International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 137
ISSN 2229-5518
Natural Dye-Sensitized Solar Cell Based on Zinc
Oxide
Salah Abdulla Hasoon1, Raad M.S. Al-Haddad2, Oras Tariq Shakir1, Issam M. Ibrahim2
Abstract— Thin Film of ZnO nanoparticles paste are spread on transparent conducting ITO coated glass using doctor-blade method, the average particle size of about 16.4nm. Natural dyes extracted from pomegranate and spinach were used as sensitizers to fabricate dye sensitized solar cells (DSSCs). The thin films were studied by absorption spectra of all dyes were performed by UV-Visible spectroscopy which showed that the dye absorbed light in the visible region from (200-325)nm for chlorophyll pigment and (500-530)nm for anthocyanin pigment. The optical band gap was found to be 3.5eV. X-Ray diffraction, Atomic Force Microscopy and Scanning Electron Microscopy were also investigated.
Index Terms— Dye-Sensitized Solar Cell, ZnO, Nanoparticles, UV-Visible Spectroscopy
—————————— ——————————
1 INTRODUCTION
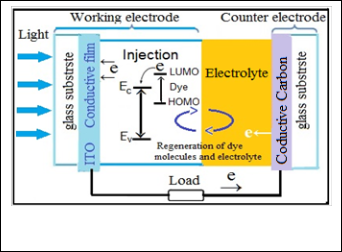
YE sensitized solar cells are a type of photoelectrochemi- cal cell. One of the main differences between dye- sensitized solar cells and other types of solar cells is the way charges are separated [1].Dye-sensitized solar cells (DSSCs) are promising, relatively low cost, and green energy photovol- taic devices. DSSCS consists of two conducting glass electrode coated with porous nanocrystalline wide-band gap semicon- ductor oxide film, such as ZnO nanoparticles which have the dye adsorbed onto their surface, whereas the counter elec- trode, is coated with platinum or graphite, there is an electro- lyte solution that contains the redox couple that regenerates the dye [2,3]. The nanostructured oxide films are particularly attractive for DSSCs as they provide a large surface area for dye anchoring. The working principle of a dye-sensitized solar cell is schematized in Figure (1). The device is comprised of two faced electrodes, a photoanode and a counter electrode, with an electrolyte in between. Both electrodes are usually made from a sheet of common float glass coated on one side with a thin transparent conductive layer of indium-doped tin oxide (ITO). Surface electrical resistivity of a few Ohm squares can be easily obtained while preserving a good optical trans-
mission over the whole visible and near infrared spectrum [4]
Thus (DSSCs) have many advantages comparing with the Si
————————————————
1 Dr. Salah Abdulla Hasoon Solid state physics College of Science for Women physics department Baghdad Iraq .Mobile: +9647801256859
E-mail:salahabd55@yahoo.com
2 Dr. Raad M. S. Al-Haddad Physics of Solid College of Science physics department Baghdad Iraq. Mobile: +9647901270618
E-mail: alhaddadraad@yahoo.com
1M.Sc Student. Oras Tariq Shakir Solid state physics College of Science for
Women physics department Baghdad Iraq. Mobile: +9647903782925
E-mail:orastariq99@yahoo.com
2Dr. Issam M.Ibrahim Solid state physics College of Science physics department Baghdad Iraq . Mobile: +9647702646902
E-mail: dr.issamiq@gmail.com
based photovoltaics, it is not sensitive to the defects in semi- conductors and it is possible to realize the direct energy trans- fer from photons to chemical energy [5]. The semiconductor substance that forms the core of the photoelectrode (PE) should be chemically stable and inert towards the electrolyte species, it should have a lattice structure suitable for dye bonding, its conduction band should be located slightly below the LUMO level of the dye in order to facilitate efficient elec- tron injection, and it should be available in nanostructure form to increases the effective surface area for dye adsorption ap- proximately by a factor of thousand, improving the sunlight harvesting efficiency [6]. ZnO is a wide-band-gap semiconduc- tor that possesses an energy-band structure and physical properties similar to those of Ti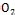 ,but has higher electronic mobility that would be favorable for electron transport, with reduced recombination loss, the direct band gap is (3.37 eV), higher exciton binding energy (60 meV) compared to Ti
