The RON , MON, and AKI (𝑅+𝑀 ) Values for both naphtha's
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 313
ISSN 2229-5518
Improvement of Octane Number of Naphtha Cut of Taq-Taq Crude Oil and Khormala Crude Oil Wells by Using Additives.
Abdulsalam R. Karim1
Two Types of additives and pyrolysis gasoline have been blended with naphtha samples in different volumetric ratios. Octane number of each blend has been measured using three different octane analyzers.
—————————— ——————————
Gasoline is a complex mixture of hundreds of volatile and combustible compounds derived from petroleum, with 5-12 carbon atoms and boiling points in the range of 30-220 °C[1] . The properties of commercial gasoline are influenced by the origin of the crude oil, the refinery processes and the presence
of additives, which are added with the purpose of improving the
environmental performance , fast , easy and accurate determination of octane numbers is important for refiners and quality inspectors as optimization of refining process and quality control at a reasonable cost is ever – increasing requirement , very soon scientists began to look for a correlation between the tendency of hydrocarbon – based fuels to knock and the composition of these fuels to calculate the octane numbers indirectly and rapidly , such as using gas
[13]
performance and reducing the emissions of automotive vehicles
[2],[3],[4],[5]. The addition of oxygenates to gasoline became
chromatography and FTIR spectroscopy
curves and partial least squares regression
[15]
, by distillation
[14], and methods
widespread after the elimination of the tetra ethyl lead compounds [6].
Specifications for gasoline properties were re – evaluated when a major change accured in the oil – automobile industry system. For example , the "oil crisis " in the 1970 s and the planned phase – out of tetra ethyl lead prompted studies of the optimum octane rating new unleaded - gasoline in the united states and Europe [7],[8],[9],[10] .
Octane number is one of the main parameters used in quality control of gasoline and provides information about the resistance to auto – ignition - this phenomenon occurs when the temperature of the fuel – air mixture under the effect of compression , leading to sufficiently increased self – detonation of the mixture without the help of a spark [11] .
Octane rating is measured at two different operational conditions, the rating measured at the more severe operating conditions (inlet temp. and RPM) is called Motor octane number (MON) and the rating measured at the mild conditions is called the research octane number, (RON), the spread between the two numbers (MON & RON) is known as the fuel sensitivity and pump octane number (PON) or Anti Nock index
(AKI) is the arithmetic average of RON and MON [12].
Currently both RON and MON are still measured in a standardized single cylinder, internal combustion engine (cooperation research fuel – CFR engine), following the standard methods ASTM D2699 (RON) and ASTM D2700 (MON), respectively. As all the standard methods are time
consuming , complicated , relatively expensive and of poor
depend on dielectric spectroscopy .
This work describes the use of automatic analyzer based on mid
– infrared spectroscopy ( zeltex 101C), and (shatox SX –
100M) which operates by measuring. The sample's dielectric properties, as well as the Ukrainian device called octane meter OKM - 2.
(i) Gas condensate from khor – mhor field, near Kirkuk with properties and specifications shown in table (1)
(ii) Naphtha produced from taq – taq crude and khormalla crude oil with specifications and properties shown in table (2) (iii) Additive (A): EPT octane Enhancer from: Enviro petro technologies pty LTD, Sydney, NSW, Australia.
Composition: Xylene, toluene, vegetable oil fatty acid, Diethyl malonate, trimethyl benzene, N- Methyl aniline, and Dimethyl carbonate.
(iv) Additive (B): octane Enhancer from: UKRZEMRESOURCE, Kyiv, Ukraine.
Composition: N – methyl aniline, MTBE, Toluene and unknown composition component.
