International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 880
ISSN 2229-5518
Multielement Analysis of Fresh Tomatoes Produced at the Tono Irrigation Scheme by Instrumental Neutron Activation Analysis
Aaron N. Adazabra, K. Appiah - Kubi, Samul A. Bamford
Abstract— Fresh tomatoes can be a source of mineral components and trace elements as well as some undesirable substances due to exposure to the environment. Instrumental neutron activation analysis was therefore used to analyze the elemental composition of fresh tomatoes, cultivated under the Tono irrigation scheme. In this study, As, Ca, K, Mg, Mn, S, Co Cu, Mo, Fe, Zn, P, Na, Cl, Br, Se and Cr were analyzed. Among the metals analyzed, P was the most abundant, ranging from 5.09g/kg in Gaani sample to 11.91 g/kg in Bonia sample, followed by K with 0.97 g/kg in Biu sample to 7.74 g/kg in Chuchuliga sample. Ca and Mg concentrations were also determined in the range, (0.048 – 0.75) g/kg and (0.15 – 0.66) g/kg respectively. Mn, As, Co Cu, Mo, Fe, Zn, Na, Cl, Br, and Se were all also found in reasonable amounts. Fortunately, toxic element (As) had very low concentration in most of the samples with concentration ranging from <
0.09 mg/kg in Yigbwania samples to 0.21 mg/kg in Wuru sample. This data therefore, should not only be helpful in explaining the nutritional value of tomatoes grown in these localities but also in establishing their base-line values in the country’s agroecosystem.
Index Terms— Fresh Tomatoes, Activation Analyis, Tono Irrigation Scheme, Upper East Region, Mineral Content, Ghana,
—————————— ——————————
1 INTRODUCTION
omato, scientifically called Lycopersicon esculentum, is a popular vegetable grown in many parts of the world. It is very nutritious and a major source of vitamins A, C and
riboflavin as well as carbohydrate, protein, calcium, carotene in our diets [1], [2]. In Ghana, tomato with its high per capita consumption is one of the most commonly grown fresh mar- ket vegetables. It forms a very important component of food consumed in Ghana as it is used in almost all Ghanaian homes. This is evident in the fact that many Ghanaian dishes have tomatoes as a component ingredient. Again, records at the Ministry of Trade and Industry indicated that in 2000
Ghana was the second largest importer of tomato in the world, second to Germany [3].
Therefore, realising the significant economic activity in this agribusiness, the Tono irrigation scheme was therefore con- structed in the Upper East Region of Ghana, where conditions are most suitable, for all – year – round cultivation of fresh tomato and other food crops by mostly small-scale farmers [4]. The region is located on the North-East corner of Ghana be- tween latitudes 10º 30´ to 11º North and longitudes 0º to 1º 30´ West within the White Volta River Basin. It covers a land sur- face area of 8,842 kilometres square representing only about 4
% of the total land mass of the country. It has two internation- al boundaries with the Republic of Burkina Faso to the North and Togo to the East. The other boundaries are Northern re- gion and Upper West region to the South and West respective- ly [5], [6]. Fig. 1 shows a map of Upper East Region in the Na- tional Context.
————————————————
• Aaron N. Adazabra is currently an Applied Nuclear Physicist at the Uni- versity for Development Studies, Tamale, Ghana. E-mail: adazabraa- ron@yahoo.com
• K. Appiah - Kubi is lecturer in parasitology at University for Development Studies and currently pursuing Doctorate degree in Clinical L aboratory Diagnosis at Jiangsu University, Jiangsu Province, China. E-mail: ap- piensis@gmail.com
• Samuel A. Bamford is a Senior Lecturer at Graduate School, University of
Fig. 1: Map of Upper East Region in the National Context.