,but has higher electronic mobility that would be favorable for electron transport, with reduced recombination loss, the direct band gap is (3.37 eV), higher exciton binding energy (60 meV) compared to Ti 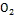 (4 meV), and higher electron mobility (200cm²V-1s-1 ) over Ti
(4 meV), and higher electron mobility (200cm²V-1s-1 ) over Ti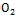 (30cm2V-1s-1). However, the efficiency of the DSSC based on ZnO nanostructures is still very low (5%) when used in DSCs [7- 9]. The dye that is used as a photosensitizer plays a main role in the process of DSSCs. The efficiency of the cell is criti- cally dependent on the absorption spectrum of the dye and the anchor of the dye to the surface of the semiconductor [10, 11]. Anthocyanin dyes are water-soluble and vacuolar pigments that may appear red, purple, or blue depending on the PH. Anthocyanins absorb light in the blue-green region between the 450 and 600 nm wavelengths; this allows many fruits and plants to reflect red, purple, or blue[12].
(30cm2V-1s-1). However, the efficiency of the DSSC based on ZnO nanostructures is still very low (5%) when used in DSCs [7- 9]. The dye that is used as a photosensitizer plays a main role in the process of DSSCs. The efficiency of the cell is criti- cally dependent on the absorption spectrum of the dye and the anchor of the dye to the surface of the semiconductor [10, 11]. Anthocyanin dyes are water-soluble and vacuolar pigments that may appear red, purple, or blue depending on the PH. Anthocyanins absorb light in the blue-green region between the 450 and 600 nm wavelengths; this allows many fruits and plants to reflect red, purple, or blue[12].
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 138
ISSN 2229-5518
prepared by adding few drops of dilute nitric acid (2.5ml) to ZnO powder and completely mixed with each other by grind- ing pestle or using a mortar, then we deposited the ZnO paste on the ITO conductive glass by doctor-blading technique in order to obtain a ZnO film with a thickness of 112nm and an area of 1cm2. The ZnO films was sintered at 400 ᵒC for 30 min and then. After cooling, the ZnO electrode was immersed in a natural dye for 15min for pomegranate juice and over night for spinach extract .The dye-sensitized ZnO electrode and a carbon dust counter electrode was assembled to form a solar cell by sandwiching a redox (I−/I3−) electrolyte solution.
Fig. 1. Shows schematic illustration of operation principle of dye sensitized solar cell [13] .
2 EXPERIMENTAL
2.1 Synthesis of ZnO Nanoparticles
In a typical synthesis, of ZnO nanoparticles is carried out by sol gel process, at (80-90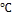 ). Solution contain 0.2M/l of zinc acetate dihydrate [(CHR3RCOO)R2RZn.2HR2RO] was prepared by dis- solving (2.195 gm) of zinc acetate dihydrate in (100ml) distilled water/ethanol, and stirred in ambient atmosphere. Potassium hydroxide KOH (1.122 gm) is dissolved in 10ml distilled water and was added to the above solution drop wise under contin- uous stirring. After few minutes solution turn into jelly form and a milky white solution was obtained, the mixture was re- fluxed for 3 h at (80-90)
). Solution contain 0.2M/l of zinc acetate dihydrate [(CHR3RCOO)R2RZn.2HR2RO] was prepared by dis- solving (2.195 gm) of zinc acetate dihydrate in (100ml) distilled water/ethanol, and stirred in ambient atmosphere. Potassium hydroxide KOH (1.122 gm) is dissolved in 10ml distilled water and was added to the above solution drop wise under contin- uous stirring. After few minutes solution turn into jelly form and a milky white solution was obtained, the mixture was re- fluxed for 3 h at (80-90)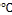 without stirring. The resulting sus- pension was centrifuged to retrieve the product, and the mix- ture was washed with distilled water in an ultrasonic bath- water and then the powder was dried at 70
without stirring. The resulting sus- pension was centrifuged to retrieve the product, and the mix- ture was washed with distilled water in an ultrasonic bath- water and then the powder was dried at 70 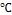 over night.
over night.