2.2 Motor and research octane numbers of prepared samples
were measured by the following devices:-
(i) octane Meter type OKM – 2 : equivalent to motor ( EN ISO
5163 : 2005 ) and research ( EN ISO 5164 : 2005 ) methods :
from Ukraine ,
(ii) octane meter – type Zeltex 101 C : portable , battery
1 Chemistry Dept., School of Science, University of Sulaimani. E-mail: abdulsalam_doctor@hotmail.com
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 314
ISSN 2229-5518
powered octane analyzer for gasoline from : (Zeltex , Inc , Hagerstown , MD .USA ) provides accuracy and repeatability equivalent to ASTM – approved CFR engines .
(iii) Octane meter type shatox Sx – 100 M: portable octane / cetane Analyzer for gasoline, diesel fuel and other petroleum products; from: SHATOX research organization for developing and manufacting of instrument in cooperation with the Petroleum chemisorptions institute of Siberian Branch of Russian Academy of science. Equivalent to ASTM D 2699 –
86, ASTM D 2700 – 86 Methods.
2.3 procedures and Methods:
The physical and chemical properties of gas condensate, and both Naphtha sample have been determined according to specific ASTM and IP standard methods. Specific gravity and API gravity by hydrometer methods (ASTM D 1298) , sulfur- total ( ASTM D 4294 ) , sulfur mercaptan (ASTM D 3227 ) ,
Reid vapor pressure (ASTM D 323 ) , salt content (ASTM D
3230) , Kinematic viscosity (ASTM D 445) , wax content (
UOP 46) , pour point (ASTM D 97) , Ash content (ASTM D
482) , Total acid number (ASTM D 664) , Water content (
ASTM D 4928) , Total nitrogen (ASTM D 3228) , metals ( IP
470) .
PIONA (ASTM D 6293), Distillation (ASTM D 86), asphaltenes (IP 143).
Some of physical and chemical properties of both Naphtha samples and gas condensate measured according to specific ASTM methods are illustrated in Table (1) (naphtha from khormala), table (2) (naphtha from taq – taq), and table (3) (gas condensate from khor – mhor)
Table (1) some physical and chemical properties of naphtha produced from khormala crude.
TEST | UNIT | METHOD | RESULT | ||
C5-80 | 80-150 | 150-193 | |||
Yield on crude | % Volume | ASTM D2892 | 6.3 | 0.5 | 7.3 |
Yield on crude | % mass | ASTM D2892 | 8.3 | 1201 | 7.8 |
Specific gravity@15.6 °C | Kg/L | ASTM D1298 | 0.6536 | 0.7395 | 0.7871 |
API Gravity | ° API | ASTM D1298 | 84.3 | 59.8 | 48.3 |
Sulpher – total | PPm Wt | ASTM D3120 | 278 | 884 | 0.2(%W) |
Total Nitrogen | PPm Wt | ASTM D3228 | - | ‹ 1 | ‹ 1 |
PIONA (*) | |||||
Total Paraffins | % Volume | ASTM D6293 | 92.9 | 60.9 | 51.4 |
Total olefins | % Volume | ASTM D6293 | ‹ 0.01 | 0.1 | 0.3 |
Total Naphthenes | % Volume | ASTM D6293 | 6.8 | 31.3 | 26.1 |
Total Aromatics | % Volume | ASTM D6293 | 0.3 | 7.9 | 22.5 |
Distillation | |||||
IBP | ° C | ASTM D86 | 30.5 | 96.3 | 157.1 |
5% | ° C | ASTM D86 | 41.3 | 102.7 | 160.8 |
10% | ° C | ASTM D86 | 43.4 | 104.4 | 161.6 |
20% | ° C | ASTM D86 | 45.8 | 106.5 | 162.5 |
30% | ° C | ASTM D86 | 48.2 | 109.3 | 163.8 |
40% | ° C | ASTM D86 | 51.0 | 112.5 | 165.1 |
50% | ° C | ASTM D86 | 53.4 | 115.9 | 166.2 |
60% | ° C | ASTM D86 | 56.4 | 120.4 | 168.0 |
70% | ° C | ASTM D86 | 59.5 | 124.5 | 169.8 |
80% | ° C | ASTM D86 | 63.0 | 130.5 | 173.5 |
90% | ° C | ASTM D86 | 66.9 | 136.1 | 176.6 |
95% | ° C | ASTM D86 | 73.2 | 145.6 | 182.7 |
E.P | ° C | ASTM D86 | 75.6 | 149.1 | 184.8 |
(*) with compliments of NRG – GLOBAL LLC.
