International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 497
ISSN 2229-5518
Mechanisms of Nutrient Removal in Moving Bed
Biofilm Reactors
Wisam Sabeeh Al-Rekabi•
Abstract—This review paper presents the problems and challenges suffered by the conventional wastewater treatment plants, because the need for clean water is rapidly increasing as the world’s population grows by each year ,in addition to the great change in the required specifications for wastewater treatment , especially when the nutrient are become a major concern in the design and operational of wastewater treatment plants, so many conventional wastewater treatment facilities are needed to being expanded to provide additional capacity with least possible cost. This review paper also intends to provide an overall vision of the Moving Bed Biofilm technology as an alternative and successful method to overcome all those challenges. Fundamental research into Moving Bed Biofilm technology is presented in three sections, The Processes Of Moving Bed Biofilm Reactors , Factors Affecting Performance Of Moving Bed Biofilm Reactor, and The Mechanisms Of Nutrient Removal in Moving Bed Biofilm Reactor . The review also includes many relevant researches carried out at the laboratory, and pilot scales plants.
Keywords— Moving Bed Biofilm Reactor (MBBR) , Biological Nutrient Removal(BNR), Integrated Fixed-Film Activated Sludge (IFAS), Nitrogen, Nitrification ,Denitrification, Nitritation , Denitritation, Phosphor. Nitrosomanas, Nitrobactor.
—————————— ——————————
1 INTRODUCTION
Wastewater containing high levels of phosphorus and nitrogen cause several problems, such as eutrophication, oxygen consumption, and toxicity, when discharged into the environment [1]. It is, therefore, necessary to remove such substances from wastewaters in order to reduce their harm to the environment [2]. From the last two decades the nutrient are become a major concern in the design and operational of wastewater treatment plants. Various treatment methods, such as physical, chemical, and biological, have been used to deal with nutrient control and removal from the discharged system. Nutrient removal can be implemented by using biological treatment system because of it is low-cost, reliable, and effective [3].
————————————————
• Wisam Al-Rekabi is currently lecturer in civil Engineering Dept. in College of Engineering in University of Basrah, Iraq, E-mail: wesam752014@gmail.com
Nitrogen is an essential nutrient for plants and animals. Approximately 80 percent of the earth’s atmosphere is composed of nitrogen and it is a key element of proteins and cells. The major contributors of nitrogen to wastewater are human activities such as food preparation, showering, and waste excretion. The per capita contribution of nitrogen in domestic wastewater is about 1/5th of that for BOD. Total nitrogen in domestic wastewater typically ranges from 20 to 70 mg/L for low to high strength wastewater [4]. Factors affecting concentration include the extent of infiltration and the presence of industries. Influent concentration varies during the day and can vary significantly during rainfall events, as a result of inflow and infiltration to the collection system. The most common forms of nitrogen in wastewater are:
• Ammonia (NH3)
• Ammonium ion (NH4+)
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 498
ISSN 2229-5518
• Nitrite (NO2‐)
• Nitrate (NO3‐)
• Organic nitrogen
Nitrogen in domestic wastewater consists of approximately
60 to 70 percent ammoni‐ani trog en and 30 to 40 p ercent
organic nitrogen [4,5]. Most of the ammon‐iani trog en is
derived from urea, which breaks down rapidly to ammonia
in wastewater influent.
Total phosphorus (TP) in domestic wastewater typically ranges between 4 mg.L-1 and 8 mg.L-1 but can be higher depending on industrial sources, water conservation, or whether a detergent ban is in place. Sources of phosphorus are varied. Some phosphorus is present in all biological material, as it is an essential nutrient and part of a cell’s energy cycle. Phosphorus is used in fertilizers, detergents, and cleaning agents and is present in human and animal waste. Phosphorus in wastewater is in one of three forms:
• Phosphate (also called Orthophosphate)
• Polyphosphate, or
• Organically bound phosphorus.
The orthophosphate fraction is soluble and can be in one of several forms (e.g., phosphoric acid, phosphate ion) depending on the solution pH. Polyphosphates are hig‐h energy, condensed phosphates such as pyrophosphate and
trimetaphosphate. They are also soluble but will not be precipitated out of wastewater by metal salts or lime. They can be converted to phosphate through hydrolysis, which is very slow, or by biological activity .
Biological processes based upon suspended biomass are effective for organic carbon and nutrient removal from municipal wastewater plants. But there are some problems of sludge settle ability and the need for large reactors, settling tanks and biomass recycling [6-8].
Moving Bed Biofilm (MBB) processes have proved to be reliable for organic carbon and nutrient removal and are without some of the problems of activated sludge processes [9]. MBBRS are especially useful when slow growing organisms like nitrifiers have to be kept in a wastewater
treatment process. Both nitrification and denitrification processes have been individually successful in the biofilm reactor [2]. There are already many different biofilm systems in use, such as trickling filters, rotating biological contactors (RBCs), fixed media submerged biofilters, granular media biofilters and fluidized bed reactors-all of which have advantages and disadvantages. For these reasons, the MBB process was developed in Norway during the late 1980s and early 1990s [10,11].
The MBB process is a promising process for the enhancement of nitrification, denitrification and phosphorus removal in conventional activated sludge systems that can be used for upgrading biological nutrient removal, particularly when they have space limitations or need modifications that will require large monetary expenses [12]. The MBBR is a highly effective biological treatment process that has been developed on the basis of conventional activated sludge and biofilter processes. It is a completely mixed and continuously operated biofilm reactor, where the biomass is grown on small carrier elements that have a little lighter density than water and are kept in movement along with a water stream inside the reactor. The movement inside a reactor can be caused by aeration in an aerobic reactor and by a mechanical stirrer in an anaerobic or anoxic reactor.
There are presently more than 400 large-scale wastewater treatment plants based on this process in operation in 22 different countries all over the world [13]. During the past decade it has been successfully used for the treatment of many industrial effluents including pulp and paper industry waste [14], poultry processing wastewater [15], cheese factory wastes [16], refinery and slaughter house wastes [17], phenolic wastewater [18], dairy wastewater [19,20] and municipal wastewater [21-27]. Moreover, sequencing batch operation of MBBR has been attempted for biological phosphorus removal [28, 29].
The aim of the present study was to presents the problems and challenges suffered by the conventional wastewater treatment plants and provide an overall vision of the Moving Bed Biofilm technology as an alternative and successful method to overcome all those challenges.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 499
ISSN 2229-5518
Fundamental research into Moving Bed Biofilm technology is presented in three sections, The Processes Of Moving Bed Biofilm Reactors , Factors Affecting Performance Of Moving Bed Biofilm Reactor, and The Mechanisms Of Nutrient Removal in Moving Bed Biofilm Reactor . The review also includes many relevant researches carried out at the laboratory, and pilot scales plants.
2.0 BIOLOGICAL NUTRIENT REMOVAL
The biological nutrient removal (BNR) process is economically feasible for simultaneous organic substrate, nitrogen and phosphorus removal from wastewater. A number of BNR processes have been developed; meanwhile, various biochemical models have also been proposed in the past decade [30-35]. Nevertheless, the biological mechanisms are not completely understood [36,37]. Moreover, there are still some conflicts that need to be resolved.
Typically, a BNR process has anaerobic, anoxic and aerobic reactors; the activated sludge is repeatedly exposed to these conditions. In the anaerobic reactor, the activated sludge releases phosphorus, which is stored as polyphosphate in the aerobic reactor, and accumulates polyhydroxylalkanoates (PHA) when the carbon substrate is more abundant. Subsequently, the released phosphorus is uptake in excess by phosphate- accumulating organisms
(PAOs) in aerobic conditions; therefore, phosphorus can be removed from wastewater. In the anoxic reactor, nitrate decreases due to denitrification. Moreover, in the aerobic reactor, nitrification, organic substrate oxidation, and phosphorus uptake occur at the same time. Consequently, organic substrate, nitrogen and phosphorus can be removed simultaneously in a BNR process.
