadelphia: W. B. Saunders,
19954.
8.Devita,Hellman and Rosenberg’s Principle and Practice Of Oncology. Ninth edition page no.1427.
9. Leek RD. The prognostic role of angiogenesis in breast cancer. Anticancer
2001;21:4325–32.
10. Martin L, Green B, Renshaw C, et al. Examining the technique of angiogenesis assessment in invasive breast cancer. Br J Cancer 1997;76:1046–54.
11. Uzzan B, Nicolas P, Cucherat M, Perret GY. Micro-vessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta analysis. Cancer Res 2004; 64:2941-
55.
6 Jensen HM, Chen I, DeVault MR, Lewis ![]()
AR. Angiogenesis induced by "normal"
human breast tissue: a probable marker for precancer . Science 1982; 218:293–5
1. Kamlesh Verma MS , Clinical fellow Tata Memorial Centre, Mumbai, India, e mail:
Corresponding Author:
Kamlesh Verma MS , Clinical fellow , Tata Memorial Centre, Mumbai, India, e mail:
IJSER © 2013 http://www.ijser.org