Table (2) some physical and chemical properties of naphtha produced from Taq – Taq crude.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 315
ISSN 2229-5518
TEST | UNIT | METHOD | RESULT | ||
TEST | UNIT | METHOD | C5-80 | 80-125 | 125-175 |
Yield on crude | % Volume | ASTM D2892 | 10.5 | 13.5 | 13.9 |
Yield on crude | % mass | ASTM D2892 | 12.5 | 15.0 | 14.6 |
Specific gravity@15.6 °C | Kg/L | ASTM D1298 | 0.6536 | 0.7111 | 0.7541 |
API Gravity | ° API | ASTM D1298 | 85.0 | 67.5 | 56.1 |
Sulpher – total | PPm Wt | ASTM D3120 | 10 | 25 | 133 |
Total Nitrogen | PPm Wt | ASTM D3228 | ‹ 1 | ‹ 1 | ‹ 1 |
PIONA (**) | |||||
Total Paraffins | % Volume | ASTM D6730 | 95.2 | 77.8 | 68.5 |
Total olefins | % Volume | ASTM D6730 | ‹ 0.001 | 0.165 | 0.402 |
Total Naphthenes | % Volume | ASTM D6730 | 4.7 | 20.0 | 12.5 |
Total Aromatics | % Volume | ASTM D6730 | 0.07 | 1.6 | 16.3 |
Distillation | |||||
IBP | ° C | ASTM D86 | 30.1 | 89.0 | 134.1 |
5% | ° C | ASTM D86 | 41.1 | 94.5 | 140.8 |
10% | ° C | ASTM D86 | 43.6 | 95.9 | 141.3 |
30% | ° C | ASTM D86 | 50.0 | 99.8 | 145.9 |
50% | ° C | ASTM D86 | 56.5 | 104.1 | 150.6 |
70% | ° C | ASTM D86 | 63.7 | 109.6 | 156.4 |
80% | ° C | ASTM D86 | 67.9 | 113.3 | 160.3 |
90% | ° C | ASTM D86 | 73.2 | 119.1 | 165.9 |
95% | ° C | ASTM D86 | 79.5 | 123.6 | 170.9 |
E.P | ° C | ASTM D86 | 87.9 | 149.0 | 178.4 |
(**) with compliments of intertek, Fujairah.
Table (3) some physical and chemical properties of gas condensate produced in khor – mhor field.
