International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 66
ISSN 2229-5518
Production of Activated Carbon from Watermelon
Peel
Gin, W.A., Jimoh, A.*, Abdulkareem, A.S., Giwa, A.
Department of Chemical Engineering, School of Engineering and Engineering Technology, Federal University of Technology, Minna, Nigeria
*Corresponding author; E-mail: fatai2011@futminna.edu.ng
Abstract— Watermelon peel is one of the several unwanted by-products generated by restaurants, fruit juice producers and food industries in Nigeria. A great quantity of this waste is got rid of indiscriminately into the environment thereby causing pollution one way or the other. In this study, instead of discharging them into the environment where they normally negatively affect the environment, watermelon peels have been carbonized at different temperatures between 200 to 350 oC for 15, 30, 45 and 60 min for the determination of optimum conditions for pre-treatment. The chemical treatment of the resulting carbon produced using the obtained optimum conditions was carried out using different concentrations of H 2 SO4 , HCl and ZnCl 2 (from 0.5 to 1.5 M). The results obtained revealed that the optimum
carbonization temperature was 300 oC while the time was 60 min. Moreover, the best watermelon peel activated carbon that produced the
highest percentage reduction of the heavy metals considered was the one treated with 1.0 M sulphuric acid. Therefore, it has been discovered that local agricultural wastes like watermelon peels should always be considered worthwhile raw materials for the production of high quality materials one of which is activated carbon that is very useful industrially.
Index Terms— Watermelon peels, activated carbon, carbonization temperature, carbonization time.
—————————— ——————————
1 INTRODUCTION
iverse types of fruits are eaten in Nigeria daily. This is because they supply the body with the needed vitamins, minerals and fibres. However, for most fruits, only the fleshy pulps of these fruits are consumed leaving behind the seeds and the peels. This result to great amount of agricultural wastes (peels) generated and discarded. Getting rid of these peels can have serious environmental impact that has been very difficult to solve. Therefore, there is the need for an in- tensified research in the development of the possible nutri-
tional and industrial potential of fruit wastes.
In Nigeria, watermelon peel is generated from restau-
rants, small scale fruit juice producers, fruit sellers, food bev-
erages processing lines and these wastes are not much being
reused. In fact, the value of watermelon peel is not recognized at present due to limited investigation into on how the waste can be converted into a more useful form, instead of being dumped as a solid waste to the environment.
Although most of the studies on watermelon fruits
have focused on the anti-nutritional (Johnson et al., 2012), phy- tochemical and anti-oxidant properties (Oseni et al., 2013) of the fruit juice, little has been done on the nutritional/quality (proximate) contents (Fila et al., 2013) of the peels which could encourage their consumptions or further use. For example, in Malaysia, watermelon peels have been analyzed and re- utilized as potential raw materials for production of jam (Mo- hamad et al., 2012). Sensory evaluation of the jam prepared from watermelon peels with different flavors was found to possess acceptable physical, chemical and rheological proper- ties.
Moreover, watermelon peels chemically contain large
amount of water with promising levels of solid matters (Mo-
hamad et al., 2012), which further makes them worthwhile to be considered for industrial production of high quality acti- vated carbon. This novel use of watermelon peels will not only minimize the wastes being discarded, but also create more income for farmers, food processors and more importantly reduce many negative environmental impacts.
The area of concern in this study is, therefore, on in-
vestigating the optimum conditions required for the pre-
treatment of watermelon peel wastes in order to make them suitable as adsorbents for heavy metal removals from indus- trial effluents. This study looked at the influence of operating parameters such as carbonization temperature and carboniza- tion time. Apart from that, chemical modifications of the re- sulting carbons were subsequently carried out using HCl, ZnCl2 and H2 SO4 at different concentrations. The developed and selected adsorbent was characterized for proximate and FT-IR analyses to know the functional groups present in it.
2 MATERIAL AND METHODS
2.1 Fruit Waste Collection and Preparation
The major raw material used in this research work was watermelon peels that were obtained from different fruit seller locations in Minna, Niger State.
Analytically graded reagents used were activating
agents like zinc chloride (ZnCl2 - 98.00%), sulphuric acid
(H2 SO4 - 98.0%) and hydrochloric acid (HCl - 36.0%).
While preparing for the carbonization experiments,
the watermelon peel samples gathered were sun-dried for 5-
7days to drastically reduce their moisture contents before they
were then crushed with a mortar and pestle to reduce their
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 67
ISSN 2229-5518
sizes.
2.2 Carbonization of Watermelon Peel
In the course of the carbonization experiments, the dried watermelon peels were crushed into powder form and