However, it is well established that tomato is the second largest crop in terms of fungicide expenses per unit area but occupies the first position in insecticide use [7]. These chemi- cals are increasingly being used at the Tono irrigation scheme year after year for tomato cultivation. Hence since these chem- icals contain toxic elements with their associated harmful ef- fect, which can be transferred from the environment to hu- mans through the food chain, public concern about the envi- ronmental impact has grown in recent decades. In addition, human existence and its survival predominantly depend on the inhalation of ambient air, intake of clean water, and inges- tion of nutritionally adequate as well as contaminant-free food. For these reasons, there is an increasing interest in the determination of mineral content of foods and diets even at
Ghana, Legon – Accra. Ghana. E – mail: bamford_s@yahoo.com
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 881
ISSN 2229-5518
very low levels. Similarly, the mineral content present in this vegetable can be in trace or even ultra-trace quantities. Hence, a suitable analytical technique with the appropriate sensitivity range is required for the precise determination of the nutrients (multielements) present in these samples. Recent advances in analytical techniques with improved sensitivity have opened up this new scope to scientists [8].
Some of the cardinal techniques employed for these analy- sis are; flame atomic absorption spectrometry (FAAS), graph- ite furnace atomic absorption spectrometry (GFAAS), induc- tively coupled plasma atomic emission spectrometry (ICP- AES), energy dispersive X-ray fluorescence (EDXRF), electron thermal atomic absorption spectrometry (ETAAS) and induc- tively coupled plasma mass spectrometry (ICP-MS) [9], [10], [11]. Besides those mentioned above, other techniques such as differential pulse cathode stripping voltamperometry (DPCSV) and Instrumental neutron activation analysis (INAA) have also been shown as excellent tools for trace and ultratrace analysis [12].
Consequently, the risk of diseases associated to chemically contaminated food has caused concern among consumers and there is an ever-increasing demand for food quality nowadays. This work therefore, applies instrumental neutron activation analysis (INAA) to investigate the multielement composition of fresh tomatoes produced at the Tono irrigation dam in other to establish the nutritional value of these widely consumed vegetable and carefully evaluate the potentially toxic chemical elements levels due to the contamination in the catchment area.
2 MATERIALS AND METHODS
2.1 Sampling
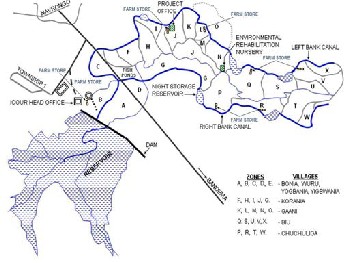
Individual fresh tomatoes samples (pectomech variety) were randomly obtained, from local farmers, from different sections in the Irrigation catchment area as shown in Fig.2. The Irriga- tion catchment area is divided into many zones lettering from A to W but these Zones were grouped into eight sections namely; Bonia, Wuru, Yogbania and Yigbwania.
The rest of the sections are Korania, Gaani, Biu and Chuchuli- ga.
The quantity of fresh tomatoes obtained was normally a few kilograms from each section. The tomatoes samples were thoroughly hand – rinsed with distilled water, shaken to re- move any excess water and then gently blotted with a paper towel. The samples were wrapped with clean polyethylene , freeze-up and then placed into clean polyethylene containers for transportation to the Ghana Research Reactor – 1 centre at the National Nuclear Research Institute of Ghana Atomic En- ergy Commission.
2.2 Sample Preparation
At the Centre, about ten sub – samples were randomly se- lected from each section mentioned above. These sub-samples were further thoroughly washed with doubly distilled water and then with distilled de-ionized water. The ten sub – samples from each section were then mixed and ground to a homogeneous fine mixture using a high-speed home- styled blender with stainless steel blades in other to reduce contami- nation. Aliquots of each homogenized sample were frozen at -
20°C and lyophilized (Christ Gamma 1-16) at -30 °C and 0.370 mbar. The lyophilized samples were milled in the vibratory disc mill (Retsch RS 100). The lyophilized homogenates ob- tained were stored in closed polyethylene bottles with screw caps and kept at -20 °C until analysis in order to prevent deg- radation of the sample. Drying by lyophilization was used because it ensured that the initial sample texture is preserved and in addition facilitates subsequent milling of samples [11], [13], [14]. Six replicates (approximately 200 mg) of the lyophi- lized from each section were weighed into clean polyethylene foils, wrapped with forceps and the foil heat-sealed.