TEST | UNIT | METHOD | RESULT |
Specific gravity@15.6 °C | Kg/L | ASTM D 1298 | 0.7073 |
API Gravity | ° API | ASTM D 1298 | 68.6 |
Total sulfur | % | ASTM D 4294 | 0.0618 |
Sulfur- Mercaptan | mg/Kg | ASTM D 3227 | 119 |
Reid vapour pressure@100 °C | psi | ASTM D 323 | 11.4 |
Salt content | PTB | ASTM D 3230 | 0 |
Characterization factor | - | ASTM D 375 | 12.2 |
Kinematic Viscosity at 40 °C | Cst | ASTM D 445 | 0.53 |
Kinematic Viscosity@100 °C | Cst | ASTM D 445 | N/A |
Pour point | ° C | ASTM D 97 | ‹ -24 |
Wax content | % mass | ASTM D 46 | ‹ 0.05 |
Ash content | % mass | ASTM D 482 | ‹ 0.001 |
Total acid number | mgkoH/g | ASTM D 664 | 0.011 |
Asphaltenes | % mass | IP 143 | 0.06 |
Water content | ppm wt | ASTM D4928 | 0.014 |
Total Nitrogen | ppm wt | ASTM D3228 | 126 |
Metals | |||
Nickel (Ni) | mg/ kg | IP 470 | ‹ 0.1 |
Vanadium (V) | mg/ kg | IP 470 | ‹ 1 |
Sodium (Na) | mg/ kg | IP 470 | 1.006 |
Lead (P3) | mg/ kg | IP 470 | ‹ 0.5 |
Iron (Fe) | mg/ kg | IP 501 | 0.224 |
As it is very clear from the tables above , naphtha produced from Taq-Taq crude oil is more paraffinic and lighter than naphtha produced from khormala crude oil , also its sulfur content is much less while nitrogen content of both naphtha samples are identical . The sulfur content of gas condensate is also low (0.06%) and it is very light (API = 68.6), and highly
paraffinic (KRwR = 12.2). Table (4) lists the general properties of
full range naphtha samples and gas condensate, octane numbers (clear) are low and close to each other , RON of the two naphtha samples are little bit higher than RON of gas condensate sample, (77.5 for khormala, 74.5 for Taq-Taq , and
65.6 for gas condensate). Fractionation of gas condensate
shows that it contains some kerosene and gas oil fractions, these amounts differ depending on the boiling range of the
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 316
ISSN 2229-5518
chosen condensate fraction, for example if these two different break downs were chosen ; [16],[17] then different amounts of
kerosene & gas oil will be obtained as shown below :-
IBP – 194 °C | Naphtha = 82.0%v |
194 – 232 °C | Kerosene = 9.1%v |
232 – 310 °C | Gas oil = 2.7%v |
Fuel and loss | 6.2%v |
IBP – 150 °C | Naphtha = 70%v |
150 – 232 °C | Kerosene = 20%v |
232 – 310 °C | Gas oil = 5%v |
Fuel and loss | 5%v |
Table (4) general properties of full range naphthas and gas condensate samples.
TESTS | UNITS | METHOD | RESULTS | ||
TESTS | UNITS | METHOD | Naphtha (Taq- Taq) | Naphtha (khormala) | Gas condensate (Khor-mhor) |
Specific gravity @ 15.6 °C | - | ASTM D1298 | 0.7121 | 0.7073 | |
A.P.I Gravity | ° API | ASTM D1298 | 67.2 | 68.6 | |
Sulfur | %wt | ASTM D4294 | 0.030 | 0.0618 | |
Viscosity @ 40°Ckg -1 | Cst | ASTM D445 | 0.670 | 0.535 | |
Distillation @ 100 °C (%v) | Cst | ASTM D86 | 32 | 58 | |
Distillation @ 14.5 (%v) | % | ASTM D-86 | 81 | 70 | |
Final b.p °C | ASTM D-86 | 166 | 315 | ||
Octane number | (Zeltex. 101C | ||||
RON | - | - | 74.5 | 77.5 | 65.6 |
MON | - | - | 70.5 | 72.7 | 63.2 |
R+M 2 | - | - | 72.6 | 74.8 | 64.4 |
Enhancements of octane number:
![]()
The RON , MON, and AKI (𝑅+𝑀 ) Values for both naphtha's
2
and gas condensate measured by zeltex 101C , shatox , and
OKM -2 instruments without additives are shown in table (5).
Table (5) octane number of naphtha's and gas condensate by zeltex 101C, shatox, and OKM – 2 instruments.