15 g of the powdered samples was weighed into six different clean and pre-weighed crucibles, which were then introduced into the hot zone of a muffle furnace. The peels were carbon- ized at different temperatures (250, 300, 350 and 400 oC). The samples were held at each of the temperatures for various times (15, 30, 45 and 60 min) in order to establish the optimum conditions for the process. The content was then removed from the muffle furnace after the set period and cooled in an open air for one hour. This process was repeated until a sub- stantial amount of carbonized sample was obtained. The car- bonaceous materials produced at different temperatures and time were then characterized.
2.3 Chemical Modification of Carbonized Materials
The activations of the carbonaceous materials pro- duced using zinc chloride and hydrochloric acid were carried out in accordance with the description reported in the work of Yalc et al. (2000) while the chemical activations using sulphuric acid of various concentrations were accomplished by employ- ing the method adopted by Kobya et al. (2005).
2.4 Proximate Analysis of Carbon Produced
The activated material produced was subjected to characterization in order to test it for properties like fixed car- bon, bulk density, ash content, volatile content and moisture content. The fixed carbon content was determined in accord- ance using the procedure of ASTM (2001) while the volatile
content, ash content, fixed carbon were conducted with the procedure laid down by the Association of Analytical Chemis- try (AOAC) (1994).
The FTIR spectra of the developed sample was rec-
orded with an FTIR spectrophotometer Leo Supra 50vp model using potassium bromide (KBr) pellet method before and after adsorption of heavy metals using the activated carbon. The standard values of the physicochemical properties of the ad- sorbent were used as references to judge the characteristics of the carbon for use for industrial effluent treatments.
3 RESULTS AND DISCUSSIONS
3.1 Effect of Carbonization Temperature and Time on
Carbon
The influences of carbonization temperature and time on fixed carbon content, charcoal yield, moisture content, volatile content and ash content were studied for watermelon peels, in the course of preparing the activated carbon from the peels, and the results obtained are as presented in Tables 1 and
2.
From Table 1 that is showing the variations of the pa- rameters (fixed carbon content, charcoal yield, moisture con- tent, volatile content and ash content) with changes in the car- bonization temperature, it can be seen that, using constant carbonization time of 15 min and constant sample mass of 5 g, all the parameters investigated were found to actually vary as the carbonization temperature was also varied. This has re- vealed the dependence of the parameters considered on the carbonization temperature of the process.
Table 1: Measured parameters of peel of watermelon material carbonized at different temperatures
S/N | Temperature | Time (min) | Mass (g) | Ash Content (%) | Volatile content (%) | Moisture Content (%) | Fixed Carbon (%) | Charcoal Yield (%) |
1 | 200 | 15 | 5 | 18.6 | 34.0 | 2.0 | 47.4 | 50.0 |
2 | 250 | 15 | 5 | 21.4 | 28.6 | 1.0 | 50.0 | 43.3 |
3 | 300 | 15 | 5 | 26.2 | 22.9 | 1.9 | 50.9 | 33.3 |
4 | 350 | 15 | 5 | 28.6 | 20.4 | 2.0 | 50.8 | 33.0 |
Shown in Table 2 are the responses from the parame-
ters investigated as the carbonization time was varied, but, this time around, keeping the carbonization temperature constant at 300 oC and the mass of the sample used for all the runs was also constant at 5 g. The effects of the carbonization time on the characteristics of the developed watermelon peel activated carbon are as given in Table 2. Similar to the observations no-
ticed in the case of varying the carbonization temperature, it
has been discovered in this case also that the considered pa- rameters were found to respond to changes in the carboniza- tion time of the process. This was an indication that the car- bonization time of the process was also among the important factors affecting this process.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 68
ISSN 2229-5518
Table 2: Measured parameters of peel of watermelon material carbonized for various times
S/N | Temperature | Time (min) | Mass (g) | Ash Content (%) | Volatile Content (%) | Moisture Content (%) | Fixed Carbon (%) | Charcoal Yield (%) |
1 | 300 | 15 | 5 | 26.2 | 22.9 | 1.9 | 50.9 | 33.3 |
2 | 300 | 30 | 5 | 27.5 | 19.5 | 2.5 | 53.0 | 33.0 |
3 | 300 | 45 | 5 | 27.8 | 12.4 | 2.9 | 59.8 | 32.4 |
4 | 300 | 60 | 5 | 26.2 | 12.2 | 3.5 | 61.6 | 32.1 |
As can be seen in Table 2, increasing the carbonization
time was found to reduce the quantity of the carbonized product (charcoal yield) obtained from the watermelon peels. This was found to be due to the excessive burning/oxidation and collapse of the pore structures of the peels which was found to predominate at long carbonization time.
From the results of the watermelon peel carbonization presented in Tables 1 and 2, it was discovered that maximum percentage fixed carbons were obtained when the carboniza- tion temperature and the carbonization time were 300 oC and
60 min, respectively. As such, these temperature (300 oC) and
time (60 min) were selected as the optimum conditions for the process.
3.2 Effect of Concentration of Activating Agents on Ad- sorption of Heavy Metals
Watermelon peel carbon obtained at optimum per- centage fixed carbon content was activated with different con- centrations of H2 SO 4 , HCl and ZnCl2 (0.5, 1.0 and 1.5 M). The resulting watermelon peel activated carbons (WPACs) were then used to remove heavy metals from the effluent acquired from an electroplating processing industry. The results of the overall percentage reduction of heavy metals using the wa- termelon peel activated carbon are as given in Tables 3, 4 and 5 for HCl, H2 SO4 and ZnCl2 treated carbons, respectively.