However, some conflicts arise when such a single sludge system is employed to remove nitrogen and phosphorus simultaneously [38]. Selecting the sludge retention time (SRT) of a process is a major task in BNR processes [39- 41]. Ammonium nitrogen can theoretically be removed by aerobic nitrification, followed by anoxic denitrification. In the sequence of reactions, the nitrification of autotrophic nitrifiers is normally a limiting reaction on nitrogen removal. Both longer SRT and higher dissolved oxygen (DO) conditions are prerequisites for improving nitrification. However, the phosphorus removal capacity corresponds to the amount of waste sludge and its phosphorus content. Under a shorter SRT and extreme anaerobic stage, PAOs can release more phosphorus and accumulate more polyphosphate in the anaerobic and aerobic stages, respectively. The process under shorter SRT yields better performance in terms of phosphorus removal. Consequently, difficulties in selecting SRT and DO arise when simultaneously removing nitrogen and phosphorus. These difficulties must be eliminated to upgrade the BNR process.
3.0 THE PROCESSES OF MOVING BED BIOFILM REACTORS (MBBRS)
Today many wastewater treatment facilities are being expanded to provide additional capacity because of increased flow and organic loading. Often these facilities have limited space due to encroaching development. As a result, processes continue to be developed to address the site constraints faced by municipal and industrial wastewater treatment facilities. The secondary treatment of the Wastewater Treatment Plant (WWTP) is usually accomplished by biological processes that can be classified as being either suspended or attached growth. Biological
treatment of domestic wastewater by conventional activated sludge process (CAS) has been practicing for more than 100 years. Since then activated sludge process modified numerous times in order to produce higher quality effluent. Even at present conventional activated sludge process considered as one of the most widely used and most economical ways of treating wastewater containing organic pollutants. Operational problems drastically reduced the efficiency of conventional activated sludge process. Most common operational problems in CAS
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 500
ISSN 2229-5518
are sludge bulking, sludge rising and Nocardia foam. Therefore generally CAS needs more attention by conducting frequent analytical tests and having an experienced crew to look after the system. Higher hydraulic retention time requirement is another drawback of CAS and this leads to higher tank volumes, finally end up in large foot print. Situation becomes worse when treating wastewater for nutrients in wastewater such as nitrogen and phosphorus, because the removal takes more time compared to other organic matter.
Due to the stringent rules and regulations in disposal of treated effluent to environment now a days there is a bloom in finding new treatment methods and process modifications to existing processes. Urbanization and increasing urban population increase the wastewater generation while reducing the available land area to build new treatment facilities. In order to face this challenge there is necessity of finding new treatment method which can produce higher quality effluent while having minimum foot print. In the last years, the idea to combine the two different processes (attached and suspended biomass) to increase the performances of an existing CAS system by increase the amount of biomass inside the reactor.
Moving Bed Biofilm Reactor (MBBR) was introduced in order to overcome some of the draw backs identified in the conventional activated sludge (CAS) process.
The idea of the MBBR process is a continuous flow process which combine the two different processes (attached and suspended biomass) by adding biofilm small high density polyethylene (HDPE) carrier elements with a large surface area and a density slightly less or heavier than 1×103 kg.m-
3 into the tank for biofilm attachment and growth has been proposed. This kind of system is usually referred as IFAS (Integrated Fixed-film Activated Sludge) process [42].
The carrier elements can be installed in either anaerobic , anoxic reactor or aeration basin,, the carrier media that is added for the growth of the attached biomass it can be fixed or freely moving inside the reactor. In this latter case, when the media is used on its own, the process is usually called moving bed biofilm reactor (MBBR) [43]. The agitation pattern in the reactor is designed to provide an upward movement of the carriers across the surface of the retention screen which creates a scrubbing effect to prevent clogging
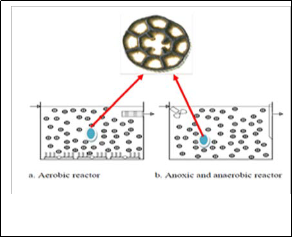
,so that the whole reactor volume is biologically active resulting in higher biomass activity. The MBBR and carrier in reactor with biofilm growth are shown in Fig.(1). The foremost difference between the MBBR and IFAS systems is the presence of a return activated sludge stream that remains central to the IFAS process. In the MBBR process, biomass is retained in the bioreactor through attachment to suspended carrier material and retention of carrier material using sieves. Nevertheless, recently in the case of moveable carrier media IFAS have been addressed as HMBBR (Hybrid Moving Bed Biofilm Reactors) process [44,45].
Fig. 1 : The MBBR and carrier in reactor with biofilm growth
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 501
ISSN 2229-5518
The Integrated Fixed-Film Activated Sludge (IFAS) process is typically installed as a retrofit solution for conventional activated sludge systems that are at or beyond capacity. IFAS upgrades offer an extremely cost-effective retrofit solution to municipal wastewater plant expansion, taking full advantage of existing systems, equipment, process knowledge, training, and operator skills. The technology is compatible with plug flow and complete mix configurations; IFAS hybrid processes are designed for complete compatibility with fine bubble aeration systems, providing demonstrated long-term operational cost savings.
The IFAS variation of the MBBR process gets its name from the integration of biofilm carrier technology within conventional activated sludge. This hybrid process (referred to as an integrated fixed-film activated sludge, or IFAS), enables activated sludge systems to achieve dramatic gains in volumetric productivity without increasing mixed liquor suspended solids (MLSS) levels in the process. By doing so, IFAS systems deliver improved performance while reducing the solids impact on clarification processes. As a result, clarification processes actually benefit from implementing IFAS technology.
Integration of fixed-film technologies and conventional activated sludge is not a ground-breaking approach in of itself. Facilities over the years have implemented caged ropes, cord media, hanging fabric, and other failed methods to increase the density of nitrifying and denitrifying bacteria populations within activated sludge systems.
The first moving bed biofilm reactor (MBBR) facility became operational in early 1990 in Norway and then was developed in Europe and United State of America. In 2000, there have been more than 400 large-scale wastewater treatment plants based on this process in operation in 22 different countries all over the word [46] .
The MBBR process is based on the biofilm principle that take advantage of both activated sludge process and conventional fixed film systems without theirs disadvantages. Reactor can be operated at very high load
and the process is insensitive to load variations and other distributances [9, 47]. Unlike most biofilm reactors, the reactor volume in the MBBR is totally mixed and consequently there is no dead or unused space in the reactor. In addition, this system has a small head loss and no need for recycling of biomass or sludge [48].
In the MBBR process the biomass grows both as suspended flocs and as attached biofilm .In this way, the carrier elements allow a higher biomass concentration to be maintained in the reactor compared to a suspended growth process, such as activated sludge. This increases the biological treatment capacity for a given reactor volume. Furthermore, the increase of the overall sludge age in the system leads to a favorable environment for the growth of nitrifying bacteria [49].Without the highly concentrated suspended bacterial population of activated sludge, the overall solids removal requirements are also reduced, allowing for the use of alternative technologies such as dissolved air flotation. In general the reactors are straightforward to install and maintain, requiring only a tank of adequate size and a bank of aerators. Odegaard et al. [50] proved that the treatment performance of MBBR is proportional to the installed biofilm surface area, so treatment upgrades can be performed by simply adding additional carriers to the same tank.
There are several different sizes and designs of carrier elements used in the MBBR process. The main four carriers shape are shown in Fig.(2) .The KMT carrier K1is the original kindles carrier that is mostly used . A lot of carriers were made of high-density polyethylene (density 0.95g.cm-
3) in order to avoid influence buoyancy differences. The surface areas given in Table(1) are estimations to the best of our ability. The total surface area consist of both inner and outer surfaces, while the effective surface area is that where biofilm seems to attach. The effective surface area of the KMT K1 and the AWT carriers were calculated as the whole inner area plus the area of the outer fins. The area between the fins was not included since visual inspection did not show any sign of growth here. For the ANOX carrier, the effective area is calculated as the inner area since there are no fins with outer area.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 502
ISSN 2229-5518
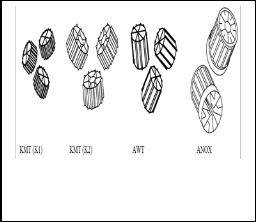
TABLE 1
CHARACTERISTIC DATA FOR THE FOUR DIFFERENT CARRIERS [9].