2.2 Natural Dye Extraction
To extraction the Anthocyanin Pigment from Pomegranate, we bought fresh pomegranate fruit, grabbing the seeds and after removing any white pith from the seeds, we put it in clean mortar to grinding the seeds, we finally got the pomegranate juice and putting it in a clean dish. In the other hand the chlo- rophylls and the different accessory pigments can be extracted from plants using solvent based methods; Green portion from Spinach leaves was taken out after cleaning the surfaces of leaves with acetone, after that we converted in to paste by grinding Pestle, and 10 ml acetone was added and again grind. A Filter paper was kept over the funnel of beaker and above paste of leaves was poured over the filter paper, and then 30 ml acetone was dropped over the past, and finally, drop by drop chlorophyll of Spinach leaf come out from the funnel and settled in beaker ultimately kept it in clean petri-dish.
2.3 Preparation of dye-sensitized solar cells

ITO conductive glass sheets (Asahi Glass, tin-doped indium oxide, sheet resistance: 15 /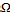 2), were first cleaned in a deter- gent solution ethanol, and then dried. Zinc Oxide Paste were
2), were first cleaned in a deter- gent solution ethanol, and then dried. Zinc Oxide Paste were
3 RESULTS AND DISCUSSION
3.1 X-Ray Diffraction Measurement and Morphological
Studies
In order to study the structural properties, the crystalline structure was examined by X-ray diffractions using. This sys- tem recorded the intensity as a function of Bragg's angle. Crys- tal structures of films were analyzed by using a (Philips PW) X-ray diffractometer system with CuKRαR target, radiation (λ=1.5406Å) with scanning angle: (20- 70°).
The optical Measurement absorbance spectra of the ZnO thin
film within the wavelength ranging from (190 - 1100 nm) at room temperature were measured using UV-Visible (SP – 8001 spectrophotometer over (Meterrech)).
The surface morphology and the size of ZnO particles were analyzed using atomic force microscope (AFM), (SPM, Model AA3000), tip NSC35/AIBS from Angstrom Advanced Inc (USA), and Scanning electron microscopy (S-4160 HITACHI) was used to inspect the size and morphology of specimens at very high magnifications. The magnification can go 100 K, and
300 nm Scale. During SEM analysis, a beam of electrons is fo-
cused on a spot volume of the specimen. The electron beam has energies that range from a few keV to 20 keV and it is fo- cused by two condenser lenses into a beam with a very fine spot size.
3.2 Structural Properties
XRD pattern of the prepared ZnO film is shown in Fig. 2. It is clear from the figure that all the diffraction peaks are highly crystalline as well as the diffraction peaks are well defined and in accordance with the typical wurtzite hexagonal structure. We have Six major diffraction peaks were seen at 2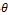 (31.79,
(31.79,
34.44, 36.26, 47.54, 56.61, 62.87) which can be assigned to dif-
fraction from (100), (002), (101), (012), (110), (013), (200), (112) planes respectively, according to the data base in JCPDS card (96-900-4180). This revealed that the resultant nanoparticles were pure ZnO with a hexagonal wurtzite structure (a=3.247Å, c=5.622Å). There is no diffraction peaks corresponding to the impurity could be detected in this pattern, which implies hexag- onal phase ZnO nanoparticles. The mean crystalline size was calculated from the full-width at half-maximum (FWHM) of
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 139
ISSN 2229-5518
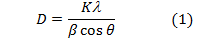
XRD lines by using the Debye-Scherrer equation (1).
Where D is the mean size of crystallites (nm), K is crystallite shape factor a good approximation is 0.9, λ is X-ray wave- length, 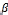 is full width at half the maximum (FWHM) in radi- ans of the X-ray diffraction peak and θ is the Bragg angle [14]. It can be seen that the average size of nanoparticles is16.44nm from table (1).
is full width at half the maximum (FWHM) in radi- ans of the X-ray diffraction peak and θ is the Bragg angle [14]. It can be seen that the average size of nanoparticles is16.44nm from table (1).