Table 3: Overall percentage reduction of heavy metals using HCl treated WPAC
WPAC
WPAC
WPAC
S/N Heavy metals

(0.5M HCL)
(1.0M HCL)
(1.5M HCL)

% Reduction % Reduction % Reduction
1 Zinc 7.9 2.8 2.6
2 Copper 91.5 94.3 31.0
3 Iron 95.1 95.1 81.9

4 Lead 100.0 100.0 100

Table 4: Overall percentage reduction of heavy metals using H2 SO4 treated WPAC
S/N Heavy metals
WPAC

(0.5 M H2 SO4 )
WPAC
(1.0 M H2 SO4 )
WPAC
(1.5 M H2 SO4 )

% Reduction % Reduction % Reduction
1 Zinc 12.9 1.4 2.0
2 Copper 96.8 99.1 16.1
3 Iron 60.8 90.6 78.8

4 Lead 40.0 100.0 100.0
Table 5: Overall percentage reduction of heavy metals using ZnCl2 treated WPAC

S/N Heavy Metals WPAC WPAC WPAC

IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 69

ISSN 2229-5518

(0.5 M ZnCl2 ) (1.0 M ZnCl2 ) (1.5 M ZnCl2 )

% Reduction % Reduction % Reduction
1 Zinc 2.3 2.6 2.6
2 Copper 50.6 10.3 35.6
3 Iron 88.0 25.3 67.5

4 Lead 100.0 0.0 20.0
From the results obtained, it was discovered that that the activated carbon treated with 1.0 M sulphuric acid showed
the best performance in terms of heavy metal removal from the industrial effluent used compared to those treated with zinc chloride and hydrochloric acid. This was an indication that the pore surface and the structure of the 1.0 M H2 SO4 treated watermelon activated carbon were the best among all the treated watermelon activated carbons.
3.3 Characterizations of Produced Activated Carbon
Material
It can be seen from this study that a carbonization temperature and time of 300 oC and 60 min respectively have been found to be appropriate for preparing high quality wa-
termelon peel carbon (WPC) with high fixed content. Further, the carbonaceous material obtained using the chosen optimum
conditions was used for subsequent chemical activation. Treat- ing the watermelon peel carbon prepared with the optimum conditions with different chemicals of different concentrations, it was also discovered that the best heavy metal reductions were obtained with the watermelon peel activated carbon (WPAC) treated with 1.0 M sulphuric acid. This activated car- bon was then later subjected to other characterizations. Given in Table 6 are the results obtained from the characterizations of the developed activated carbon from the watermelon peel used.

