International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 96
ISSN 2229-5518
Vijay D. Jadhav*; Nirmala V. Jadhav; Avinash D. Bholay; Sucheta N. Patil*
Present work is based on biodegradation of organophosphorus pesticide dimethoate in liquid MSM medium with micro organisms. The Actinomycete sp was found to be capable of utilizing dimethoate as sole carbon and energy source and could rapidly utilized dimethoate beyond 100 ppm and showed maximum growth in a MSM. The concentration of the dimethoate in the solution decreased exponentially with the increased exposure time. Actinomycetes sp. could tolerate dimethoate upto 900ppm. In current study, several factors influencing dimethoate degradation were investigated. Complete disappearance of dimethoate was detected after 3 days of incubation. UV-Visible spectroscopic analyses revealed the complete mineralization of dimethoate. Changes in pH of MSM medium to basic range supported the biological transformation. The optimal pH and temperature growth conditions were 8.5 and 30°C, respectively. The microbial consortia could prove to play a valuable role for the bioremediation of dimethoate contaminated soil.
Keywords: Actinomycetes sp, biodegradation, pesticide, dimethoate, soil pollution
![]()
During recent years, owing to the widespread use of pesticides and insecticides in agriculture, the amounts of these compounds in soil have increased Vijay D. Jadhav1*, Asst. Professor; (Biotech)
08275584586, K.T.H.M. College, Nashik
Mail-jdvijaybiotech@gmail.com Nirmala V. Jadhav2, K.T.H.M. College, Nashik
Avinash D. Bholay3, Asst Professor;
(Micro) K.T.H.M. College
Sucheta N. Patil4* Associate Professor (Micro) K.T.H.M College, University of Pune,
significantly. Several hundred pesticides of various chemical natures are used worldwide for agricultural and non agricultural purposes. Indeed, pesticides constitute major pollutants of the aquatic and soil environment, and their presence and persistence is of great concern because of their potential toxicity towards animals and humans[7]. The persistence of such compounds into the environment has increased interest in studying microbes
involved in their biodegradation[3].
Organophosphorus compounds (OPs) used in agriculture as pesticides represent an attempt to maximise insecticide activity
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 97
ISSN 2229-5518
and minimize environmental persistence. They have replaced organochlorine compounds which persist and accumulate in the environment. This group of pesticides has been used in large quantities throughout the world since the first introduction of a synthetic insecticide, dimethoate, for use in crop protection for long time. Different pathways of organophos-phate decomposition such as hydroly-sis, photolytic oxidation, microbial transformations and other biological processes have been reported recen-tly. Problems of contamination resulti-ng from surplus pesticides and wastewater from pesticide factories have become obvious.
Dimethoate is an insecticide used to kill mites and insects systemically and on contact. It is used against a wide range of insects, including aphids, plant hoppers and whiteflies on ornamental plants, grape, corn, cotton, sorghum, soybeans, tomatoes, and many more vegetables. Dimethoate is highly toxic to fish and to aquatic invertebrates. It undergoes rapid degradation in the environment and in
sewage treatment plants[2]. Because
dimethoate is highly soluble in water and it adsorbs only very weakly to soil particles. It subject to significant hydrolysis, especially in alkaline waters. The bioconversion of pesticides in the
environment results from physicoc-
hemical reactions as well as from the activity of cellular or extracellular components of the Effective Microorganisms. In the environment, pesticides are exposed to various degradative forces. Biode-gradation is
known to play a vital role in this respect[8].
It contributes not only in the disappearance of the original form of pesticides, but also changes their physicochemical properties, and thus affects their transport and distribution behaviour among various compartm-ents in the environments. Most forms of living organisms are capable of directly interacting with pesticides and some of them are capable of metabolizing
even very recalcitrant compounds[4]. The
techno-logy of effective microorganisms commonly termed EM Technology was developed by Dr. Teruo Higa in 1970's at the University of Ryukyus, Okinawa, Japan. This technology includes three principles types of organisms commonly found in all ecosystem, namely (lactic acid bacteria, yeast, actinomyces, and photosynthetic bacteria). Thus, it may be possible to take advantage of EM to bioremediate environmental pollution by
OPs[1].