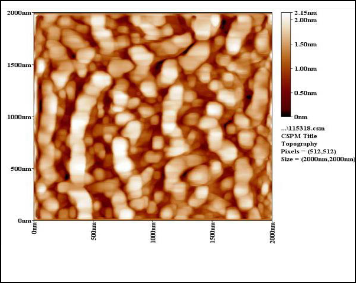
the average diameter 65.70nm with roughness 0.353nm, this means that the producers from ZnO particles within the range of nanoscale.
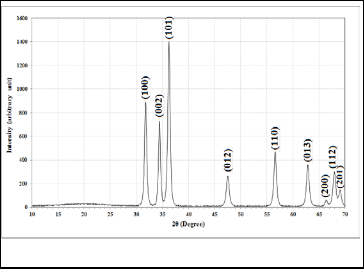
Fig. 3A .shows the 2D, 3D surface plot AFM picture of the nanocrystal- line ZnO film.
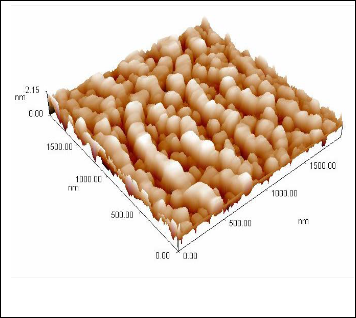
Fig. 2.XRD Spectrum of the Nanocrystalline ZnO Thin Film.
TABLE 1
STRUCTURAL PARAMETERS OF THIN



3.3 Atomic Force Microscope (AFM)
Atomic force microscope (AFM) is a high resolution scanning probe microscopy. It is a useful tool used by the research community in material science to investigate the surface pro- files in three dimensions at the nanoscale level and it is also used in manipulating atoms. Figures 3A and 3B illustrates two and three dimensional AFM images (2 μm × 2μm) of the ZnO paste were deposited by using doctor-blade method. It is clear,
Fig. 3B .shows the 3D surface plot AFM picture of the nanocrystalline
ZnO film.
3.4 Scanning Electron Microscope (SEM) of ZnO Film
The key points for DSSC application is surface morphol- ogy. Thus it is significant to study the Surface morphology of the ZnO film.
An SEM micrograph was used to examine the topogra- phy and morphology of the samples. It provides essential in- formation about shape, size, and the growth mechanism of the ZnO nanoparticles. Figure (4) shows the low and high magni- fication SEM images of the sample grown on the glass sub- strate. The figure clearly indicates the morphology of the par- ticles to be roughly spherical, non-homogeneous. Some of the particles are agglomerates with an average grain size of ap- proximately (50.62) nm, this consequence almost similar to
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 140
ISSN 2229-5518
AFM measurement.
However, it was observed that there was a difference between
the size measurement using XRD and SEM. The reason is well known. In SEM, the grain size was measured by the differ- ences between the visible grains boundaries while in, the XRD method, the measurement was confined to the crystalline re- gion that diffracted x-ray coherently. This was a more strin- gent criterion and led to smaller grain size.

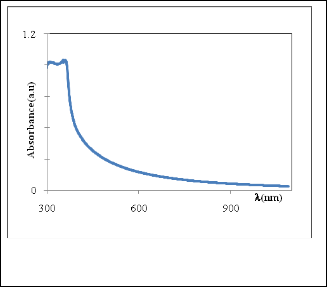
Fig. 5. shows the optical absorbance spectra of ZnO powders prepared from 0.2M Zinc acetate.
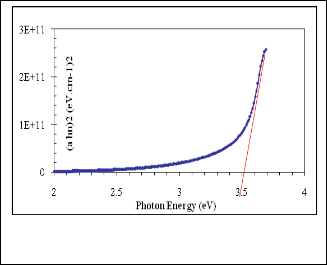
Fig. 4A. shows SEM images of the ZNO film, at low magnifica- tion.