Table 6: Characterization of WPAC obtained at optimum percentage fixed carbon

S/N | Properties | Unit | WPAC at 300 oC, 60 min with 1.0 M H2 SO4 | Literature value |
1. | Moisture Content | % | 1.4 | 2-8 |
2. | Ash Content | % | 18.6 | ≤ 8 |
3. | Volatile Content | % | 10.3 | < 20 |
4. | Fixed Carbon | % | 66.0 | >75 |
5. | Pore Volume | cm3 | 0.93 | 1.109 |
6 | Porosity | mL/g | 0.204 | 0.214 |
7. | Bulk Density | g/cm3 | 0.25 | 0.4-0.5 |
8. | Charcoal Yield | % | 34.0 | 39.99- 55.44 |

It was indicated in the results presented in Table 6 that the moisture content of the activated carbon form water- melon peel obtained was 1.4%, which was not within the
range 2-8% recommended by Tchobanoglous et al. (2002). However, according to Jabit (2007), for many purposes, mois- ture content does not really affect the adsorptive power of ac- tivated carbon. For the volatile content of the WPAC as shown in Table 6, the value obtained was 10.3%, and it was found to be within the range specified by the British Standard as re- ported by Paddon (1987). Also shown in Table 6 is the fixed carbon content (the non-volatile fraction of the sample) of WPAC that was found in this work to be 66.0% for the devel- oped activated carbon, as against the recommended range (>75) required by the British Standard as reported also by Paddon (1987). The carbon yield of the developed activated carbon was also obtained to be 34.0%. This value of the carbon
yield of the activated carbon was discovered to be close to the finding of Savova (2001) who reported carbon yield of 39.99 to
55.44 % for most activated carbons.
3.4 FT-IR Analysis of Produced Activated Carbon
The FT-IR spectra of the developed watermelon peel activated carbon before and after adsorption of metal ions were carried out as a qualitative analysis to gain better in- sights into the surface functional groups available on the sur- face of the investigated adsorbent because the chemical struc- ture of an adsorbent is of vital importance in understanding its adsorption nature. The FT-IR spectra of the watermelon peel activated carbon (WPAC) measured within the range of 500 –
4500 cm−1 recorded before and after adsorption are as shown
in Figures 1 and 2, respectively.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 70
ISSN 2229-5518
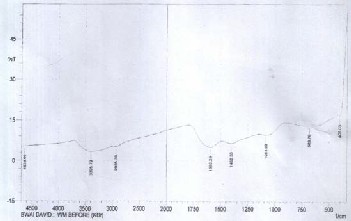
In addition, new bands were discovered to emerge at 3796.04,
4359.27 and 4449.93 cm-1 in the spectrum which were due to
the changes in the nature of the binding after the interaction of the activated carbon with the metal ions of the effluent. The new band at 3796.04 cm-1 was found to be associated with hy- droxylated compounds while the bands observed at 4359.27 and 4449.93 cm-1 were overtones or combinations of the fun- damental stretching bands which were found to occur in the
3000-1700 cm-1 region, according to Weyer and Lo, 2002. The
bands above 4000 cm-1 were found to be overlapped, which made them less useful for qualitative analysis. However, based on the information obtained from the work of Stuart (2004),
Figure 1: FT-IR spectrum for activated carbon from watermel-
the bands involved were usually C − H , stretching.
N − H or
O − H
on peel before adsorption
The FT-IR spectra of unloaded WPAC shown in Fig- ure 1 was discovered to show a band at 3395.79 cm-1, which
The FT-IR spectra obtained for the developed water- melon peel activated carbon were found to be similar to the ones reported for the studies carried out on commercial granu- lar activated carbons by Park and Jang (2002) and activated
was due to O − H
stretching of water. The band observed at
carbon made from cherry stones by Olivares (2006). The band
2935.76 cm-1 was found to correspond to methylene asymmet-
in the region of approximately 1600 cm-1 has been noticed by
ric, C − H
stretching. The band given at 1592.29 cm-1 was an
many previous researchers but has not been definitely inter-
indication of the presence of pyridine,
C = N
stretching
preted. However, for most carbonaceous materials, C=C
while the band at 1402.3 cm-1 was ascribed to azo compound,
stretching adsorption was found to frequently occurs at this
N = N
stretching. Finally the band at 1084.03 cm-1 was found
region.
to be due to aliphatic
C − N
stretching while the band at
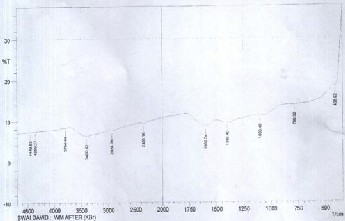
683.79 cm-1 was due to P = S stretching.
Figure 2: FT-IR spectrum for watermelon peel activated carbon after adsorption
