The research paper published by IJSER journal is about Production and Application of Keratin-Based Organic Fertilizer from Microbially Hydrolyzed Feathers to cowpea 1
ISSN 2229-5518
Production and Application of Keratin-Based Organic Fertilizer from Microbially Hydrolyzed Feathers to cowpea (Vigna unguiculata).
* Adetunji C. O1 , Makanjuola O.R1 ,Arowora K.A1, Afolayan S.S1 , Adetunji J. B 2
Abstract— This experiment was carried out using potted plants to assess the effect of keratin-based organic fertilizer produced from microbially hydrolysed feathers on cowpea’s. The fungus isolated and used for the hydrolysis of feather during the production of organic fertilizer was identified as Aspergillus niger. The results showed that the organic fertilizer was very effective on plants with soils amended with the hydrolysed feathers, as the plants exhibited better yields and productivity. Data for plant height (cm), weight of plant, number of leaves per plant and number of pods per plant were taken at weekly interval for a period of 9 weeks.. The various treatments used were 1kg/10kg of soil, 2kg/10kg of soil, 3kg/10kg of soil while 0kg/10kg served as control. Based on observation, the treated plants were less susceptibile to diseases compared to the non-treated plants. It was revealed that organic fertilizers application rate of 3kg/10kg had the highest performance in terms of growth and yield, and least susceptibility to diseases, the converse was observed for control. The result of this study was indicative that feather which is cheap, readily available and environmentally friendly offers a promising prospect in agriculture both as an organic fertilizer and in the control of disease in cowpea if applied at recommended rates and time.
Index Terms—Organic farming , Keratin, Aspergillus niger , bioreactor , Vigna unguiculata
—————————— ——————————
1 INTRODUCTION
Organic farming is a form of agriculture which excludes the use of synthetic fertilizers and pesticides, plant growth regulators and livestock feed additives [6].Organic farmers depend on crop rotation, crop residues, animal manures and
----------------------------------
1Nigerian Stored Product Research Institute, Km 3 Asa dam road, P.M.B. 1489, Ilorin, Nigeria. Email: charliguitar@yahoo.com
2University of Ilorin, Department of Biochemistry, P.M.B. 1515,
Ilorin, Kwara State.
mechanical cultivation to maintain soil productivity and to control weeds, insects and other pests [4]. The role of organic agriculture, either farming processing, distribution, consumption is to sustain and enhance the health of ecosystem and organism [17]. Approximately
31 million hectares (75 million acres) worldwide
are grown organically (IFOAM) “International
organic farming organization."
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Production and Application of Keratin-Based Organic Fertilizer from Microbially Hydrolyzed Feathers to cowpea 2
ISSN 2229-5518
Keratin is insoluble structural protein and difficult to digest by humans and animals, this protein is resistant to degradation
by common proteolytic enzymes such as trypsin, pepsin and papain due to the composition and molecular conformation of the amino acids found in keratin [9] ,[1]. However, some microorganisms are able to degrade it. Keratin can be degraded by some species of saprophytic and parasitic fungi [15], [2], [12] a few actinomyces [3] and Bacillus species. The mechanical stability of keratin and its resistance to microbial degradation depend on the tight packing of the protein chain in α-helix (α-keratin) and β -sheet (β-keratin) structures and their linkage by cystine bridges due to high degree of cross-linkage by disulfide bonds, hydrogen bonding and hydrophobic interactions. Despite the recalcitrant character of keratins, diverse bacteria, actinomycetes, and fungi have been identified and reported to degrade and utilize keratin because they produce keratinolytic enzymes [9], [5].
The protein rich concentrate feather meal
generated for poultry feed can also be applied for organic farming as a semi slow-released fertilizer [11], [7]. Organic farming relies on the use of nitrogen-rich organic amendment that serve the dual purpose of improving plant growth and
intensifying microbial activity in soil, guano has
been widely used as fertilizer in organic farming [11]. However, owing to high expenses, there is a need to search for more suitable alternatives. Feather meal being nitrogen-rich (15%N), inexpensive and readily available sources as a potential substitute to guano. The microbially hydrolysed feather meal can further edge over the steamed meal as fertilizer due to its high nutritive value, easy production and economic feasibility.