2.3 Standard Preparation
Six replicate samples of compositionally appropriate standard reference material (SRM) obtained from National Institute of Standards and Technology (NIST), Tomato leave (1573 a) [15] was used as a standard for gamma spectrum evaluation using the relative method of standardization for neutron activation analysis. Another six replicate of SRM, Oyster Tissue (1566b) [16] standard also obtained from NIST, was used to check the accuracy and precision of the analytical method used. These standards were accurately weighted di- rectly into pre – clean 2.0 mL polyethylene vials, capped and heat – sealed. The polyethylene vials were pre – cleaned by thoroughly washing them with distilled water before soaking them in 1:4 reagent grade HCl for a day. The vials were again rinsed with distilled de-ionized water and then air – dried in fumehood. To obtain reproducible geometry, all vials were half - filled. However, the weights of the materials ranged from 100 mg to 200 mg depending on their densities. All the vials were then placed into 7.0 mL polyethylene irradiation vials, capped and heat sealed again.
Fig.2: Map of Tono irrigation dam and its catchment areas.
2.4 Samples and Standard irradiation, Counting and
Analysis
The standards were irradiated in the isotropic neutron field of the inner (No. 2) irradiation site of Ghana Research Reactor
IJSER © 2013
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 882
ISSN 2229-5518
– 1 (GHARR -1) facility which is a miniature neutron source reactor. The reactor was operated at a thermal power of 15 kW that has a corresponding thermal neutron flux of 5.0 x 1011 n.cm-2s-1 in the inner irradiation sites. All the vials were sent to the irradiation site of the reactor (GHARR – 1) by means of pneumatic transfer system.
The irradiated standards and samples after appropriate de- cay periods were assay for gamma activities using a gamma- ray spectrometry system. The system consists of an N-type HPGe coaxial detector (model GR 2518) with an 8 k MAES- TRO Multichannel Analyser (MCA) Emulation software and a Pentium II computer (for spectrum and data evaluation and analysis). The detector operates at a bias voltage of -3000 V, which has a resolution of 1.8 keV (FWHM) for 60Co gamma- ray energy of 1332 keV, and a relative efficiency of 25% to NaI detector. By means of the MCA card, the spectra intensities were accumulated for a preset time. Care was taken to account for the counting losses by keeping the dead time around 10% at the start of counting of the samples. This was maintained by choosing suitable duration of irradiation and keeping the sample-to-detector distance at 7.2 cm from the top of the de- tector surface during measurement.
Irradiation, decay and counting times were varied depend- ing on the radionuclides present. The counting times were chosen not to exceed 0.2 times the half-life of the radionuclide of interest. The activities of radionuclides were followed as a function of time to ensure purity and identity.
3 RESULTS AND DISCUSSION
3.1 Quality Assessment
Detailed results of the quality assessment of neutron activa- tion method are presented in Table 1 below. The validation of the analytical method used for this study was performed by
Table 1. Analysis of NIST SRM Oyster Tissue (1566b) in mg/kg by INAA
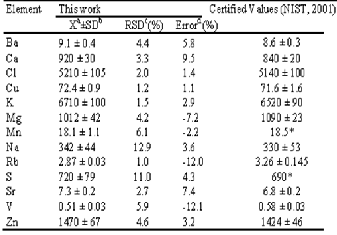
* Recommended or non – certified values, a Mean value, b Standard deviation, c Relative standard deviation, d Relative error, SD= Standard Deviation; X= mean concentration
analyzing six replicates of certified reference material SRM,
Oyster Tissue (1566b) from NIST –USA for thirteen (13) differ- ent elements under the same experimental conditions. The precision and accuracy of the measurement was assessed in terms of the percentage relative standard deviation (%RSD) and percentage relative deviation (Error) respectively. It is evident that in most of the cases the deviations (%RSD) from the certified values are within 10% signifying high order of accuracy and precision of the analytical method. The results therefore, showed good agreement with the certified values.