Fuel | By zeltex 101C | By shatox | By OKM -2 | ||||||
Fuel | RON | MON | AKI | RON | MON | AKI | RON | MON | AKI |
Naphtha from Taq-Taq | 74.5 | 70.5 | 72.6 | 78.0 | 75 | 76.4 | 77.6 | 62.1 | 69.8 |
Naphtha from khormala | 77.5 | 72.5 | 74.8 | 80 | 77 | 78.5 | 79 | 68.8 | 74 |
Gas condensate | 65.6 | 63.2 | 64.4 | 72.1 | 67.4 | 69.7 | - | - | - |
Addition of additive (A): EPT octane Enhancer:
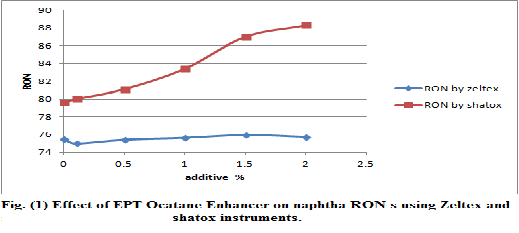
According to data shown in table (6) and fig (1) the addition of additive (A) (EPT octane Enhancer) is not suitable for naphtha
by measuring octane number with zeltex 101C, but by
measuring shatox, the ONS increased suitably for naphtha samples.![]()
Table (6) EPT octane Enhance effect on the ONS value of naphtha sample from Taq-Taq crude oil by using zeltex 101C and shatox instruments.
Additive %V Measuring by zeltex Measuring by shatox
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 317
ISSN 2229-5518
RON | MON | AKI | RON | MON | AKI | |
0 | 75.5 | 71.5 | 73.5 | 79.6 | 76.6 | 78.1 |
0.1 | 75 | 71.7 | 73.8 | 80.0 | 77 | 78.5 |
0.5 | 75.4 | 71.6 | 73.5 | 81.1 | 77.5 | 79.3 |
1 | 75.6 | 71.2 | 73.4 | 83.4 | 78.5 | 80.9 |
1.5 | 75.9 | 71.5 | 73.7 | 87.0 | 80.1 | 80.7 |
2 | 75.7 | 71.1 | 74.4 | 88.3 | 80.7 | 84.5 |
According
Enhance
pyrolysis gasoline to meet national fuel determination therefore we blended our naphtha with pyrolysis gasoline of (RON = 98)
and general properties shown in table (7), in deferent ratios,
erent volume ratios and two instruments zeltex
101C, and shatox. These results are shown in tables (8 – 14)
and figs. (2 - 9).
Table (7) general properties of pyrolysis gasoline
Tests | Method | Units | Results |
Specific gravity @ 15.6 °C | ASTM D1298 | - | 0.8036 |
API Gravity | ASTM D1298 | °API | 44.5 |
IBP | ASTM D – 86 | °C | 30 (Min) |
FBP | ASTM D – 86 | °C | 200 (Max) |
RVP | ASTM D – 323 | Psi | 9 (Max) |
RON | ASTM D 2699 | - | 98 |
Total sulfur | ASTM D5453 | Wtppm | 300 |
Aromatic content | G .C | wt% | 40 |
Benzene content | G .C | wt% | 15 |
Existed Gum | - | mg/100ml | 3816 |
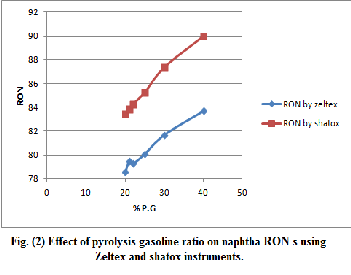
Table (8) octane number of blended naphtha and pyrolysis gasoline (P.G) using zeltex 101C instrument.
Octane No. | Pyrolysis gasoline | naphtha | 20% P.G | 21% | 22% | 25% | 30% | 40% |
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 318
ISSN 2229-5518
80% Nap. | 79% | 78% | 75% | 70% | 60% | |||
RON | 98.3 | 74.1 | 78.6 | 79.5 | 79.3 | 80.1 | 81.7 | 83.7 |
MON | 84.1 | 78 | 72.9 | 73.6 | 73.5 | 73.6 | 74.9 | 75.9 |
R + M 2 | 91.2 | 72.4 | 75.8 | 76.6 | 76.4 | 76.6 | 78.3 | 79.8 |
Table (9) octane number of blended naphtha and pyrolysis gasoline (P.G) using shatox instrument.