Cowpea is a leguminous crop grown through the
African continent as well as in parts of south East Asia and Latin America. The aim of this experiment was to produce a Keratin-Based Organic Fertilizer from Microbial Hydrolysis of Feathers and its application to Cowpea.
2. Materials and Methods
2.1 Sources of Keratin
Feathers, the keratin carbon and nitrogen source used in this study were obtained from a commercial poultry farm. (Tuns Farms) in, Oshogbo, Osun State, Nigeria. The feathers were thoroughly washed to remove dirt and blood stains, sun-dried for several days, and then oven- dried at 75°C for 8 hours. The dried feathers were then grinded using a ball mills. Standard sieve of
60 mesh particle size was used to obtain very fine
feather substrate. The powders were kept at room temperature and used for further studies.
2.2 Source of Cowpea
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Production and Application of Keratin-Based Organic Fertilizer from Microbially Hydrolyzed Feathers to cowpea 3
ISSN 2229-5518
The cowpea used for this experiment was obtained from federal ministry of Agriculture
,Ilorin, kwara state Nigeria.
2.3 Isolation of Microorganisms
Feather-degrading fungus was isolated from the soil samples originally from the same site where the keratin substrates were obtained. The soil samples were collected in a sterile polythene bags. Minimal medium was prepared as follows (g/l): NaNO3, 2; NaCl, 2; KH2PO4, 2; MgSO4 0.05; FeSO4.7H2O, 0.1; CaCO3, 0.1; keratin substrate,
20; and agar-agar 20. The medium was sterilized at 121°C for 15 minutes. The medium was supplemented with 0.05g/l of sterile tetracycline to inhibit the growth of bacteria. 1g of soil samples was dispensed in 9ml of distilled water. About 0.2ml of the aliquot was used to inoculate the minimal medium for the selective growth of the isolate using the pour plate method. The plates were labeled and incubated for 3days. Distinct colonies observed using morphological features were selected, isolated and purified using yeast extract agar to obtain pure cultures. The pure cultures were stored on agar slant of yeast extract agar and stored at 4°C.
2.4 Inoculum Development
Inoculum was developed by transferring loopful of inoculum into the prepared inoculum medium (0.2% yeast extract, 1% feather substrate, pH 6.0).
25ml of the isolated was dispensed in a 150ml capacity bottle. Incubation was done at (30± 1°C) on a thermostatic shaking water bath at 100rpm for 24 hours.
2.5 Characterization of pure cultures of the
microorganism
Fungal identification was carried out according to the procedure described [18].
2.6 Preparation of Microbially Hydrolysed
Feathers
Whole feathers were microbially hydrolysed in a bioreactor containing sterilized the basal medium (g/3l): NaNO3 6; NaCl, 6; CaCO3, 0.3; KH2PO4, 2; MgSO4, 0.15; FeSO4.7H2O, 0.03; feather meal 60, pH 5.7. An inoculum size of 15% (v/v) of a
24hour-old culture was used to inoculate the basal medium, and incubation was carried out at room temperature (30±2°C). Aeration was supplied to the bioreactor to allow agitation and hydrolysis was allowed to take place for 7 days. After hydrolysis, feathers were dried in the oven for 2 days at 70 °C and ground to a particle size
1mm. These products were designated as
microbially hydrolysed feathers.
2.7 Experimental design and plant propagation Experiments were conducted in Nigerian stored product research institute, using potted plants to assess the effects of an organic fertilizer on
Cowpea (Vigna unguiculata).
