International Journal of Scientific & Engineering Research, Volume 3, Issue 2, February-2012 1
ISSN 2229-5518
Magnetic Susceptibility of Superconducting
MgB2 Nanomaterials
Reynante C. Embalzado, Gil Nonato C. Santos
Abstract — Superconducting MgB2 nanomaterials were synthesized using the Horizontal Vapor Phase Crystal Growth
(HVPCG) technique with growth temperatures of 900℃, 1000℃, 1100℃ and 1200℃ with heating time of 2 to 12 hours with a
two- hour increment. The grown structures were characterized by its surface morphology, crystal structure and critical
temperature. Results revealed that the critical temperature of MgB2 nanomaterials fabricated at 1000°C was at 33K. SEM images revealed nanomaterials of different structures such as nanowires, nanobelts, nanorods and nanoparticles. Crystal structure of the synthesized materials revealed that the nanomaterials have growth orientation of (100), (101), and (110), which indicated that the synthesized structures are polycrystalline.
Index Terms— Nanomaterials, Horizontal Vapor Phase Crystal Growth, Superconducting, Magnetic Susceptibiility
—————————— ——————————
1 INTRODUCTION
he studies of the MgB2 and its derivative have been focused on the bulk, thin films, and wires. Those forms of materials can be productively synthesized with well-
established techniques and their commercial products have been successfully manufactured. Since the discovery of car- bon nanotubes in 1991, the worldwide nanotechnology re- search has been extended to space-restrained physical phe- nomena. Fabrication and investigation of nanoscale devices of MgB2 provide the fundamental understanding of the ef- fect of dimensionality and size on superconductivity. MgB2 is a promising superconductor for practical applications in the field of superconducting magnets for MRI, due to its high Tc and relatively low material costs. The development of tapes and wires was much faster than for many others HTS and LTS materials and commercial wires and tapes became available only a few years after its discovery. There have been limited reports on the fabrication of MgB2 nano- materials. Most of the synthesis methods used to grow MgB2 nanomaterials are multi-steps. Lai et al [1] fabricated MgB2 nanowires using vapor transport and reaction process, Zhao et al [4] synthesized nanowires through reactive sintering, Zhou et al [5] fabricated MgB2 tubelike nanostructures using thermal evaporation of MgB2 particles precursors. The other synthesis methods to grow MgB2 nanowires are the direct pyrolysis from MgB2 nanoparticles [6] or the pyrolysis from the mixed gel in the diborane-N2 atmosphere [7]. The pur- pose of the study is to synthesize and characterize Magne- sium Diboride (MgB2) nanomaterials using horizontal vapor phase crystal growth technique. The study also aims to in-
————————————————
Reynante Embalzado, Master of Science in Physics, De La Salle University
-Manila Philippines, E-mail:
Gil Nonato C. Santos, Doctor of Philosophy in Materials Science and En- gineering, De La Salle University-Manila, Philippines. E-mail: san-
tosg@dlsu.edu.ph
vestigate the optimum growth parameters on fabricating nanomaterials. The effect of nanoscale crystal size on the superconducting property of Magnesium Diboride will be examined.
2 EXPERIMENTAL SECTION
2.1 Synthesis of MgB2 Nanomaterials
Thirty-five milligrams (35mg) of Magnesium Diboride powder from Accumet Materials Co. was used and loaded into a clean quartz tube and was sealed at one end. The tube was then connected to a high vacuum system and sealed at
10-6 Torr. The tube was then heated in the horizontal tube furnace with varying temperature of 900°C, 1000°C, 1100°C
and 1200°C with growth times of 2, 4, 6, 8, 10 and 12 hours
and with constant ramp time of 80 minutes. The tube was then allowed to cool down to room temperature, after which it was opened for investigation. The grown materials were then characterized using the scanning electron microscope, X-ray dispersive spectroscopy and the HALL apparatus.