Fig. 6. Evolution of the (αhυ)2 vs. hυ curves of ZnO thin film pre- pared from (0.2M) Zinc acetate.
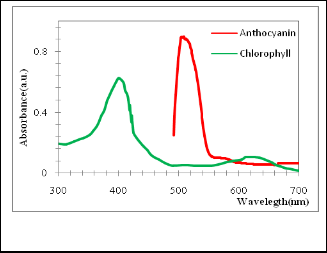
Fig. 4B. shows SEM images of the ZNO film, at high magnifica- tion.
4 OPTICAL CHARACTERIZATIONS
4.1 Optical Energy Gap (E0 ) For ZnO Films
The absorption spectrum of ZnO nanopowder (colloidal na- noparticles) is shown in Figure (5), the sample show strong UV absorption in visible region with peak maximum at 357nm indicating band- to-band excitation of ZnO, which is used for calculation of optical band gap of nanoparticle by using equa- tion (2).[15]
Fig. 7. Shows the absorption spectra of chlorophyll and antho- cyanin pigment .
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 141
ISSN 2229-5518
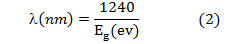
The optical absorption provides the dependence of the absorp- tion coefficient (α) on the photon energy (hν) for direct al- lowed [16]
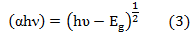
we applied Tauc plot to find the optical band gap by plotting (αhν)2 vs photon energy using the data obtained from optical absorption spectra and extra plotted to α = 0 as shown in fig- ure (6) . The optical band gap determined from this curve is
~3.5eV.
4.2 UV-Visible Spectroscopy for the Dye
The absorption spectra of chlorophyll and anthocyanin pig- ment are shown in figure (7), the absorption spectra showed the presence of distinct absorption peaks in the visible region at about 400 nm and a small peak at about 617 nm for the ex- tract of chlorophyll Huizhi Zhou et al. [17]. The absorption spectra showed also the presence of peak at (503-510 nm) for anthocyanin pigment dye [18]. Results show that the absorp- tion intensity of anthocyanin dye is highest that than chloro- phyll, it can be seen the absorption of two dyes in the visible region (approximately 400 - 620 nm). This proves the possibil- ity used as sensitizers to fabricate dye-sensitized solar cells (DSSCs) as mentioned by Chang et al. [19].
4.3 Photovoltaic Properties
Photovoltaic tests of the fabricated DSSCs using these natural dyes as sensitizers were performed by measuring the I–V curve of each cell under irradiation with white light (100 mW/cm2) from xenon arc lamp and at room tempera- ture(22.3 with cell area 1cm2.. The values of fill factor (FF) was calculated by applying the following generalized equat
with cell area 1cm2.. The values of fill factor (FF) was calculated by applying the following generalized equat
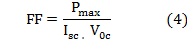
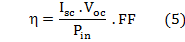
Where 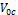 and
and 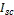 are respectively the open circuit voltage and short circuit current.
are respectively the open circuit voltage and short circuit current.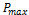 is the maximum power delivering point. The I-V characteristics of the prepared DSSCs taking pomegranate juice, chlorophyll extract from spinach as the natural dye by using Doctor-blade with ZnO nanoparticles is shown in figure (8). The photoelectric conversion efficiency (η) of DSSC is given as
is the maximum power delivering point. The I-V characteristics of the prepared DSSCs taking pomegranate juice, chlorophyll extract from spinach as the natural dye by using Doctor-blade with ZnO nanoparticles is shown in figure (8). The photoelectric conversion efficiency (η) of DSSC is given as
Where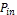 is the incident light power. Table (2) shows the data acquired from measuring the photoelectric conversion effi- ciency of the DSSCs
is the incident light power. Table (2) shows the data acquired from measuring the photoelectric conversion effi- ciency of the DSSCs
Fig. 8. Photocurrent voltage characteristics spinach and pome- granate dye.