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Production and Application of Keratin-Based Organic Fertilizer from Microbially Hydrolyzed Feathers to cowpea 4
ISSN 2229-5518
Three seeds of cowpea were sown per 10-liter (50cm diameter) plastic pot filled with sandy- loamy soil. Prior to potting, however, the soil was steam-sterilised at 121oC for 1hour. The plants were thinned down to two per pot after germination. The microbially hydrolysed feather powder was applied to the soil at 1kg/10kg soil,
2kg/10kg soil and 3kg/10kg soil and labeled treatment A, B and C respectively while a non- application rate of 0kg/10kg soil served as the control. The application of the microbially hydrolysed feather substrate to the soil was carried out at the 6th day of planting. Each treatment was replicates. Data were collected from the first week of germination of cowpea for the following parameters:
2.7.1 Plant height
The plant height was obtained by placing the
ruler at the base of each plant (ground level) to the terminal bud in cm at weekly intervals after planting.
2.7.2 Number of leaves
This was taken by mere counting of the leaves
per plant.
2.7.3 Number of pods per plant
The number of pods that appeared on each plant was counted, the average was recorded and this represented the number of pods per plant.
2.7.4 Weight of cowpea seeds
The pods were dried and weighed with the aid of
an electronic balance. Threshing was done manually before weighing to get the dried seed weights in gram.
2.7.5 Disease severity
Disease severity was rated using a modified 0 to
5 scale from Smith (2003): 0 = no visible symptoms,1 = leaves with small necrotic flecks, but no stem lesions(0-5%), 2 = discrete lesions on leaves and/or stem, some plant wilting(6-25%); 3
= lesions 40.5 cm of stem’s circumference and leaf tissue with necrotic lesions, more severe wilting of plant and top leaves(27-75%); 4 = girdling stem lesions and total leaf necrosis(76-100%), and 5 = plant death or girdled and falling over
2.8 Statistics
Data collected from this study were analysed using SPSS package (version 12.0).Means of the treatments were separated at 5% level of significance.
3. Result and Discussion
The fungus isolated and used for the hydrolysis of feather during the production of organic fertilizer was identified as Aspergillus niger in a bioreactor as shown in figure 5 below, its characteristics is as shown in the table below. This fungus has been characterized as the
producer of several proteolytic enzymes, which
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Production and Application of Keratin-Based Organic Fertilizer from Microbially Hydrolyzed Feathers to cowpea 5
ISSN 2229-5518
have been reported to be responsible for the key events involved in the physiology of Aspergillus niger (GradiŠar et al., 2000). Keratinases have several important uses in biotechnological processes, the use of these enzymes as an alternative to dehairing, catalyst in leather industry, in slow release nitrogen fertilizers, cosmetics and biodegradable films (Riffel et al.,
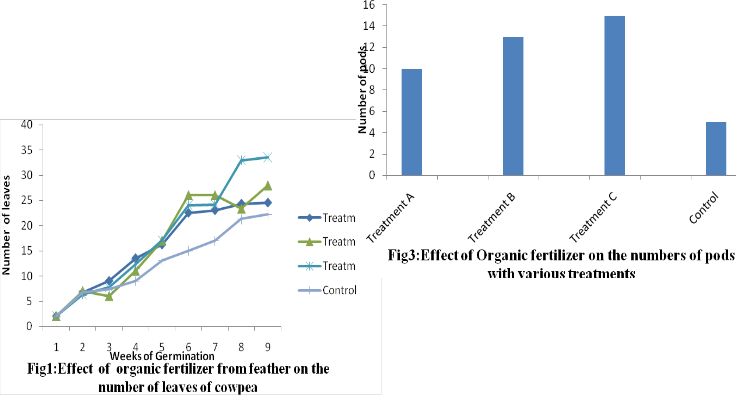
2003). Keratinases have been in several species of fungi, e.g. Aspergillus spp, Rhizomucor spp. [8], the A. niger was characterized by having Colonies consisting of a compact white or yellow basal felt covered by a dense layer of dark-brown conidial heads. Mycelial hyphae are septate and hyhaline. Condiosphores are typically 900-1600µm long, smooth walled and terminate in pale brown coloured globose, vesicles 40-60µm in diameter. The presence of highest number of leaves in a plant could be indicative of higher yield in a particular plant .Treatment C had the highest value number of leave is treatment C with
160.2±3.86 while the control had lowest value of
113.5±2.30 . Statistical analysis of the result using
ANOVA showed that there was significant differences (P<0.05) between the treatments and the control. The result of the growth of cowpea with respect to the number of leaves at the end of
9th week were 141.7±2.81, 146.24±3.33, 160.2±3.86 and 113.5±2.30 for treatment A, B, C and Control respectively according to the graph below.