Fig. 2: The main four biofilm carriers [9].
The MBBR system finds several uses in both industrial and municipal wastewater treatment as:
stand-alone biological treatment process for BOD removal, nitrification and/or de-nitrification.
pre-treatment system ahead of an existing activated sludge system for increased organic matter removal.
Several configurations are possible to meet different treatment objectives. The different flow diagrams and treatment objectives are presented in Figure (2). As can be seen from the last example in Fig. (2) (flow scheme k) the MBBR carriers can also be used in a hybrid configuration with activated sludge.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 503
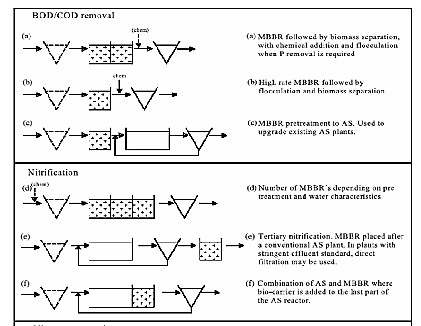
ISSN 2229-5518
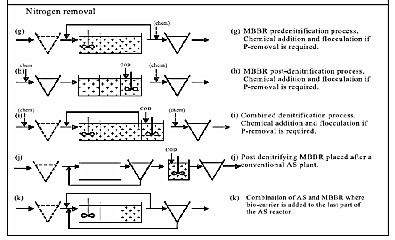
(a)
(b)
Fig.( 2):Typical flow schemes for various applications of the MBBR Process
(a: organic carbon and ammonia removal processes , b: nitrogen removal processes) [11].
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 504
ISSN 2229-5518
MBBR systems have been very useful in upgrading schemes. The small footprint of the reactor saves the cost of acquiring land at a high premium for conventional reactors. It has also been used in treating industrial wastewaters from food processing and paper and pulp industries [9,51]. Where phosphorus removal is desired, chemical coagulation is incorporated in a pretreatment or post treatment step. If coagulation is used in the pretreatment stage, it was found that suspended solids were removed, leaving low molecular weight soluble organic matter in the influent wastewater stream. The low influent suspended solid concentration increases the overall system efficiency [9].
According to Metcalf & Eddy[3], and Pastorelli et al. [29]
the advantages of MBBR includes:
1- Compact units with small size.
2- Increased treatment capacity.
3- Complete solids removal.
4- Improved settling characteristics.
5- Operation at higher suspended biomass
concentrations resulting in long sludge retention
times.
6- Enhanced process stability.
7- Low head loss.
8- No filter channeling.
9- No need of periodic backwashing.
10- Reduced sludge production, and no problems with
sludge bulking.
3.1 Factors Affecting Performance Of MBBR
The high specific area of the carrier media, which allows very high biofilm concentrations in a small reactor volume, controls the system performance. It was reported that typical biofilm concentrations range from 3000 to 4000 g TSS .m-3 [9], which is similar to values obtained in activated sludge processes with high sludge ages. It was inferred that, since the volumetric removal rate in the MBBR is several times higher than that in the activated sludge process, the biomass in the former are much more viable [9]. The percent of reactor volume comprised of media is limited to 70%, with 67% being typical [9]. However, the percentage of media required is based on wastewater characteristics and specific treatment goals. Values lower than 67% are frequently used.
Other factors reported to affect performance are flow and mixing conditions in the reactor. Adequate turbulence is ideal for efficient system performance. The nature of the carrier media used requires development of a very thin, evenly distributed and smooth biofilm to enable transport of substrate and oxygen to the biofilm surface. In this regard, thick and fluffy biofilms are not desired for this system. Adequate turbulence sloughs off excess biomass and maintains adequate thickness of biofilm. Biofilm thickness less than 100 μm for full substrate penetration is
usually preferred. Adequate turbulence also maintains flow velocities necessary for effective system performance [9]. Extremely high turbulence detaches biomass from the carrier and therefore is not recommended. In addition, collision and attrition of media in the reactor causes biofilm detachment from the outer surface of the Kaldnes media (carrier media used in experiment). Because of this, the MBBR carrier media is provided with fins on the outside to protect biofilm loss and promote growth of biofilm. The surface area of the fins does not contribute to the specific area reported [9]. The effective area of the MBBR carrier medium is reported to be 70% of the total surface area due to less attachment of biofilm on the outer perimeter of the media. Biomass density was determined from mass of biomass per media and volume of biomass per media, which were experimentally determined. Biofilm thickness was determined by slicing biofilm-containing media in a manner to enable good magnification and clear pictures of the biofilms to be taken. The sliced media pieces were viewed with an Olympus BH-2 phase contrast microscope supplemented with an Olympus PM-6 35-mm camera. Pictures of 3 different sliced media from each of the reactors were taken to enable an average biofilm thickness to be determined. Pictures were taken with an objective lens providing 4x magnification and with an ocular providing
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 505
ISSN 2229-5518
15x magnification, giving a magnification of 60x. Using the same magnification as the biofilm, a picture was taken of a slide with a metric scale, which enabled measurements to
20 μm. This enabled direct measurement of the biofilm thickness [52]. Fig. (3) shows a picture of a typical biofilm attached to a piece of sliced media and the metric scale.
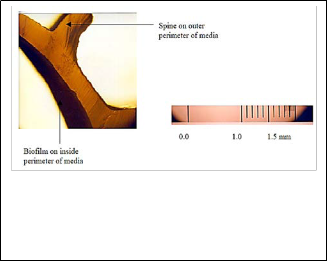
Due to the uncertainty in the actual area covered by the biofilm on the surface of the carrier, it is preferred to report system performance in terms of reactor volume instead of media surface area, which would have been appropriate.
The reactor volume assessment, however, enables the system to be compared with other systems, which use the entire reactor volume for treatment.
a b
4.0 THE PROCESSES OF NITROGEN REMOVAL IN MBBR
Nitrogen removal from domestic wastewater is a hot research topic during the last two decades. By using conventional primary and secondary treatment processes some part of the organic nitrogen which is associated with settleable solids can be removed. Most of the dissolved and colloidal organic and dissolved inorganic forms of nitrogen will be in the wastewater without affected. Nitrogen can be removed from the wastewater by advance biological processes, but addition of the tertiary treatment unit will increase the overall treatment cost as well as the land requirement. The removal of nitrogen can be achieved by two main processes; namely assimilation and nitrification- denitrification. In assimilation part of the total nitrogen is converted in to cell biomass by microorganisms. In nitrification-denitrification nitrogen removal takes place by two steps. In the first step (nitrification) ammonium nitrogen converts into nitrite by autotropic microorganisms called Nitrosomanas and further oxidized into nitrate by Nitrobactor. The second step (denitrification) nitrate is first converted to nitrite (NO2-) and then to nitrous oxide or laughing gas (N2O), nitric oxide (NO), and finally to nitrogen gas(N2) [53]. Usually, nitrite oxidation proceeds
Fig. 3: (a) Sliced MBBR media with biofilm.(b) Metric scale used to determine biofilm thickness [52].
faster than ammonia oxidation, so that nitrite rarely increases in the environment. This is very likely due to a minimum substrate concentration capable of steady state biomass and relatively high substrate uptake rate of the nitrite oxidizers [54]. Denitrification occurs under anoxic or/and anaerobic condition (dissolved oxygen concentration <0.5 mg.L-1). The transformation steps of the nitrogen removal presents in Fig.(4). Most of the biological nitrogen removal plants contain aerobic and anaerobic/anoxic processes separately.
Biological nitrification-denitrification over nitrate is considered as an efficient process characterized by a relatively easy operation and moderate costs [3].It is generally used for the treatment of wastewater containing low nitrogen concentration (<100 mg.L-1). This conventional biological nitrification and denitrification process is considered as more favorable than the chemical nitrogen removal by magnesium-ammonium-phosphate (MAP) precipitation or by air stripping for the removal of ammonium nitrogen from the wastewater [55].