3 RESULTS AND DISCUSSION
It was observed that varying the growth parameters re- sulted to the formation of different nanostructures which include nanowires, nanobelts, nanorods, nanosheets and nanochains, with nanowires as the dominant structures. Ta- ble 1 shows the optimum growth parameters of each nanos- tructure. For the as-synthesized material, the dominant peak from (101) plane can be observed while weaker diffraction peaks are seen from (100) and (110) planes. This polycrys- talline nature of the material can be verified from the SEM images. The miller indices of the dominant MgB2 peaks cor- respond to a hexagonal crystal structure with lattice parame- ters of a and c – axes of 3.085 Å and 3.524 Å, respectively, which is identical that of the bulk sample. The impurities MgO and MgB4 are also observed in the synthesized mate-
IJSER © 2012
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 2, February-2012 2
ISSN 2229-5518
rial and their intensities seemed to increase. The additional MgO and MgB4 could be the result of the reaction of the trapped oxygen in the quartz tube during heating.
Table 1. Optimum Growth parameters for MgB2 nanomate- rials
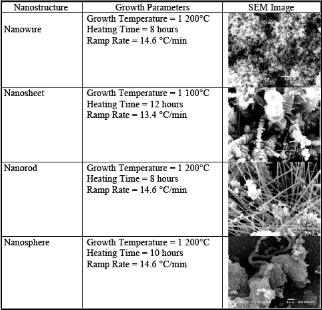
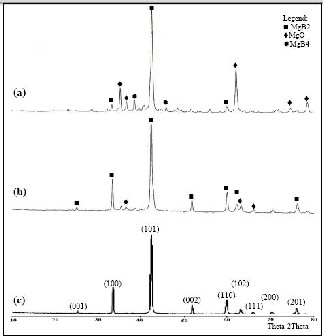
Figure 1. X-ray diffraction patterns of the (a) synthesized nanomaterial, (b) initial sample and (c) high purity powder.
The ρ-T curve of the synthesized material is shown in Figure 2b. The nanomaterial displays semiconductor behav- ior, the same with the bulk counterpart. The zero resistance transition temperature deduced from the measurement is
13K with an onset temperature of 33K, which is lower com- pared to their bulk counterparts. The rough curve could be the result of the additional MgO present in the sample. EDX test showed that the oxygen content in the fabricated mate- rial has increased by a certain amount. This was obtained during the heating process where the sample reacted with the oxygen that was trapped in the quartz tube during sealing. The low Tc (33K) of the nanomaterial was due to the in- crease of the grain boundaries of the material which resulted to less connectivity and more resistance. This result is con- sistent as reported in the literature, where the decrease in the crystal size of MgB2 resulted to the decrease in its critical temperature. The result suggests that the critical temperature of MgB2 superconductor depends on its grain size. The smaller the grains are, the lesser the critical temperature. By changing the grain size to nanoscale the Tc has reduced by
1K. Materials synthesized at heating temperature at 1100°C and 1200°C did not show superconductivity, instead the materials became insulator. This is due to the increase of impurities present in the material.
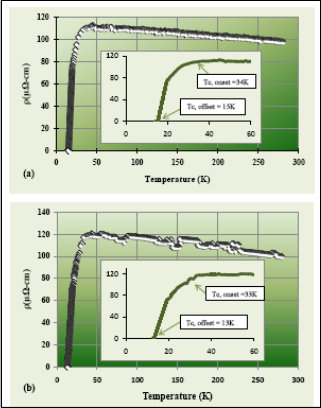
Figure 2. The resistance versus temperature curves of (a)
bulk sample and (b) nanomaterials fabricated at 1000°C in
6h.