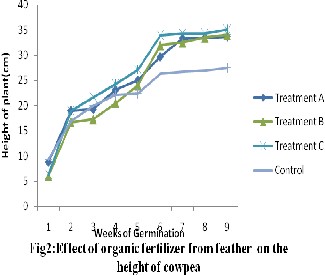
Statistical analysis of the result using ANOVA shows that there was significant difference between the treatments and control. Plant height is one of the major indicators that are considered for growth, from the results (Fig 2) treatment C had the highest value for plant height with
236.2±3.22 cm, while the control had lowest value of 198.4±1.99 . The result of the growth of cowpea with respect to the height at the end of
9th week showed 225.4±2.82, 216.5±3.25,
236.2±3.22 and 198.4±1.99 for treatment A, B, C and Control respectively according to the graph below. This observation was similar to the study carried out by Ofori (1993) reported that improved varieties of cowpea can be grown with organic fertilizer.
The rate of production of pod varied from one treatment to another after the application of the organic fertilizer.Treatment C had the highest number of pods among all the treatments while
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Production and Application of Keratin-Based Organic Fertilizer from Microbially Hydrolyzed Feathers to cowpea 6
ISSN 2229-5518
the control showed the lowest value as shown in Fig.3 below. The cowpea weights also followed the same trend with treatment C having the significantly highest (P<0.05) weights of
26.4±1.2g, while the control had a lower weight of 8.2±3.4g.The early application of the organic fertilizer from feather at five days before planting was found to be effective in this study. This was attributable to its ability to confer on the plants added nutrients and a longer release period. This finding is in agreement with [13], who showed that organic manures have a slow and long term release of nutrients and have a tendency for sustainable production.
The result of disease severity showed that control had the value of 72.2% which was the highest number of diseases because it showed lesions of
40.5 cm of stem’s circumference and leaf tissue with necrotic lesions, more severe wilting of plant and top leaves of cowpea plant. Treatment C exhibited the lowest value of 1.4% disease severity which was significantly (P<0.05) lower than control. The green of leaves in treatments A, B,and C suggested biocontrol as compared to the yellowish colour exhibited by the control at the end of the experiment.
4. CONCLUSIONS
The study showed that application of organic
fertilizer from feather at the rate of 3kg/10kg of soil played a significant role in suppressing pathogenic effects of disease severity of cowpea and also had highest growth. In the light of aforementioned, treatment C can be used as an effective cultured measure in the management of diseases of cowpea and also as organic fertilizer.
5. References
[1] Brandelli. A,, D. J. Daroit, and A. Riffel,. “Biochemical features of microbial keratinases and their production and applications,” Applied Microbiology and Biotechnology, vol. 85, no. 6, pp. 1735–1750,
2010.
[2] Bahuguna S, Kushwaha RKS ,. Hair
perforation by keratinophilic fungi. Mycoses ; vol.32,pp.340–3, 1989.
[3] Bressollier P, Letourneau F, Urdaci M,
Verneuil B. Purification and characterization of keratinolytic Serine proteinases from Streptomyces albidoflavus. Appl Environ Microbiol; vol.65:pp.2570–6.
,1999.