TABLE 2
PHOTOELECTROCHEMICAL PARAMETERS OF THE DSSCS
SENSITIZE BY VARIOUS NATURAL DYES

Natural dye pigment | Imax (mA) | Vmax (mV) | Isc (mA) | Voc (mV) | FF% | η% |
pomegrante juice(antho ocyanin) | 0.15 | 450 | 0.255 | 600 | 0.44 | 0.07 |
spinach chlorophyll | 0.04 | 410 | 0.052 | 590 | 0.53 | 0.0164 |

Fig. 9. Shows the power-voltage characteristics curves of the DSSCs sensitized by: spinach and pomegranate using Doctor-blade method..
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 142
ISSN 2229-5518
The DSSC output power was calculated using the I-V data. Figure (9) shows the power as a function of V for the DSSC sensitized by spinach and pomegranate juice.
The lowest Pmax value come from the DSSC sensitized with
spinach which may be assign to weak bonding between the dye molecule and ZnO particles. good electric conversion abil- ity in a DSSC is highly dependent on disponible bonds be- tween the dye molecules and ZnO particles, in which electrons can transport from excited dye molecules to ZnO, chlorophyll dye is lower than anthocyanin dye, therefore, this is Compati- ble with the results of Chang et al.[10, 19].
Some research workers have found that the energy conversion
efficiency was 0.0277%. with anthocyanin dye Extracted from strawberry [20] However, our results were 2.5 times higher than in others, this may have influenced the results of the ex- periment since plants contain different components which equally produce different results [20].
5 CONCLUSIONS
Hexagonal ZnO nanopartical were synthesis successfully without using any capping agent through sol gel process at 80-
900 C. we were also able to make dye-sensitized ZnO solar
cells by using natural dye (pomegranate, chlorophyll) by using
an electrolyte with a KIR3R redox couple and a carbon dust coun- ter-electrode. Natural dyes as alternative sensitizers for DSSCs are expected to be promising because of many reasons such as the simple preparation technique and low cost. The Prepara- tion and characterization of ZnO nanoparticles using XRD, AFM and SEM, are presented in this work. The SEM picture of the ZnO film shows that the nanoparticles are non- homogeneous and has a porous agglomerate structure consist- ing mainly of spherical crystalline particles with about
50.62nm diameter.
ACKNOWLEDGMENT
The authors wish to thank College of Science and college of Science for women /Department of Physics /University of Baghdad to allow us for providing Instrumental and laborato- ry facilities to carry out this work
REFERENCES
[1] B. E. Hardin, H. J. Snaith and M. D. McGehee. ” The renaissance of dye-sensitized solar cells,” Nature photonics, vol. 6, pp. 162-169, 2012.
[2] T.M. El-Agez, A.A. El Tayyan, A. Al-Kahlout, S.A. Taya, and M.S.
Abdel-Latif. ” Dye-Sensitized Solar Cells Based on ZnO Films and Natural Dyes,” International Journal of Materials and Chemistry, vol.2, no.3, pp.105-110, 2012.
[3] J. Wu, Z. LAN, S. Hao, P. Li, J. Lin, M. Huang, L. Fang, and Y.
Huang,”Progress on the electrolytes for dye-sensitized solar cells,”
Pure Appl. Chem., vol. 80, no. 11, pp.2241–2258, 2008.
[4] M. Graetzel.” Solar Energy Conversion by Dye-Sensitized Photovol- taic Cells, Inorg.Chem” vol.44, no.20, pp. 6841-6851.2005.
[5] D. Wei. ” Dye Sensitized Solar Cells,” Int. J. Mol. Sci., vol.11, pp. 1103-
1113. 2010.
[6] M. Gratzel. ” Conversion of sunlight to electric power by nanocrystal- line dyesensitized solar cells,” Journal of Photochemistry and Photobiolo- gy: Chemistry. vol. 164, no. 1-3, pp. 3-14, 2004.