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 506
ISSN 2229-5518
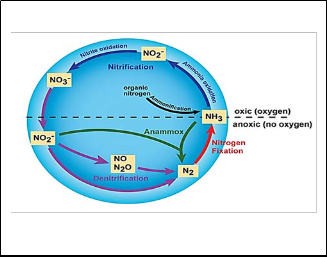
At present, treating domestic wastewater using only biological processes in an urban city is a challenging task due to the land scarcity and higher effluent discharge standards. Researchers in the previous studies developed the MBBR which combine the two different processes (attached and suspended biomass) is an attractive solution due to its compactness in foot print and higher effluent quality.
Fig. 4: The transformation steps of the nitrogen removal
4.1 Nitrification
Nitrification is the process that converts ammonia to nitrite and then to nitrate under aerobic conditions and using oxygen as the electron acceptor. The need for nitrification in wastewater treatment arises from water quality concerns over the effect of ammonia on receiving water with respect to DO concentration and fish toxicity, from the need to provide nitrogen removal to control the eutrophication, and in the control for water-reuse applications including groundwater recharge [3].
Nitrogen in raw sewage is found as ammonia and organic bound nitrogen in particulate matter. While nitrogen found
in particles can be removed by particle removal processes, ammonia must be converted to nitrate as the first step of the nitrogen removal process. Ammonia is converted to nitrate by autotrophic nitrification. This is a two-step process performed by autotrophic bacteria, where ammonia first is oxidised to nitrite and nitrite thereafter is oxidised to nitrate. The nitrification process is performed by a limited group of bacteria: Nitrosomonas and Nitrobacter. Nitrosomonas perform the oxidation of ammonia to nitrite, while Nitrobacter oxidise nitrite to nitrate. The stoichiometry of the two steps and the total reaction are given below in Equations 1, 2 and 3, respectively:
+
NH 4
3

+ O2 → NO2
2
+
+ H 2 O + 2 H
(1)
−
NO2
1

+ O2 → NO3
2
(2)
+
NH 4
+ 2O2 → NO3
+
+ 2 H
+ H 2 O
(3)
These autotrophic microorganisms derive energy for growth from the oxidation of inorganic nitrogen compounds, using in organic carbon as their source of
cellular carbon. In addition, the amount of alkalinity required to carry out the reaction (Eq. 3) can be estimated as equation (4):
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 507
ISSN 2229-5518
+
NH 4
−
+ 2 HCO3
+ 2O2 → NO3
+ 2CO2 + 3H 2 O
(4)
In the above equation, for each g of ammonia (as N) converted, 7.14 g of alkalinity as CaCO3 will be required [calculated as 2×(50 g CaCO3/eq)/14] [31].
Nitrifiers are mesophilic bacteria and have an increasing growth rate up to about 35 – 40 ºC, where the growth rate rapidly declines. The temperature dependency of the nitrification rate in the interval 10 – 22 oC can be described approximately by a simplified Arrhenius equation (Eq. 3.5).
rN-T2 = rN-T1 · θ(T2 – T1) (5)
Where:
T1, T2: temperatures, (oC)
rN-T1 : rate at temperature T1
rN-T2 : rate at temperature T2
θ : temperature coefficient
In previous studies with a MBBR, a value of 1.09 has been found for the temperature coefficient[46]. Pastorelli et. al [6] reported a value of 1.124.
The optimal pH for the nitrification process is between 8 and 9, [56]. In wastewaters with low alkalinity, the alkalinity consumption and corresponding drop in pH due to nitrification can lead to lower nitrification rates.
The nitrification rate is strongly dependant on the oxygen concentration. In activated sludge systems a DO- concentration of at least 2 mg DO/L is normally used in nitrifying processes. In biofilm processes, the nitrification rate can show a dependency on the DO concentration for much higher DO concentrations.
In previous experiments in MBBRs with a constructed wastewater an DO concentrations in the range 1 – 12 mg DO.L-1, it was found that the nitrification rate had a first order dependency on the DO concentration [57]. Later experiments with primary and secondary effluent showed lower nitrification rates, but the same increasing trend with increasing DO-concentrations [57]. Pastorelli et. al. [6,7] reported that the nitrification rate was nearly first order
with respect to DO concentration in pilot scale tests with
MBBRs.
The organic loading rate on the process also has a significant effect on nitrification. Heterotrophic bacteria have higher growth rates and will win the competition for space and oxygen in a given process configuration. In activated sludge systems the nitrifiers will be washed out as the fraction of heterotrophs increase. In a biofilm system there is a limited amount of growth area available. Heterotrophic growth will also consume oxygen and reduce the oxygen available for nitrification. In a biofilm process this can have a pronounced effect as will be discussed further below in relation to nitrification kinetics in a MBBR.
Nitrifying bacteria can be inhibited by compounds in the wastewater. The nitrifiers are probably not more sensitive than other bacteria, but because nitrification is performed by a limited group of bacteria with slow growth rates compared to heterotrophic bacteria, the effect of inhibitory compounds can be more pronounced for nitrification activity than for degradation of organic matter.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 508
ISSN 2229-5518
Previously Hem et al. [20]; Odegaard et al. [9]; Rusten et al. [25] studied the nitrification kinetics in a MBBR. The nitrification rate in a MBBR can be limited by the diffusion of ammonia or oxygen in to the biofilm. The ratio of oxygen concentration to ammonia concentration can be used to evaluate which substrate that is rate limiting. The nitrification rate is oxygen limited at DO/NH4-ratios < 2 mg DO / (mg NH4-N) and ammonia limited at DO/NH4-
ratios > 5 mg DO /(mg NH4-N) [20]. For DO/NH4- ratios between 2 and 5 there will be a transition from oxygen to ammonia limitation. A ratio of 3.2 mg DO (mg NH4-N)-1 has been reported as the transition ratio in the absence of organic matter [25]. In situations where ammonia is the limiting substrate, the nitrification rate may be calculated by Equation (6).
Where:
rN : nitrification rate, (g NH4-N m-2d-1)
rN = k · (SN )n (6)
k : reaction rate constant (g NH4-N m-3) (1-n) m-2d-1
SN : concentration of ammonia in the reactor, (g NH4-N m-3)
n : reaction order constant
Without limitation caused by liquid film diffusion, the reaction rate in a biofilm process changes from first order at low concentrations to half order at higher concentrations, with respect to the limiting substrate. An effect of liquid film diffusion is to increase the observed reaction order, which may then be higher than 0.5 at higher concentrations [56].
Liquid film diffusion has been found to be of importance in the MBBR process and Hem found a reaction order for Equation 5 of 0.7 [57]. The rate constant in Equation (6) can be determined from nitrification rates measured under ammonia limiting conditions. Rate constants in the range
0.7 – 1.0 were found in experiments with a secondary
effluent and oxygen concentrations of 4.5 – 5 mg DO /L
[57]. The maximum nitrification rate at a given bulk DO concentration and temperature can be found from Equation (6), by using the ammonia concentration at the point where the oxygen concentration becomes rate limiting. When the
transient ammonia concentration in the absence of organic matter is calculated, the heterotrophic oxygen consumption should be subtracted from the bulk oxygen concentration before calculation of the transient ammonia concentration.
An estimate of the heterotrophic oxygen consumption can be found from plots of nitrification rate versus oxygen concentration under conditions where the oxygen concentration is rate limiting. An oxygen consumption due to heterotrophic activity of 2.5 – 3 mg DO /L was found with a removal of soluble organic matter of 0.6 – 1.9 g SCOD m-2d-1. Nitrification rates of 1.01 g NH4-N m-2d-1 and 1.24 g NH4-N m-2d-1 were predicted for pre- and post- denitrifcation systems, respectively. The reported rates were for conditions with low organic loading rates, a temperature of 10 oC and an oxygen concentration of 10 mg DO.L-1, [25]. Odegaard et al. [9] and Pastorelli et al. [7] reported that oxygen levels from 2 to 3 mg O2.L-1 were needed in order for nitrification to take place.