3.1 Elemental Compositions of Fresh Tomatoes
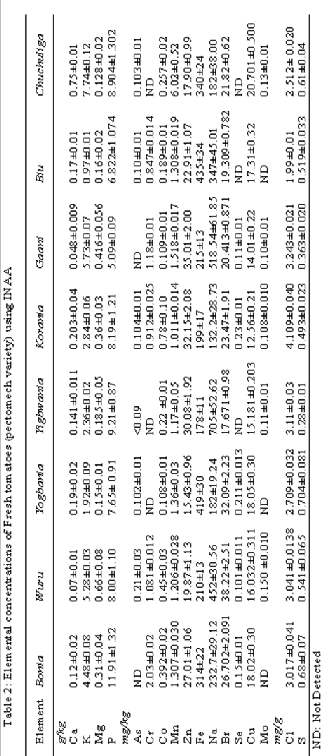
The arithmetic mean concentrations of 17 elements (As, Ca, K, Mg, Mn, S, Co Cu, Mo, Fe, Zn, P, Na, Cl, Br, Se and Cr) mostly amenable to thermal neutron activation analysis procedure determined in various different sections of the Tono irrigation dam were analyzed and presented in Table 2. Elements such as As, Cu and Se were analyzed by the single comparator method since certified or recommended values for these ele- ments are not reported in NIST 1573 a, Tomato leave. P was determined by proximate analysis. The procedure and valida- tion of method is presented elsewhere [17]. The results showed that, fresh tomatoes have the ability to accumulate essential and trace elements as well as potentially toxic or heavy metals.
A perusal of Table 2 shows that the major constituents of fresh tomatoes in this locality are; Ca, P, Mg and K. These re- sults are in agreement with those presented by other author’s for the same plant species [11], [18]. One explanation for these high concentrations may be due to the preferential addition of chemical fertilizers such as; calcium nitrate, potassium nitrate, magnesium sulphate and phosphates in the production pro- cess of this vegetable. The concentrations of Cl and S were also measured in terms of mg/g. The rest of the elements were measured in mg/kg. The concentrations of elements were ob- served to differ in magnitude and from one location to anoth- er. These differences may be attributed to the preferential ab- sorbability of the vegetable plant for a specific element and the differential elemental composition of the soil where the plants were cultivated [19].
The biological effects of chemical elements in living system strongly depend upon their concentration and thus should be carefully monitored at regular intervals especially when con- sumed by humans. Cr is implicated in maintenance of blood sugar, prevention of arteriosclerosis and control of cholesterol levels. Human studies suggest that chromium picolinate en- hances insulin sensitivity, glucose removal and may improve lipid ratios in obese and type 2 diabetics [20]. The highest con- centration of this element was found in at Bonia. The concen- tration of Mn range from 1.307 mg/kg to 1.17 mg/kg. Mn is a component of several enzymes including manganese-specific glycosyltransferase and phosphoenolpyruvate carboxykinase and essential for normal bone structure. Mn deficiency can manifest as transient dermatitis, hypocholesterolemia and in- creased ALP level [21].
Concentrations of Mo, Se and Co were very low (0.10 –
0.78) mg/kg. Mo acts as a detoxification agent in the liver as a part of the sulphite oxidase enzyme and it possibly retards
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 883
ISSN 2229-5518
degenerative diseases, cancer and aging [21].
Se as glutathione peroxidase inhibits the replication of tu- mour virus and prevents the malignant transformation of cells [22]. Ionic Co is not an essential nutrient for humans, but it is an integral component of vitamin B12 , which is essential. The concentration of S ranged (0.28 – 0.704) mg/g. Sulphur con- taining metabolites, is believed to play a role in homeostatic control mechanisms. As is a toxic element and it can cause
poisoning at elevated levels. The highest concentration was found at Yogbania. Cu is universally important cofactor for many hundreds of enzymes. A Cu deficiency can result in a decrease in the tinsel strength of arterial walls, leading to an- eurysm formation and skeletal maldevelopment [23]. Lowest concentration of this element was found at Korania. The bio- logical effect of Br is not well established.
Zn (15.43 – 35.01)mg/kg is involved in the hydrolysis of bicarbonate, hydrolysis of proteins in digestion, hydrolysis of phosphate esters, energy metabolisms, carbohydrate metabo- lism, oxidation of alcohols, detoxification of reactive oxygen species [24]. Appropriate balance and intake of manganese plays a vital role in preserving bone density and thus prevent- ing osteoporosis. It is also noted to play a key role in prevent- ing diabetes, reducing symptoms related to premenstrual syn- dromes in women and preventing epilepsy [25]. High concen- tration of Fe has the potential of been toxic. It involves in many enzymatic reactions (Redox reactions). Calcium acts as the main structural element for bones and teeth, it is also es- sential for the formation of fibrinogen [14]. P is a major inter- cellular cation in the human body. It helps maintain the cardi- ac rhythm [26]. Na (132.2 – 705) mg/kg, K (1.92 – 7.74) g/kg and Cl (1.99 – 4.109) mg/g functions as electrolytes that main- tain normal fluid balance inside and outside cells.