ON | P.G | Nap. | 20% | 21% | 22% | 25% | 30% | 40% |
ON | P.G | Nap. | 80% | 79% | 78% | 75% | 70% | 60% |
RON | 96.6 | 78.2 | 83.5 | 83.9 | 84.3 | 85.3 | 87.4 | 90.0 |
MON | 86.6 | 75.2 | 78.5 | 78.7 | 78.9 | 79.3 | 80.2 | 81.8 |
R + M 2 | 91.6 | 76.6 | 81.0 | 81.3 | 81.6 | 82.3 | 83.8 | 85.9 |
Table (10) ONS of blended naphtha (80%) and P.G 20% with different doses of EPT octane Enhancer by zeltex 101C , and shatox
instrument.
EPT additive %V | Measurement by zeltex | Measurement by shatox | ||||
EPT additive %V | RON | MON | AKI | RON | MON | AKI |
0.1 | 78.9 | 73.1 | 76 | 84.8 | 79.1 | 82.1 |
0.5 | 79.4 | 73.3 | 76.4 | 86.5 | 79.9 | 83.2 |
1 | 78.7 | 72.8 | 75.8 | 89.4 | 81.5 | 85.4 |
1.5 | 79 | 72.6 | 75.8 | 90.4 | 82.1 | 86.2 |
2 | 79.2 | 72.6 | 75.9 | - | - | - |
Table (11) ONS of bended naphtha 78% and P.G 22% with different doses of EPT octane Enhancer by zeltex 101C and shatox instruments.
EPT additive %V | Measurement by zeltex | Measurement by shatox | ||||
EPT additive %V | RON | MON | AKI | RON | MON | AKI |
0.1 | 79.3 | 73.5 | 76.4 | 86.5 | 79.9 | 83.1 |
0.5 | 79.5 | 73.2 | 76.4 | 86.9 | 80.0 | 83.5 |
1 | 80.0 | 73.5 | 76.8 | 88.8 | 81.0 | 84.9 |
1.5 | 79.5 | 73.2 | 76.3 | 89.9 | 81.8 | 85.9 |
2 | 59.5 | 72.8 | 76.1 | 91.0 | 82.5 | 86.7 |
Table (12) ONS of bended naphtha 75% and P.G 25% with different doses of EPT octane Enhancer by zeltex 101C and shatox
instruments.
EPT additive %V | Measurement by zeltex | Measurement by shatox | ||||
EPT additive %V | RON | MON | AKI | RON | MON | AKI |
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 319
ISSN 2229-5518
0.1 | 79.4 | 73.5 | 76.5 | 88 | 80.5 | 84.3 |
0.5 | 79.6 | 73.3 | 76.5 | 88.5 | 80.8 | 84.7 |
1 | 80.6 | 74 | 77.3 | - | - | - |
1.5 | 80.1 | 73.5 | 76.8 | 90.9 | 82.4 | 86.6 |
2 | 80.5 | 73.1 | 76.8 | 91.6 | 82.9 | 87.2 |
Table (13) ONS of bended naphtha 70% and P.G 30% with different doses of EPT octane Enhancer by zeltex 101C and shatox instruments.
EPT additive %V | Measurement by zeltex | Measurement by shatox | ||||
EPT additive %V | RON | MON | AKI | RON | MON | AKI |
0.1 | 82.2 | 75 | 78.6 | 88.7 | 81 | 87.2 |
0.5 | 81.5 | 74.8 | 78.2 | - | - | - |
1 | 81.4 | 74.2 | 77.8 | 90.7 | 82.3 | 86.5 |
1.5 | 81.7 | 74.5 | 78.1 | 91.6 | 82.9 | 87.2 |
2 | 81.4 | 74.1 | 77.8 | 92.2 | 83.3 | 87.8 |
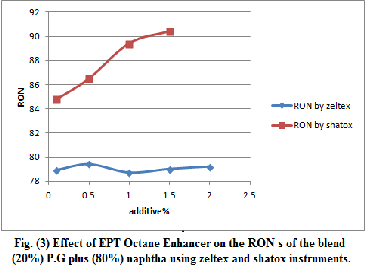
Table (14) ONS of bended naphtha 60% and P.G 40% with different doses of EPT octane Enhancer by zeltex 101C and shatox instruments.