Dimethoate is an organophosphorus insecticide and acaricide used for the control of houseflies, as well as a wide range of insects and mites on a variety of
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 98
ISSN 2229-5518
fruit, vegetable, field and forestry crops. Its solubility in water at 21°C is 25 g/L. Dimethoate released to the environment does not adsorb onto the soil and is subject to considerable leaching. The half-life of dimethoate in soil ranges from four to 16 days. It is relatively stable in aqueous media at pH 2 to 7.5 Reported half-lives for dimethoate in raw river water range from 18 hours to eight weeks. Dimethoate is degraded in the environment to another more toxic pesticide, omethoate; the proportion of omethoate in the total residue reaches about 50% after five weeks.![]()
The organophosphate insecticide dimethoate (O, O-dimethyl S-methylcarb- amoylmethyl phosphorodithioate), is manufactured by Kafr El-Zyat pesticides and chemicals Co. (kz), was employed in this investigation. Samples have been prepared in deionised water using ethyl acetate. Dimethoate was used in emulsifiable concentrate.
Effective Microorganisms (EM) were isolated from Grape field soil, Nashik, Maharashtra, India[6]. The enrichment and propagation were carried out in sterilized
250 ml Erlenmeyer flasks containing 50 ml Minimal Salt Medium (MSM)with following salts ZnSO4 : 0.01mg; CaCl2 .2H2 O:10mg; MgSO4 . 7H2 O:0.5g; K2 HPO4 :0.5g; (NH4 )2 SO4 : 0.5g and FeCl3 .H2 O:10 mg per litre in D H2 O, supplemented with 100 ppm dimethoate and 1 ml EM inoculums of O.D. 1.0. The pH value of the culture solution was adjusted to 7.0 with 1.0 NaOH or 1.0 N HCl.
Isolation and characterization of the degrading bacteria were carried out by streak method and morphological and biochemical methods (Bergey’s Bacteriology). After the incubation period at 30°C, the single colonies were picked and grown again in liquid mineral salt media for at least 5 days. This procedure was repeated until getting identical colonies. The isolated strains were characterized and identified by biochemical methods
Three flasks containing MSM media were
adjusted to different range of pH (5,7,9). The flasks were incubated on a rotary shaker at room temperature and 100 rpm. After time interval of 12 h, 2 ml, sample
was taken to determine bacterial growth[9].
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 99
ISSN 2229-5518
Depending on the optimal pH; the temperature values were adjusted to 25, 30 and 32°C with previously mentioned procedures and conditions.
The microorganisms were enriched and
cultivated on dimethoate containing MSM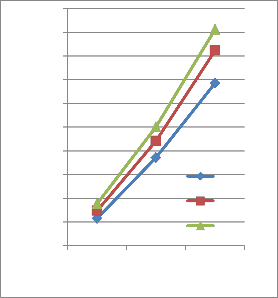
temperature[10].
100
90
80
70
60
50
40
30
20
10
0
12 h
24 h
36 h
media. The microorganism grew well by
utilizing dimethoate, as was evident from the increase in the optical density at 660 nm; and the simultaneous loss of dimethoate from the culture was observed by calculating percent biodegradation (Fig.
1and 2). When the microorganisms were grown in medium with dimethoate as the only carbon source, the milky colour of the medium was appeared. The disappearance of the dimethoate was monitored by spectrophotometric analysis. The mineralization of dimethoate by Actinomycetes sp was very promising and the concentration of dimethoate almost vanished and utilized as a sole of carbon and energy source after 36 h. The optimal pH for the growth was determined as 9.0 with optimal growth temperature of 30°C. The biodegradation of the dimethoate was promising at these optimal conditions as shown in Fig.1 and 2. Actinomycetes sp
could be well grown at the optimal pH and
5 7 9
pH
FIG. 1. Growth of Actinomycetes sp in MSM with dimethoate as a sole source of carbon and energy for pH optima.
When the bacteria grow at optimal pH and temperature the transport of the substrates will be ideal through the membrane, hence the growth rate increased. It can be observed that growth over the higher growth Temp-eratures, the transport of the substrates is impaired.