A careful scrutiny of Table 2, shows that all the potential toxic elements (Br, Fe, Zn, Cu and Co) were found in trace levels (mg/kg) hence, these elements are generally within their safe levels [27] . Except for As, toxic elements such as Cd, Ni, Hg and Pb could not be detected by the method and hence require further investigations.
4 CONCLUSION
In this study, concentrations of 17 elements were determined in fresh tomatoes fruits samples cultivated under the Tono irrigation scheme using INAA. The results obtained showed differences among the elemental concentrations determined at the different sections of the same irrigation scheme. However, Ca, K, Mg and P were generally found in high concentrations with slight variation by a factor of 1 or 2 in all sections of the irrigation scheme. The presence of these elements in high con- centrations confirms the accession that tomatoes fruits are very nutritious. Results obtained in the analysis of certified reference materials shows that the percentage relative devia- tion and percentage relative standard deviation are mostly within 10% that indicated good precision and accuracy of the method applied in this work. The results obtained in this work will not only provide data on nutritional surveillance pro- grammes, but also on the contamination brought in by several indiscriminating activities.
7.2 Acknowledgments
We wish to express our profound gratitude to the staff of Ghana Research Reactor – 1 Neutron Activation Analysis La- boratory for their innumerable hours spend preparing, irradi- ating and counting of our quiet bulky samples. Our thanks also goes to the Director General, Ghana Atomic Energy Commision, Prof., B.J.B Nyarko for his logistical support in
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 884
ISSN 2229-5518
carrying our this research. Lastly, we wish to thank all our colleagues, friends and family members for their moral sup- port.
REFERENCES
[1] K..O. Purseglove, Tropical Crops Dicotyledons. Longmans, London, pp: 199 –
237 , 1979.
[2] D. Bull, A Growing Problem: Pesticide and the Third World poor Farmers. Oxford
Press, pp: 207. 1989.
[3] E. Aryeetey, “ISSER-Merchant Bank Development Seminar Series”
:http://www.ghanaweb.com/GhanaHomePage/election2008/artikel.php?I D=101256. 2006.
[4] Irrigation Company of Upper Region (1995). ICOUR Information Handbook.
ICOUR Ltd, Ghana
[5] Aquastat, “Food and Agriculture Organisation’s Information System on Water and Agriculture. Water Report No. 29, 2005, FAO, Rome.” http://www.fao.org/nr/water/aquastat/countries/ghana.stm. 2005.
[6] Maxwell Okrah, Economic Dimensions of Inland Fisheries of the Upper East Region of Ghana. M.Sc., Thesis, Kwame Nkrumah University of Science and Technol- ogy , Kumasi, Ghana. 2010.
[7] E. M. Neves, L. Rodrigues, M. Dayoub, D. S, “Dragone, Bataticultura: Dispêndios com defensivos agrícolas no qüinqüênio”. http://www.abbabatatabrasileira.com.br/revista06_018.htm. 2012.
[8] B. J. B. Nyarko, E. H. K. Akaho, J. J. Fletcher, B. Zwicker, and A. Chatt, “Simul- taneous Determination of Short-to-Medium Lived Nuclides in Ghanaian Food Items Using Inaa and Compton Suppression Counting,”. J. Radioanal. Nucl. Chem., Vol. 270, No.1,pp. 243–248. 2006
[9] D. Joseph, M. Lal, H.N. Bajpai, and P.K. Mathur, “Levels of Trace Elements of a Few Indian Spices by Energy Dispersive X-Ray Fluorescence,” J. Food Sci. Tech., 36, 264-265. 1999.
[10] J. Scancar, R. Milacic, I. Falnoga, M. Cemazar, and P. Bu- kovec, “Use of Nitric Acid in Sample Pretreatment for Determination of Trace Elements in Various Biological Samples by ETAAS,” J. Pharmaceutical and Biomedical Analysis,
22, 993-1002. 2000. (doi:10.1016/S0731-7085(00)00305-8)
[11] D.K. Adotey, Y. Serfor-Armah, J.R. Fianko and P.O. Yeboah, 2009. “Essential Elements Content In Core Vegetables Grown and Consumed in Ghana by In- strumental Neutron Activation Analysis,” Afr. J. Food Sci., 3(9), pp. 243-249.