4.2 Denitrification
Denitrification is performed by heterotrophic bacteria that use nitrate and nitrite as electron acceptor when organic matter is oxidised. This processes occurs under anoxic or/and anaerobic condition (dissolved oxygen
concentration <0.5 mg.L-1). Biological denitrification is coupled to the respiratory electron transport chain, and nitrate and nitrite are used as electron acceptor for the oxidation of a variety of organic electron donors. A wide
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 509
ISSN 2229-5518
range of bacteria has been shown as capable of denitification, but similar microbial capability has also been found in algae or fungi. Bacteria capable of denitrification are both heterotrophic and autotrophic. Most of these heterotrophic bacteria are facultative aerobic organisms with the ability to use oxygen as well as nitrate or nitrite, and some can also carry out fermentation in the absence of nitrate or oxygen [3].
Biological denitrification involves the biological oxidation of many organic substrates in wastewater treatment using nitrate or nitrite as the electron acceptor instead of oxygen. In the absence of DO or under limited DO concentrations, the nitrate reductase enzyme in the electron transport respiratory chain is induced, and helps to transfer hydrogen and electrons to the nitrate as the terminal electron acceptor. The nitrate reduction reactions involve the different reduction steps from nitrate to nitrite, to nitric oxide, to nitrous oxide, and to nitrogen gas.
NO → NO → NO → N O → N
(7)
3 2 2 2
The electron donor as an organic substrate is obtained through: the easily biodegradable COD in the influent wastewater (equation 8) or produced during endogenous
decay, or an exogenous source such methanol (equation 9) or acetate (equation 10). Different electron donors give different reaction stoichiometries as observed below.
−
10 NO3
+ C10 H19O3 N → 5 N 2 + 10CO2 + 3H 2O + 10OH
+ NH 3
(8)
−
6 NO3
+ 5CH 3OH → 3 N 2 + 5CO2 + 7 H 2 O + 6OH
(9)
−
8 NO3
+ 5CH 3COOH → 4 N 2 + 10CO2 + 6 H 2 O + 8OH
(10)
The term C10H19O3N is often used to represent the biodegradable organic matter in wastewaters. In all the above heterotrophic denitrification reactions, one equivalent of alkalinity is produced per equivalent of N- NO3- reduced, which equates to 3.57 g of alkalinity (as CaCO3) production per g of nitrate nitrogen reduced. So, one-half of the amount destroyed by nitrification can be recovered [3].
The pH increases during denitrification and reduces the pH reduction in the total nitrogen removal process. The optimal pH for denitrifying bacteria is in the same range as
typical for other heterotrophic bacteria, pH: 7 – 9. A pH
value lower than 7 increases the production of N2O2 [9].
The effect of other environmental factors such as temperature and nutrient requirements are in general about the same for denitrifying bacteria as for aerobic heterotrophic bacteria. Oxygen will be detrimental for the denitrification process because aerobic growth will consume organic matter needed for denitrification and because many denitrifying bacteria are facultative and will use oxygen as electron acceptor when this is available.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 510
ISSN 2229-5518
A sufficient amount of a carbon source (C-source) that is easily biodegradable is a prerequisite for denitrification. The minimum theoretical COD consumption for denitrifying respiration with acetic acid as C-source is 2.87 mg CODHAc /(mg NO3-N).
Volatile fatty acids (VFA) such as acetic acid give high denitrification rates, but a wide variety of C-sources can be used. In practice one tries to utilise the organic matter in the wastewater as C-source (internal C-source) as opposed to adding an external C-source. For this reason the denitrifying reactor(s) are often placed before the nitrifying reactors in a nitrogen removal plant. This process configuration is termed pre-denitrification and has a recycle of nitrified wastewater, as opposed to post-denitrification, where the denitrifying reactor are placed after the nitrifying reactors. The wastewater composition and effluent standards in a given case will be deciding for which process configuration that will be most favourable.
Denitrification in a MBBR process has been investigated in previous studies. Rusten et. al. [26] studied nitrogen removal from municipal wastewater in a pilot plant operated in pre-denitrification mode and later in post- denitrification mode. In pre-denitrification mode, the denitrification rates were limited by the availability of C- source and the maximum denitrification rates were as low as 0.4 g NOx-N. m-2.d-1. In postdenitrification mode with pre-precipitated wastewater, maximum denitrification rates of up to 2.2 g NOx-N. m-2.d-1 were measured with addition of additional C-source. Sodium acetate was used as additional Csource. The optimal C/N-ratio was fund to be 4 mg COD added/ (mg NOx-Neq). The reported C/N- ratio use nitrate equivalents (NOx-Neq) and therefore includes COD consumed by nitrite and oxygen as well as nitrate. Calculation of nitrate equivalents was done using
the following conversion factors 1 mg DO /L = 0.35 mg
NO3-Neq.L-1 and 1 mg NO2-N .L-1 = 0.6 mg NO3-Neq. .L-
1 [26].
Aspegren et. al. [5] reported a maximum denitrification rate of around 2.5 g NOx-N. m2.d-1 at 16 oC with ethanol ac C- source in a MBBR process for post-denitrification. The C/N-ratio required for complete denitrification was 4 – 5
COD added (mg NOx-Neq).L-1.
Pastorelli et. al. [6] reported an average denitrification rate of 2.2 g NOx-N. m-2.d-1 in batch tests with acetate as C- source and an average temperature of 20.1 oC.
Helness and Gisvold [58] studied nitrogen removal in a MBBR operated with intermittent aeration to achieve simultaneous nitrification and denitrification. The results showed that a C/N-ratio of about 3.5 mg CODNaAc-added
/ (mg NO3-Neq.) was required for a nitrogen removal
efficiency of 80 % or higher. A removal efficiency of 80 %
could be achieved with an anoxic NOx-N loading rate of about 1 g NOx-N. m-2.d-1. Complete nitrification was achieved at least up to an aerobic ammonia loading rate of
0.5 g NH4-N. m-2.d-1, indicating that the non-aerated phase should be about one third of the total cycle of non-
aeration – aeration.
To achieve simultaneous nitrification – denitrification, a relatively thick biofilm is needed in order to maintain anoxic conditions in the deeper layers of the biofilm. The total COD loading rate on the process is therefore of importance as there will be a correlation between the steady state biomass concentration and the total COD loading rate. The results indicated that a total COD loading rate of about 5 g COD. m-2.d-1 was required to achieve 80
% removal efficiency for NOx-N [18].
4.3 Nitritation and Denitritation
Nitritation process is basically identical to nitrification process. The only difference is that the step for the oxidation of nitrite to nitrate in nitrification process is
omitted in nitritation process. Conversely, denitritation is a process which directly removes nitrite instead of nitrate in the removal of nitrogen. In order to prevent nitrite
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 511
ISSN 2229-5518
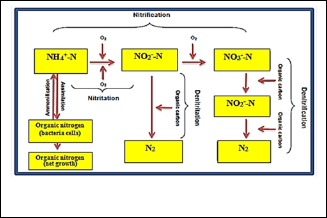
oxidation, aerobic and anoxic periods were alternated at pre-set time intervals. It was reported that the prevention of nitrite oxidation saves the oxygen required for the nitrification process (up to 25% total oxygen demand) and
40% of the organic carbon required in denitrification process [59]. Fig. (5) shows the processes involved in the removal of nitrogen species.
Fig. 5: Removal of nitrogen species.
5.0 THE PROCESSES OF PHOSPHOR REMOVAL IN MBBR
Phosphorus can be removed both chemically and biologically. Chemical phosphorus removal is done by precipitation of phosphate and coagulation – flocculation of particulate phosphorus using a metal salt of calcium, aluminum or iron. The main disadvantages of chemical phosphorus removal are the cost of chemicals and the relatively large sludge production that increases the cost of sludge treatment and the problems and cost of sludge disposal. Biological phosphorus removal offers an alternative to chemical treatment methods that has a potential for reduced sludge production. Biological phosphorus removal is performed by phosphate accumulating micro- organisms (PAO) that have the ability to accumulate phosphate over and above what is required for growth. This biological process is referred to as bio-P or enhanced biological phosphate removal (EBPR). Although the biochemical mechanism was not understood at the time, EBPR in activated sludge processes was reported as
early as 1965 [60]. Since then research in the field has progressed to identify some of the bacteria which are involved and also to clarify the biochemical mechanisms behind EBPR. However, there are still unresolved questions regarding both the bacteria responsible for EBPR and the biochemical mechanisms of EBPR.