[7] Q. Q. Zhang, C.S. Dandeneau, X. Zhou, and G.Cao, ”ZnO Nanostruc-
tures for Dye-Sensitized Solar Cells, ”. Adv. Mater., vol. 21, pp.4087–
4108, 2009.
[8] J. Han, F. Fan, C. Xu , S. Lin., M. Wei, X. Duan and Z.L. Wang,” ZnO nanotube-based dye-sensitized solar cell and its application in self- powered devices,” Nanotechnology, vol. 21, no.1,7, 2010.
[9] M. K. Erhaima, R. A.AL-ansari, N. F.Habubi,” Structural and Optical
Properties of Cobalt-Doped Zinc Oxide Thin Films Prepared By
Spray Pyrolysis Technique,” Baghdad Science Journal, vol.7, no. 1, pp,
69-75, 2010.
[10] H. Chen, Z. Duan, Y.G. Lu, A. D. Pasquier, ” Dye-sensitized solar
cells combining ZnO nanotip arrays and nonliquid gel electrolytes, ” Minerals Metals & Materials Society, Journal of Electronic Materials, vol. 38, no. 8, pp. 1612-1617, 2009.
[11] T. M. El-Agez, A. A. El Tayyan, A. Al-Kahlout, S. A. Taya, M. S. Ab- del-Latif, ” Dye-Sensitized Solar Cells Based on ZnO Films and Natu- ral Dyes, ”. International Journal of Materials and Chemistry. vol. 2, no. 3, pp. 105-110, 2012.
[12] M.N. Merzlyak, O. B. Chivkunova, A. E. Solovchenko and K. R.
Naqvi, ” Light absorption by anthocyanins in juvenile, stressed, and senescing leaves, ”. Journal of Experimental Botany., vol. 59, no. 14, pp.
3903-391, 2008.
[13] Q. Zhang, G. Cao,” Nanostructured photoelectrodes for dye- sensitized solar cells,” Nano Today., vol. 6, pp. 91—109, 2011.
[14] A. Monshi, M.R. Foroughi, and M.R. Monshi,” Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD,” World Journal of Nano Science and Engineering, vol. 2, pp. 154-
160, 2012.
[15] M. K. Patra, K. Manzoor, M. Manoth, V. S. Choudhry, S. R. Va- dera, N. Kumar,” Optically transparent colloidal suspensions of sin- gle crystalline ZnO quantum dots prepared by simple wet- chemistry,” Journal of Optoelectronics and advanced materials, vol. 10, no. 10, pp. 2588 – 2591 2008.
[16] R. S. Mane, W. J. Lee, H.M. Pathan, and S. H. Han, ”Nanocrystalline TiO2/ZnO thin films Fabrication and application to dye-sensitized solar cells, ” The Journal of Physical Chemistry B., vol. 109, no. 51, pp.
24254–24259, 2005.
[17] H. Zhou, L. Wu, Y. Gao, and T. Ma, ” Dye-sensitized solar cells using 20 natural dyes as sensitizers, ” Journal of Photochemistry and Photobiology A: Chemistry., vol. 219, pp. 188–194. 2011.
[18] S. M. Milenkovic, J. B. Zvezdanovic, T. D. Andelkovic, and D. Z.
Markovic,” The identification of chlorophyll and its derivatives in the pigment mixtures: HPLC-chromatography, visible and mass spectroscopy studies,” Advanced technologies, vol. 1, no.1, pp. 16-24,
2012.
[19] Ho. Chang, M. J. Kao, T. L. Chen, H. G. Kuo, K. C. Choand, and X.
P. Lin, ” Natural Sensitizer for Dye-Sensitized Solar Cells Using Three Layers of Photoelectrode Thin Films with a Schottky Barrier, ” Am. J. Engg. & Applied Sci., vol. 4, no. 2, pp. 214-222, 2011.
[20] J. Etula,”Comparison of three Finnish berries as sensitizers in a dye- sensitized solar cell,” European Journal for young scientists and Engi- neers, vol.1, pp. 5-23, 2012.
IJSER © 2015 http://www.ijser.org