2009.
[12] G.R.K. Naidua, H.O. Denschlag, E. Mauerhofer, N. Porte, T. Balaji, “Determi- nation of Macro, Micro Nutrient and Trace Element Concentrations in Indian Medicinal and Vegetable Leaves Using Instrumental Neutron Activation Analysis,” Appl. Radiat. Isot. 50 (1) 947-953, 1999.
[13] M. Hoenig,. “Preparation Steps in Environmental Trace Element Analysis- Facts and Traps,” Talanta, 54: 1021-1038. 2001.
[14] K. Emmanuel Quartey, S. Nicholas Opata, M. Harry Amoatey and Y.P.
George Klu, “Elemental Composition in Fruits of Gamma-Radiation Induced Variant Lines of Solanum pimpinellifolium L”, Asian Journal of Agricultural Sciences 4(3): 198-204, 2012.
[15] National Institute of Standards and Technology, Certificate of Analysis, Standard Reference Material 1573a Tomato Leaves, 1995.
[16] National Institute of Standards and Technology, Certificate of Analysis, Standard Reference Material 1566b Oyster Tissue, 2001.
[17] B.J.B. Nyarko, E.H.K., Akaho, S., Serfor – Armah, 2003. “Application of NAA Standardization Methods Using a Low Power Research Reactor,” J. Radio- anal. Nucl. Chem. 257, pp: 361-366. 2003.
[18] J. O. Olaniyi, W. B. Akanbi, T. A. Adejumo and O. G. Akande, “Growth, Fruit
Yield and Nutritional Quality of Tomato Varieties,” Afri. J. Food Sci. Vol. 4(6), pp. 398 – 402. 2010.
[19] J. H. Zaidi, i. Fatima, i. H. Qureshi, m. S. Subhani, Radiochim. Acta, 92, 363.
2004.
[20] W.T. Cefalu, Z.Q. Wang, X.H. Zhang, L.C. Baldor and J.C. Russell, “Oral Chromium Picolinate Improves Carbohydrates and Lipid Metabolism and Enhances Skeletal Muscle Glut-4 Translocation in Obese, Hyperinsulinemic (Jcr-La Corpulent) Rats,” J. Nutr., 132 (6): 1107-1114. 2002.
[21] Ellahi Bukhsh, Salman Akbar Malik and Sheikh Saeed Ahmad, “Estimation of Nutritional Value and Trace Elements Content of Carthamus Oxyacantha, Eruca Sativa And Plantago Ovata,”. Pak. J. Bot., 39(4): 1181-1187, 2007.
[22] L. A. Daniels, Biol. Trace Elem. Res., 54, 185. 1996.
[23] M.D. Tilson, “Decreased Hepatic Copper Level. A possible Chemical Marker for the Pathogenesis of Aortic Aneurysms in Man,” Arch. Surg.; 117(9): 1212-
1213. 1982
[24] Kaim, W. and B. Schwederski,” Bioanorganische Chemie,” 2nd ed.. B. G.
Teubner, Stuttgart: 460, 1995.
[25] Y. Zhao, T.D. Oberley, L. Chaiswing, S.M. Lin, C.J. Epstein, T.T. Huang and D.
Clair, “Manganese Superoxide Dismutase Deficiency Enhances Cell Turnover Via Tumour Promoter-Induced Alterations in Ap-1 And P53-Mediated Pathways in a Skin Cancer Model”. Oncogene, 21(24): 3836-3846. 2002.
[26] T. C. G. Martins, E. A. De Nadai Fernandes, A. A. Ferrari, F. S. Tagliaferro, M.
A. Bacchi, “Chemical Characterization of Agricultural Supplies Applied to
Organic Tomato Cultivation,” J. Radioanal. Nucl. Chem, Vol. 278, No.2, 517–
520. 2008.
[27] NRC, Recommended Dietary Allowances, “NRC, Recommended Dietary
Allowances _RDA” 10th ed. Washington, DC: National Research Council,
1989.
IJSER © 2013 http://www.ijser.org