In order to facilitate selection of the bacteria responsible for EBPR in a treatment plant, the biomass must be exposed to alternating anaerobic and aerobic or anoxic conditions. This can be done by alternating the conditions in a reactor, as in a sequencing batch reactor (SBR), or by moving the biomass from one reactor to another in a continuous process. Currently all full scale processes that remove both phosphorus and nitrogen biologically are based on activated sludge. One of the reasons for this is that exposing the biomass to alternating anaerobic and anoxic or aerobic conditions required for EBPR, can be achieved by
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 512
ISSN 2229-5518
circulating the biomass through reactors with different conditions. EBPR can be combined with nitrogen removal by adding an anaerobic stage in front of the nitrogen removal process. Several process configurations have been developed to optimise the combined process, including hybrid processes with use of suspended biofilm carriers in the activated sludge to enhance nitrification and achieve a more compact process with a lower hydraulic retention time (HRT).
Since EBPR is achieved by incorporation of phosphorus in the biomass, a high concentration of phosphorus accumulating biomass in the process is an advantage. However, phosphorus is removed from the process by withdrawal of excess sludge. Efficient phosphorus removal will therefore depend on efficient separation of the biomass even if efficient selection of PAO and a high biomass concentration are achieved.
An activated sludge process for EBPR will be heavily dependent on effective separation of biomass to achieve a high biomass concentration in the process, and a low effluent total phosphorus (tot-P) concentration since this is correlated to the effluent suspended solids concentration. The normal sludge separation method in activated sludge is settling in clarifiers. Biological activity can deplete the oxygen concentration in the settled sludge and lead to anaerobic conditions in the sludge collection part of the clarifiers. As will be discussed later, anaerobic conditions lead to phosphorus release from an EBPR-sludge. Efficient phosphorus removal in an activated sludge process for EBPR will therefore also be dependent on avoiding secondary phosphorus release from the sludge in the clarifiers.
In a pure biofilm process for EBPR the concentration of suspended solids in the influent to the sludge separation will be much lower than in an activated sludge process. This is a potential advantage with respect to the problems stated above because a lower amount of settled sludge in the clarifier reduces the problem of secondary phosphorus release. Dissolved air flotation (DAF) is an alternative sludge separation method that can ensure aerobic
conditions in separated sludge. The lower suspended solids concentration in the influent to the sludge separation with a biofilm process also makes use of flotation as separation method more attractive because the required amount of air for separation and thereby the cost is reduced compared to flotation with an activated sludge process. Also, in a biofilm process it can be easier to achieve a high biomass concentration than in an activated sludge process because there is no limitation due to sludge separation. The main disadvantage with a pure biofilm process for EBPR is that submitting the biomass to alternating anaerobic and aerobic/anoxic conditions, essential for selection of the PAO, requires a sequencing operation. In a biofilm process, this can be done by operating the reactor(s) as a SBR with alternating anaerobic and aerobic/anoxic conditions in a time sequence or by having several biofilm reactors, i.e. fixed bed filters, in a series with a sequence that changes at set intervals. This type of operation may be more complex and require more piping and valves than a continuous activated sludge process. A SBR process may also require additional volume, compared to a continuous process, to compensate for the time for filling and drawing the reactors. However, in a biofilm SBR one can empty the reactor completely after each cycle and therefore achieve an efficient utilization of the volume. Previously, simultaneous nitrification, denitrification and phosphate uptake in the aerated phase of the operating sequence has been reported in laboratory experiments with fixed bed filters [61]. Simultaneous nitrification, denitrification and phosphate uptake gives a potential for reduced hydraulic retention time in a pure biofilm process for EBPR and nitrogen removal because it can remove the need for a separate anoxic phase.
There are therefore potential advantages that suggest that a pure biofilm process can be an interesting alternative to activated sludge processes for biological phosphorus and nitrogen removal. With a moving bed there could be a possibility to achieve a process with continuous feed and exposure of the biomass to alternating conditions if the biofilm media could be moved through anaerobic and anoxic and/or aerobic zones in the reactor. One way of moving the media from one zone to another could be to
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 513
ISSN 2229-5518
pump the media from one compartment to another by an air-lift pump. However, this would probably cause problems when moving the media to an anaerobic or anoxic compartment because of the resulting carry-over of oxygen.
Another possibility could be to move the media hydraulically. In such a reactor the different zones could be separated by vertical walls with openings at the top and bottom. The media would then have to be moved in a vertical loop over and under the partitioning walls. This would probably be quite difficult to achieve in a satisfactory way, especially when one considers that the retention time should probably be different in the anaerobic and anoxic or aerobic zones. One could also build the reactor as a carrousel and move the media in a horizontal loop in the same principle as carousel activated sludge plants. By placing aerators and mixers at appropriate intervals it could be possible to obtain both good vertical mixing and horizontal movement of the media.
An alternative to a continuous process with a moving bed would be a sequencing process with the biofilm media in a
moving bed SBR or in a stationary filter bed. A moving bed SBR would probably resemble an activated sludge SBR in many aspects. However, use of a biofilm process would facilitate simultaneous nitrification and denitrifying phosphate uptake, and an efficient utilization of the reactor volume as discussed previously. In a process with filters one would remove biomass by back flushing of the filters and thus ensure that the biomass leaving the plant is phosphorus enriched. Also, one would probably not need a final clarifier after the biological reactors. However, a unit for separation of sludge and back flushing water would still be required.
A process based on a SBR or use of filters would need a more sophisticated control strategy than a moving bed carousel, and it would also probably be more expensive to build because of more piping and valves. However, a cost analysis would be required to conclude in this respect.
5.1 EBPR process
The enhanced biological phosphorus removal consists of incorporating the phosphorus present in the influent into cell biomass, which subsequently is removed from the process as a result of sludge wasting. The organisms responsible for this task are the phosphorus accumulating organisms (PAOs). To incorporate the phosphorus into the cell biomass it is necessary to apply two different conditions, anaerobic and aerobic, in order to encourage the biomass to grow and consume phosphorus.
EBPR has three main characteristics: anaerobic organic matter uptake and storage, anaerobic phosphate release and aerobic phosphate uptake far in excess of cell growth requirement. All the same time, three are storage compounds which play an important role in the metabolism of EBPR process. These are polyphosphate,
glycogen and poly-hydroxy-alcanoates (PHA). PHA can be found as poly-hydroxybutyrate (PHB) or poly- hydroxyvalerate (PHV).
Under anaerobic conditions, PAOs can accumulate Volatile Fatty Acids (VFAs) mainly acetate, produced by COD fermentation. Then, the VFA is stored inside the cell as poly-hydroxyalkanoates (PHAs), basically poly- hydroxybutyrate (PHB). The energy (in the form of Adenosine Tri-Phosphate, ATP) for this process is obtained from the degradation of stored polyphosphate (polyp) and glycolysis of the glycogen uilisation giving reducing power (NADH2). The poly-phosphate degradation results in the release of orthophosphate in the liquid media Fig. (6).
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 514
ISSN 2229-5518
Whereas under aerobic or anoxic conditions, the PHA is metabolized providing energy (NADH2) and carbon source, to produce more cells and replenish the glycogen pool. Thus, the energy, NADH2 is converted into ATP. The energy from ATP is used by PAOs to grow, take up the excess soluble orthophosphate in order to recover and increase the polyphosphate (polyP) pool in the cell, and to
form glycogen Figure (6), in turn leading to a net phosphate
removal from the wastewater.
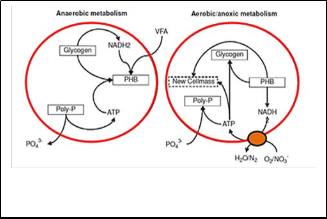
The main difference between aerobic and anoxic phosphate uptake is that for the formation of ATP under anoxic conditions, nitrate is used. The rest of the metabolism of PAOs under aerobic and anoxic conditions remains identical.
Fig. 6: Schematic diagram of the metabolism of polyphosphate- accumulating organisms [62].