4 CONCLUSION
In this study, Magnesium Diboride nanomaterials were fabricated using Horizontal Vapor Phase Crystal Growth method. SEM characterization revealed nanomaterials of
IJSER © 2012
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 2, February-2012 3
ISSN 2229-5518
different sizes are grown inside the quartz tube. In a 900°C heating temperature, unreacted magnesium diboride powder was observed and no nanomaterial was formed. When the temperature was increased to 1000°C, some microstructures particularly microspheres were observed. At higher tempera- tures most especially, more nanostructure materials were observed. Dense and tangled nanowires are predominantly observed on higher heating times. These nanowires are sev- eral micrometers in length and 60 -170 nm in diameter. The diameter, moreover, seems to correlate the heating time, therefore, more heating time resulted in thinner diameters of MgB2 nanowires. XRD patterns of the synthesized material reveal dominant MgB2 phase and trace amounts of impurity phases. Miller indices are indicated in each peak. The dif- fraction peaks of (100), (110), and (110) correspond to MgB2 hexagonal structures with calculated parameters of a and c –axes of 3.085 Å and 3.524, respectively, which is a match with the bulk sample. The presence of the impurity is most likely due to the reaction of the initial material with the air atmosphere. In addition, during heating, the trapped oxy- gen in the quartz tube reacted with the powder, thus, produc- ing MgO and MgB4. Electrical resistivity of the fabricated nanomaterials at 1000°C was measured using four-point probe method. The result shows that the critical temperature of the synthesized material is 33K which is below the Tc of the bulk counterpart. The transition width is 20K, which is very broad. The low critical temperature of the material is due to the increase of the grain boundaries of the sample which resulted to less connectivity and more resistance. The broadened transition width is indicative of large amount of defects and impurities within the sample. Materials synthe- sized at heating temperature of 1 100°C and 1 200°C did not show superconductivity, due to the increase of impurities present in the material. The findings suggest that the opti- mum growth parameters are 12 hours and 1 200°C. More nanomaterials are grown in the heating time of 12 hours and growth temperature of 1 200°C such as nanowires, nanorods and nanobelts. The study shows that the growth temperature is vital to control the size and number of the nanostructures. The higher the temperature, the more materials sublimed and traveled in the colder region of the quartz tube that resulted to the formation of more nanomaterials. The XRD test shows that the crystal structure of the fabricated material is hexagonal, which is the same with the initial material. Four- point resistivity measurements imply that the nanomaterials are still superconductors. However, the critical temperature has decreased by 1K. This is because of the size dependence of Tc of the superconducting material. The smaller the crys- tal size of MgB2 powder, the lower its Tc.
5 RECOMMENDATION
Further characterization should be done on the materials synthesized by this method such as the hall mobility, mag- netic susceptibility, critical current density and critical field. It is also recommended the use high resolution imaging so as
to fully investigate the surface morphology and growth mechanism of the synthesized materials. It is also proposed the use of Energy Dispersive X-ray (EDX) that can quantify Boron element so as to study the effect of varying growth time and temperature on the stoichiometric composition of the synthesized materials.
REFERENCES
[1] Lai, S.H., Liu,S.C., Lan, M.D. (2007). Fabrication of MgB2 Nanowire and its Superconductivity, Journal of Crystal Growth, 304, 460-463.
[2] Zhao, Q., Liu, Y. C., Shi, Q., Gao, Z. (2007). Character- istic and synthesis mechanism of MgB2 nanoparticles in solid-state reactive sintering. Physica C 467, 145-149.
[3] Zhou, S. M., Wang, P., Li, S., Zhang, B., Gong, H. C., Zang, X. T. (2009). Superconducting Single Crystalline MgB2 Nanotubes, Materials Letters, 1680-1682.
[4] Bando, Ma. R., Mori, T., Golberg, D. 2003. Direct Py- rolysis Method for Superconducting Crystalline MgB2
Nanowires, Chemical Materials, 15 (16), 3194–3197.
[5] Nath, M., Parkinson, B.A. (2006). A simple Sol-Gel Synthesis of Superconducting MgB2 Nanowires, Ad- vanced Materials, 18, 1865-1868.
[6] Glowacki, B.A., Majoros, M., Vickers, M., Evetts, J.E., Shi, Y. and McDougall, I. 2001. Superconductivity of Powder-In-Tube MgB2 Wires, Superconductor Science and Technology, 14, 193.
[7] Cui,C., Liu, D., Shen, Y., Sun, J., Meng, F., Wang, R., Liu, S., Greer, A.L., Chen, S., Glowacki, A.B. (2004). Nanoparticles of the superconductor MgB2: structural characterization and in situ study of synthesis kinetics. Acta Materialia, 52, 5757-5760.
[8] Park, S.C., Chung, J.K., Lim, Y. J., Kang, S.G., Song, K.J., Kim, C.J. 2008. Synthesis and characterization of nano-sized MgB2 powder by spray pyrolysis method, Physica C, 468, 1817-1820.
[9] Yang, C.C., Li, S.(2007). Size Dependence of Critical
Transition Temperature of MgB2. Proceedings 31st An- nual Condensed Matter and Materials Meeting.
[10] Varin, R.A., and Chiu, C. 2006. Synthesis of nanocrys- talline magnesium diboride (MgB2) metallic supercon- ductor by mechano-chemical reaction and post- annealing. Journal of Alloys and Compounds, 407, 268–
273.
IJSER © 2012
http://www.ijser.org