It was found that it was possible to apply
the microbial activities and/or their biocatalysts, for the remediation of natural soil containing millimolar concentration of toxic, persistent aromatic pesticides. It is
expected that pesticides will be
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 100
ISSN 2229-5518
100
90
80
70
60
50
40
30
20
10
0
26 28 30
Temperature
12 h
24 h
36 h
quality of soil, water and water resources and eliminating aromatic pesticide residues dissolved or dispersed in water and soil. As a conclusion microbial processes in the various kinds of aerobic and anaerobic systems for treating industrial, agricultural and municipal wastes are very important because these systems represent the hot spots of discharge of many chemicals into
environment[10]. Soil microorganisms as
Actinomycetes sp play a vital role in the in
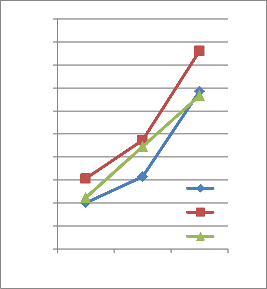
FIG. 2. Growth of Actinomycetes sp in MSM with dimethoate as a sole source of carbon and energy for temperature optima.
transformed into biodegradable compounds and mineralized into O2 , H2 O and CO2 , by using these micro-organisms for an appropriate duration. This recent tools in biotechnology methods can be considered very efficient and much pH and temperature dependence of dimethoate biodegradation by Actinomycetes sp. Biological techniques are more efficient
than chemical ones for improving the
situ biodegradation of organophosphates and aromatic pesticides in environments, where ambient temperatures often present[11]. The effective and stable biodegradation capacity of these microorganisms in utilizing and degrading these compounds reflected their potential
in biotechnological application at room temperature bioremediation of organophosphate compounds contaminated sites[12].
[1] A. N. Moneke*, G. N. Okpala and C.
U. Anyanwu (28 June, 2010); Biodeg- radation of glyphosate herbicide in vitro using bacterial isolates from four rice fields; African Journal of Biotechnology Vol. 9 (26), pp. 4067-
4074
[2]Feitkenhauer, H. (1998). ‘Biode-grada- tion of aliphatic and aromatic hydrocar- bones at high tem-perature: kinetics and application.’ Dissertation Technis-che Universität Hamburg-Harburg
[3]K. K. Sailaja and K. Satyaprasad
(2006); Degradation of glyphosate in
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 101
ISSN 2229-5518
soil and its effect on fungal population; Journal of Environ. Science &Engg. Vol. 48,.No 3, P.189,190
[4] Malachowesky, K., Phelps, T. J., Minni-kin, D. E., and White, D. C., (1994), ‘Aerobicmineralization of trichl-oroehy-elene vinyl chloride and aroma-tic compounds by Rhodococcus speci-es’. Appl. Environ. Microbiol. 60:
542-548.
[5] Meer, J., De-Vos, W., Haryama, S., Zehhnder, A. (1992). ‘Molecular Mechanisms of genetic adaptation to Xenbiotc compounds’. Micrbiological Rev. 677-694.
[6] Merkel, G., Stapleton, S. S., and Perry, J. J. (1978), ‘Isolation and pept- idoglycan of Gram negative hydroca- rbon utilizing thermophilic acteria’. J. Gen. Microbiol. 109: 141-148
[7] Meister, R.T. (ed.). 1992. Farm Chemicals Handbook '92. Meister Publishing Company, Willoughby, OH. Cheminova, A. S. 1991,
‘Material Safety Data Sheet : Dimethoate’. Cheminova, Lemvig, Denmark.
[8] Mohapatra Oudamini & Awasthi M.P. (1998), ‘Degradation of Metalaxyl in soil and Soil microbial culture’. Proc. Natl, symp. Frontiers in Appl. Environ, Microbiol. 11-13 Dec.1995. SES CUSAT Cochin @1998, pp.59-
64
[9] Nedwell, B. D., and Rutter, M. (1994).
‘Influence of temperature and growth rate and composition between psychrtolerant Antractic Bacteria: Low temperature diminishes affinity for substrate uptake’. Environ. Microbiol. 60: 1984-1989
[10] Patil Darmendra S. et. al. ( 1998 ),
‘Biodegradation of Dimethyl Terphtha-late by Pseudomonas acidovorans Du’. Proc. Natl, symp. Frontiers in appl Environ. Microbiol.
11-13, Dec 1995 SES, CUSAT Cochin c 1998 pp. 69-71
[11] Varanasi, U., Gmur, D. J., Reichert, W. L. (1981), ‘ Effect of environmental Temperature on naphthalene metabolis-m by juvenile starry floun-der (Platichthys stellatus)’. Arch. Envi-ron. Cont-am. Toxicol. 2: 203–214
[12] Stucki G, Leisinger T (1983),
‘Bacterial degradation of 2-chloroeth- anol proceeds via chloroacetic acid’. FEMS Microbiol Lett 16:123-126
IJSER © 2013 http://www.ijser.org