Under anoxic conditions, however, approximately 40% less ATP is formed per amount of NADH2 than under aerobic conditions. This low ATP/NADH2 ratio means an end result of lower biomass production under anoxic conditions[63].
As well as the energy needed for growth, extra energy is also necessary to execute and maintain this cycle. Because of this the metabolism of PAOs requires more energy than that of other heterotrophic microorganisms (non-PAOs). In an aerobic activated sludge process, PAOs would not be able to survive like the other heterotrophic microorganisms. An anaerobic phase and rapid uptake of substrate in the
anaerobic phase constitute the key factors in maintaining PAOs in a biological phosphorus removal process. Conditions for this rapid uptake are glycogen and polyphosphate cycles. In the aerobic/anoxic phase the recovery of glycogen and polyphosphate for PAOs may be more important than bacterial growth [63].
The performance of EBPR can become unstable, especially when it is applied in combination with the biological nitrogen removal process. This instability could be explained by competition with Glycogen Accumulating Organisms (GAOs) or the introduction of nitrate into the anaerobic phase [64].
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 515
ISSN 2229-5518
6.0 CONCLUSIONS
Recent studies have combined suspension and attach growth systems by using the MBBR technology as an alternative way to resolve the difficulty in selecting SRT and DO. The combined system can provide two kinds of bacteria populations in the process: suspended activated sludge bacteria for enhancing phosphorus removal and biofilm bacteria with a long sludge age for improving nitrification-dentrification. The combined system seems highly promising for simultaneous removal of nitrogen and phosphorus.
Researchers have proven that MBBR possesses many excellent traits such as high biomass, high chemical oxygen demand (COD) loading, strong tolerance to loading impact, relatively smaller reactor and no sludge bulking problem .
MBBR systems continue to draw significant research attention. While this review is not an all-encompassing documentation for the this processe , it does provide an opportunity to reflect on what biofilm and hybrid biofilm systems may still have to offer the wastewater and environmental research and engineering community.
ACKNOWLEDGEMENTS:
Firstly I would like to express my sincere gratitude to my supervisor, Professor He Qiang, for his guidance, encouragement, and support throughout this research. Thanks should also go to the staff members of my college and all my Chinese classmates at my supervisor office for
their cooperation and help. I also would like to thank the CSC China Scholarship Council for providing me with opportunity to come and study in Chongqing university , I also would like to thank the Iraqi government for support and facilities especially the staff of the embassy in Beijing.
REFERENCES:
[1] Luostarinen S, Luste S, Valentin L, Rintala J (2006). Nitrogen removal from on-site treated anaerobic effluents using intermittently aerated moving bed biofilm reactors at low temperatures. Water Res. 40: 1607-1615.
[2] Wang XJ, Xia SQ, Chen L, Zhao JF, Renault NJ, Chovelon JM (2006).
Nutrients removal from municipal wastewater by chemical precipitation
in a moving bed biofilm reactor. Process Biochem. 41: 824-828.
[3] Metcalf and Eddy, Inc., Wastewater Engineering: Treatment, Disposal and Reuse, 4th Ed.; Tchobanoglous, G., Burton, F.L., and Stensel, D.H..; McGraw-Hill: New York, 2003.
[4] Tchobanoglous, G., F. L. Burton, and H.D. Stensel.. Wastewater Engineering:
Treatment and Reuse. New York, NY: McGraw ‐H ill,2003.
[5] Crites R. and G. Tchobanoglous.. Small and Decentralized Wastewater
Management Systems. New York, NY: McGraw Hill,1998.
[6] Pastorelli G, Andreottola G, Canziani R, Darriulat C, de Fraja Frangipane E, Rozzi A (1997a). Organic carbon and nitrogen removal in moving-bed biofilm reactors. Water Sci Technol. 35: 91-99.
[7] Pastorelli G, Andreottola G, Canziani R, de Fraja Frangipane E, de Pascalis F,
Gurrieri G, Rozzi A (1997b). Pilot-plant experiments with moving bed
biofilm reactors. Water Sci Technol. 36: 43-50.
[8] Pastorelli G, Canziani R, Pedrazzi L, Rozzi A (1999). Phosphorus and nitrogen removal in moving-bed sequencing batch biofilm reactors. Water Sci Technol. 40: 169-176.
[9] Odegaard, H., Rusten, B. and Westrum, T. (1994) A new moving bed biofilm
reactor — Applications and results. Water Science and Technology, 29(10–
11), 157–65.
[10] Odegaard, H., Rusten B, Siljudalen J (1999). The development of the moving bed biofilm process-from idea to commercial product. Eur. Wat. Manage. 2: 36-43.
[11] Odegaard H. (2006). Innovations in wastewater treatment: the moving bed bioreactor. Water Science and Technology, 53 (9), 17-33.
[12] Hooshyari B, Azimi A, Mehrdadi N (2009). Kinetic analysis of enhanced biological phosphorus removal in a hybrid integrated fixed film activated sludge process. Int J Environ Sci Tech. 6: 149-158.
[13] Rusten B, Eikebrokk B, Ulgenes Y, Lygren E (2006). Design and operations of the Kaldnes moving bed biofilm reactors. Aquacult Eng. 34: 322-331.
[14] Jahren SJ, Rintala JA, Odegaard H (2002). Aerobic moving bed biofilm
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 516
ISSN 2229-5518
reactor treating thermomechanical pulping whitewater under thermophilic conditions. Water Res. 36: 1067-1075.
[15] Rusten B, Siljudalen JG, Wien A, Eidem D (1998). Biological pretreatment of
poultry processing wastewater. Water Sci Technol. 38: 19-28.
[16] Rusten B, Siljudalen JG, Strand H (1996). Upgrading of a biological- chemical treatment plant for cheese factory wastewater. Water Sci Technol. 34: 41-49.
[17] Johnson CH, Page MW, Blaha L (2000). Full scale moving bed biofilm reactor results from refinery and slaughter house treatment facilities. Water Sci Technol. 41: 401-407.
[18] Hosseini SH, Borghei SM (2005). The treatment of phenolic wastewater
using a moving bed bio-reactor. Process Biochem. 40: 1027-1031.
[19] Andreottola G, Foladori P, Ragazzi M, Villa R (2002). Dairy wastewater
treatment in a moving bed biofilm reactor. Water Sci Technol. 45: 321-328.
[20] Rusten B, Odegaard H, Lundar A(1992). Treatment of dairy wastewater in a novel moving bed biofilm reactor. Water Sci Technol. 26: 703-711.
[21] Andreottola G, Foladori P, Ragazzi M, Tatano F (2000a). Experimental
comparison between MBBR and activated sludge system for the treatment of municipal wastewater.
Water Sci Technol. 41: 375-382.
[22] Andreottola G, Foladori P, Ragazzi M (2000b). Upgrading of a small wastewater treatment plant in a cold climate region using a moving bed biofilm reactor (MBBR) system. Water Sci Technol. 41: 177-185.
[23] Andreottola G, Foladori P, Gatti G, Nardelli P, Pettena M, Ragazzi M (2003). Upgrading of a Small Overloaded Activated Sludge Plant Using a MBBR System. J Environ Sci Health. Part A. A 38: 2317-2328.
[24] Rusten B, Siljudalen JG, Nordeidet B (1994). Upgrading to nitrogen removal
with the KMT moving bed biofilm process. Water Sci Technol. 29: 185-195.
[25] Rusten B, Hem L, Odegaard H (1995a). Nitrification of municipal
wastewater in moving-bed biofilm reactors. Water Environ Res. 67: 75-86.
[26] Rusten B, Hem L, Odegaard H (1995b). Nitrogen removal from dilute wastewater in cold climate using moving bed biofilm reactors. Water Environ Res. 67: 65-74.
[27] Rusten B, Kolkinn O, Odegaard H (1997). Moving bed biofilm reactors and chemical precipitation for high efficiency treatment of wastewater from small communities. Water Sci Technol. 35: 71-79.
[28] Helness H (2007). Biological phosphorous removal in a moving bed biofilm reactor. Doctoral Dissertation, Norwegian University of Science and Technology, Norway.
[29] Pastorelli G, Canziani R, Pedrazzi L, Rozzi A (1999). Phosphorus and nitrogen removal in moving-bed sequencing batch biofilm reactors. Water Sci Technol. 40: 169-176.