As observed from Figure 2, after the adsorption of the metal ions present in the effluent by the developed activated carbon, the FT-IR spectra was found to show shifts in some of the bands, and there were also appearances of new bands. For instance, the bands at 406.03, 683.79, 1084.03, 1403, 1592.29,
2935.76, and 3395.79 cm-1 were shifted to 408.92, 786.02,
1099.46, 1399.4, 1599.04, 2934.79 and 3400.62 cm-1, respectively.
The shifts in the bands confirmed the participations of the functional groups in the adsorption of the metal ions of the effluent onto the developed activated watermelon peel carbon.
4 CONCLUSIONS
The results obtained from this work that was carried out to develop an adsorbent from watermelon peel for use in wastewater treatment, specifically for the adsorption of heavy metals, have revealed that the optimum carbonization temper- ature and time for the preparation of watermelon peel activated carbon with high fixed content were 300 oC and 60 min, respectively. In addition, the watermelon peel activated carbon treated with 1.0 M sulphuric acid was found to be the best among the treated ones because it has relatively the highest percentage reduction of the heavy metals investigated in this work.
NOMENCLATURES
AOAC Association of Analytical Chemistry
ASTM American Society for Testing and Materials
WPAC Watermelon peel activated carbon
WPC Watermelon peel carbon
REFERENCES
[1] AOAC (Association of Official Analytical Chemists). (1994). Official methods of analysis, 14th Ed. Association of Official Analytic Chemists, Washington DC.
[2] ASTM (2001). Activated carbon standards. American Soci-
ety for Testing and Materials. Online. Fritz Publication. Http: //www.Fritz.Com.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 71
ISSN 2229-5518
[3] Fila, W.A., Ifam, E.H, Johnson, J.T, Odey, M.O., Effiong, E.E., Dasofunjo, K., and Ambo, E.E (2013). Comparative proximate compositions of watermelon Citrullus Lanatus, Squash cucurbita pepo’l and Rambutan, Nephelium Lap- paceun. International Journal of Science and Technology, 2(1),
81-88.
[4] Park, S.J., and Jang, Y.S. (2002). Pore structure and surface
properties of chemically modified activated carbon for ad- sorption mechanism and rate of Cr(VI). Journal of Colloid and Interface Science, 249(2), 458-463.
[5] Johnson J.T., Iwang E.U., Hemen J.T., Odey, M.O., Effiong,
E.E., and Eteng, O.E, (2012). Evaluation of anti-nutritional
content of watermelon Citrullus lanatus. Annals of Biologi- cal Research, 3(11), 5145-5150
[6] Kobya, M., Demirbas, E., Senturk, E., and Ince, M. (2005).
Adsorption of heavy metal ions from aqueous solutions
by activated carbon prepared from apricot stone. Biore-
source Technology, 96, 1518-1521.
[7] Jabit, N.B. (2007). The production and characterization of
activated carbon using local agricultural waste through chemical activation process: Thesis submitted in fulfillment of the requirements for the degree of Master of Science, Univer- siti Sains Malaysia, Penang, Malaysia.
[8] Olivares, M. (2006). Preparation of activated carbons from
cherry stones by activation with potassium hydroxide.
Applied Surface Science, 252 (17), 5980-5983.
[9] Oseni, O.A., and Okoye, V.I. (2013). Studies of phyto-
chemical and anti-oxidant properties of the fruit of wa- termelon (Citrullis Lanatus), Journal of Pharmaceutical and biomedical Science, 27(27), 508-514.
[10] Paddon, A. (1987). Review of the available data concern-
ing the amount of charcoal and fuelwood in Sudan. Field
Project Document No.22. FAO, Khartoum, 1987.
[11] Savova, D. (2001). Biomass conversion to carbon adsor-
bents and gas. Biomass and Bioenergy, 21, 133-142.
[12] Mohamad, S.A., Saheed, O.K., and Jamal, P. (2012). Physi-
co-chemical analysis of jam preparation from watermelon
waste. International Conference on Chemical, Environ- mental and Biological Sciences (ICCEBS'2012) Penang, Malaysia, 74-77.
[13] Stuart, B. (2004). Spectral analysis. In Infrared Spectroscopy:
Fundamentals and Applications. John Wiley & Sons, Ltd.
[14] Tchobanoglous, G., Burton, F.L., and Stensel, H.D. (2002).
Waste water engineering, treatment and re-use. 6th Ed.
John Wiley.
[15] Weyer, L.G., and Lo, S.C. (2002). Spectra-structure correla-
tions in the near-infrared. In Handbook of Vibrational Spec-
troscopy. Wiley, United Kingdom.
[16] Yalc, N., and Sevinc, V. (2000). Studies of the surface area
and porosity of activated carbons prepared from rice husks. Carbon, 38, 1943–1945.
IJSER © 2014 http://www.ijser.org