[4] Bullock, D.G. Crop rotation. Critical
Reviews in Plant Sciences. Vol.11.pp:309-
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Production and Application of Keratin-Based Organic Fertilizer from Microbially Hydrolyzed Feathers to cowpea 7
ISSN 2229-5518
326,1992.
[5] Brandelli, A.Bacterial keratinases:
useful enzymes for bioprocessing agroindustrial wastes and beyond. Food Bioprocess. Technol. Vol.8, pp.105–116,
2008.
[6] Crookston, R.K. The rotation effect:
What causes it to boost yields? Crops Soils
vol.36,no6,pp.12- 14,1984.
[7] Choi, J.M., Nelson P.V. Developing a slow release nitrogen fertilizer from
organic sources using poultry feathers. Journal of American Society of Holticultural Science vol.121,pp.639-643,1996.
[8] Friedrich, J., Gradisar, H., Mandin, D., Chaumont, J.P. Screening fungi for synthesis of keratinolytic enzymes. Lett. Appl. Microbiol. vol28,pp.127–130, 1999.
[9] Gupta R., Ramnani P. Microbial keratinases
and their prospective applications: an overview. Applied Microbiology and Biotechnology, vol.70 p. 21-33, 2006.
[10] Gradisar, H., Kern, S., Friedrich, J.
Keratinase of Doratomyces microsporus. Doratomyces microsporus to some known
proteases. Appl. Environ. Microbiol. Vol.3, pp3420-3426,2000.
[11] Hadas, A., Kautsky, L.Feather meal, a
semi-slow-release nitrogen fertilizer for organic farming. Fertil Res vol.38 pp.165-
170, 1994.
[12] Malviya, H.K., Rajak, R.C., Hasija, S.K.
Synthesis and regulation of extracellular keratinase in three fungi isolated from the grounds of a gelatin factory, Jabalpur, India. Mycopathologia vol.120, pp.1–4,1992.
[13] Mbagwu, J. S. C. Sub-soil
productivity of an Ultisoil in Nigeria as effected by organic Waste and inorganic fertilizer Amendments. Soil Sci. vol.140,no6pp.436-44, 1988.
[14] Riffel, A., Ortolan, S., Brandelli, A.
Unhairing activity of extracellular proteases produced by keratinolytic bacteria. J. Chem.Technol. Biotechnol. vol.78, pp.855–859,2003.
[15 ] Safranek WW, Goos RD. Degradation
of wool by saprophytic fungi. Can J Microbiol ;vol.28,pp.137–40. 1982.
[16] Singh S.R. and Rachie K. O. Cowpea
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Production and Application of Keratin-Based Organic Fertilizer from Microbially Hydrolyzed Feathers to cowpea 8
ISSN 2229-5518
research, production and utilization. John
Wiley and Sons. pp. 217- 231,1985.
[17] Singh BB, Mohan Raj DR, Dashiell KE, Jackai
Len. Advances in cowpea research. IITA-JIRCAS, Ibadan, Nigeria.1997.
[18] Samson RA, Van Reenen-Hoekstra ES
. Introduction to foodborne fungi. 3rd edition. Centraal bureauvoor
Schimmdoultur Baarn. pp. 2-89,1982.
Treatments | Weight (g) |
A | 18.5±2.9 |
B | 21.3±2.4 |
C | 26.4±1.2 |
Control | 8.2±3.4 |
Table 1: Weight of dried cowpea seeds after
harvest.
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Production and Application of Keratin-Based Organic Fertilizer from Microbially Hydrolyzed Feathers to cowpea 9
ISSN 2229-5518
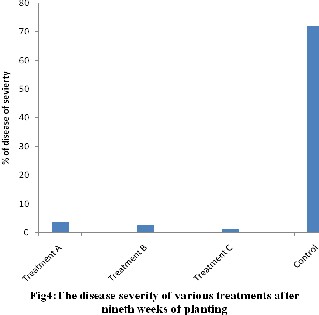
Fig. f feat f orga
IJSER © 2012
http://www.ijser.org