[30] Comeau, Y., K. J. Hall, R. E. W. Hancock, and W. K. Oldham (1986)
Biochemical model for enhanced biological phosphorus removal. Wat.
Res., 20(12), 1511-1521.
[31] Cooper, P., M. Day, and V. Thomas (1994) Process options for phosphorus and nitrogen removal from wastewater. J. IWEM, 8(February), 84-92.
[32] Mino, T., W. T. Liu, F. Kurisu, and T. Matsuo (1995). Modeling glycogen storage and denitrification capability of microorganisms in enhanced biological phosphate removal processes. Wat. Sci. Tech., 31(2), 25-34.
[33] Randall, C. W., J. L. Barnard, and H. D. Stensel (1992). Design and Retrofit
of Wastewater Treatment Plants for Biological Nutrient Removal, Chap. 1-
4. Technomic Publishing Company, Inc., Lancaster, PA, U.S.A.
[34] Smolders, G. J. F., M. C. M. van Loosdrecht, and J. J. Heijnen (1995). A metabolic model for the biological phosphorus removal process. Wat. Sci. Tech., 31(2), 79-93.
[35] Wentzel, M. C., L. H. Lotter, G. A. Ekama, R. E. Loewenthal, and G. v. R.
Marais (1986) Metabolic behavior of Acinetobacter spp. in enhanced
biological phosphorus removal - a biochemical model. Water SA, 12, 209-
224.
[36] Gujer, W., M. Henze, T. Mino, T. Matsuo, M. C. Wentzel, and G. v. R.
Marais (1995) The activated sludge model No. 2: biological phosphorus removal. Wat. Sci. Tech., 31(2), 1-11.
[37] Satoh, H., T. Mino, and T. Matsuo (1994) Deterioration of enhanced biological phosphorus removal by the domination of microorganisms without poly-phosphate accumulation. Wat. Sci. & Tech., 30(6), 203-211.
[38] Su, J. L. and C. F. Ouyang (1996) Nutrient removal using a combined
process with activated sludge and fixed biofilm. Wat. Sci. Tech., 34(1-2),
477-486.
[39] Fukase, T., M. Shibata, and Y. Miyaji (1984) The role of anaerobic stage on
biological phosphorus removal. Wat. Sci. Tech., 17, 69-80.
[40] Daigger, G. T. and S. R. Dolson (1991) Design and operation of biological phosphorus removal facilities, In: Phosphorus and Nitrogen Removal from Municipal Wastewater, pp. 167-201. R.I. Sedlak Ed. Lewis Publisher, New York, NY, U.S.A.
[41] Orhon, D. and N. Artan (1994) Modelling of Activated Sludge Systems, pp.
61-69, 397-410. Technomic Publishing Company, Inc., Lancaster, PA,
U.S.A.
[42] Sriwiriyarat, T. & Randall, C. W.( 2005). Evaluation of integrated fixed film activated sludge wastewater treatment processes at high mean cells residence time and low temperatures. J. Environ. Eng. ASCE 131(11),
1550–1556.
[43] Germain, E., Bancroft, L., Dawson, A., Hinrichs, C., Fricker, L. & Pearce, P. (2007). Evaluation oh hybrid processes for nitrification by comparing MBBR/AS and IFAS configurations. Water Sci. Technol. 55(8–9), 43–49.
[44] Di Trapani, D., Mannina, G., Torregrossa, M. & Viviani, G.(2008 a). Hybrid
moving bed biofilm reactors: a pilot plant experiment. Water Sci. Technol.
57(10), 1539–1545.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 517
ISSN 2229-5518
[45] Di Trapani, D., Odegaard, H. & Viviani, G.( 2008 b). Municipal wastewater treatment in a hybrid activated sludge/biofilm reactor: a pilot plant experience. Proceedings of the “1st IWA National Young Water Professional Conference”. Mexico City, Mexico. April, 9–11, 2008.
[46] Maurer, M., Fux, C., Graff, M., Siegrist, H., (2000). Moving bed biological
treatment (MBBT) of municipal wastewater: denitrification. Water Sci. & Tech., 43(4-5): 337-344.
[47] Delenfort, E., and Thulin, P., (1997). The use of Kaldnes suspended carrier process in treatment of wastewaters from the forest industry. Wat.Sci.Tech., 35(2-3):123-130.
[48] Xiao, L.W., Rodgers, M., Mulqueen, J., (2007). Organic carbon and nitrogen removal from a strong wastewater using a denitrifying suspended growth reactor and a horizontal-flow biofilm reactor. Bioresource Technology., 98: 739–744.
[49] Randall, C. W. & Sen, D. (1996). Full-scale evaluation of an integrated fixed- film activated sludge (IFAS) process for enhanced nitrogen removal. Water Sci. Technol. 33–12, 155–162.
[50] Odegaard, H., Gisvold, B., & Strickland, J. (2000). The Influence of Carrier Size and Shape in The Moving Bed Biofilm Process. Water Science and Technology , 41 (4-5), pp. 383-391.
[51] Rusten, B., Johnson, C. H., Devall, S., Davoren, D., Cashion, B. S., (1999).
Biological Pretreatment of a Chemical Plant Wastewater in High-Rate
Moving Bed Biofilm Reactors. Water Sci. Tech. Vol. 39, No. 10-11, pp. 257-
264.
[52] Kofi Asidu .(2001). Evaluating Biological Treatment Systems ( Moving Bed Biofilm Reactor Versus Biological Aerated Filtration And Sulfide- Induced Corrosion In Anaerobic Digester Gas Piping).Master Thesis. Department of Civil and Environmental Engineering, Virginia Polytechnic Institute and State University.
[53] Wang, J., and Yang, N., (2004). Partial nitrification under limited dissolved
oxygen conditions. Process Biochemistry., 39:1223-1229.
[54] Rittmann, B.E., and McCarty, P.L., (2001). Environmental biotechnology:
principles and applications. McGraw-Hill, New York: 470-4.
[55] Siegrist H. (1996). Nitrogen removal from digester supernatant— Comparison of chemical and biological methods. Water Science and Technology, 34(1-2), 399 - 406.
[56] Henze M., Harremoës P. la Cour Jansen J. and Arvin E. (1990): Spildevandsrensing biologisk og kemisk. Polyteknisk Forlag, Lyngby, Denmark. In Danish.
[57] Hem, L. J., Rusten, B., Odegaard, H., (1994). Nitrification in a Moving Bed
Biofilm Reactor. Water Research vol. 28 (6) pp. 1425 – 1433 .
[58] Helness, H. and Odegaard, H. (2001): Biological phosphorus and nitrogen removal in a sequencing batch moving bed biofilm reactor. Wat. Sci. Tech, Vol. 43, No. 1, pp. 233-240.
[59] Fux C, Velten S, Carozzi V, Solley D Keller J (2006). Efficient and stable nitritation and denitritation of ammonium-rich sludge dewatering liquor using an SBR with continuous loading. Water Res. 40 , 2765–2775.
[60] Levin G. V. and Shapiro J (1965): Metabolic uptake of phosphorus by
wastewater organisms. J. WPCF., Vol. 37, pp. 800-821.
[61] Garzón-Zúñiga M. A. and González-Martínez S. (1996): Biological phosphate and nitrogen removal in a biofilm sequencing batch reactor. Wat. Sci. Tech., Vol. 34, No. 1-2, pp. 293-301.
[62] Zhiguo Yuan , Steven Pratt ,Damien J Batstone ,(2012) . Phosphorus recovery from wastewater through microbial processes . Biochemical Engineering Journal .23 ,878–883.
[63] Janssen, P.M.J.,Meinema, K.,van der Roest, H.F. (2002) Biological phosphorus Removal: Manual for design and operation, 1st. IWA Publishing, Amersfoort, Netherlands.
[64] Saito, T., Brdjanovic, D., Van Loosdrecht, M.C.M. (2004). Effect of nitrite on phosphate uptake by phosphate accumulating organisms. Water Research, 38, 3760-3768.
IJSER © 2015 http://www.ijser.org