EPT additive %V | Measurement by zeltex | Measurement by shatox | ||||
EPT additive %V | RON | MON | AKI | RON | MON | AKI |
0.1 | 83.9 | 75.8 | 79.9 | 90.3 | 82.0 | 86.1 |
0.5 | 84.3 | 76 | 80.1 | 91.1 | 82.5 | 86.8 |
1 | 83.6 | 75.6 | 79.6 | 92.5 | 83.5 | 88 |
1.5 | 84.1 | 75.2 | 79.6 | 93.6 | 84.3 | 88.9 |
2 | 84.4 | 75.5 | 79.9 | 95.4 | 85.4 | 90.4 |
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015
ISSN 2229-5518
320
100
80
-
"' "'
94
_.,
-
60 ...
z
88 - -
0 so
a::
20
10
0
a::
. RON by shatox 84
82
80
78
...............
-- .......
RON by zeltex
. RON by shatox
0 0.5 1 LS 2 2.5
additive%
0 0.5 LS
additive%
2 2.5
Fig. (4) Effect ofEPT Octane Enhancer on the RON s of the blend
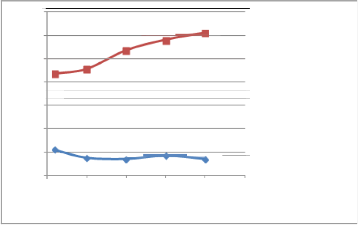
(22%) P.G plus (78%) naphtha using Zeltex and shatox instruments.
94
92
90
Fig. (5) Effect of EPT Octane Enhancer on the RON s of the blend
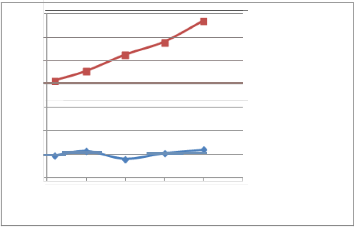
(25%) P.G plus (75%) naphtha using Zeltex and shatox instruments.
96
94
92
z88
0
a: 86
84
82
RON by zeltex
_._RON by shatox
z90
0
a: 88
86
84
RON by zeltex
_._RON by shatox
80
0 0.5 1 1.5 2 2.5
additive%
Fig. (6) Effect of EPI Octane Enhancer on the RON s of the blend
(30%) P.G plus (70%) naphtha using Zeltex and shatox instruments.
82
0 0.5 1 1.5 2 2.5
additive%
Fig. (7) Effect of EPT Octane Enha ncer on the RON s of the blend
(40%) P.G plus (60%) naphtha using Zeltex a.nd shatox instrument.
98
97.5
97
96.5
96
z
Naphtha from
85
84
83
82
81
z 80
Naphtha from
a0:: 95.5
95
94.5
94
93.5
93
0
2 4 6 additive% (B)
Khormala
-+-Naphtha from TaqT·aq
8
79
78
77
76
75
74
0 2 4 6
additive% (BI
khormala
-+-Naphtha from Taq-Taq
8
Fig. (8) Effect of additove (B) on the RON s of both naphtha samples using OKM:- 2 instruments.
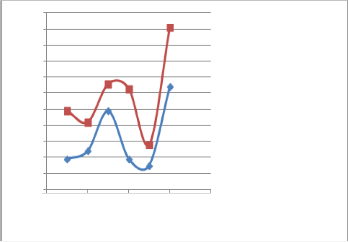
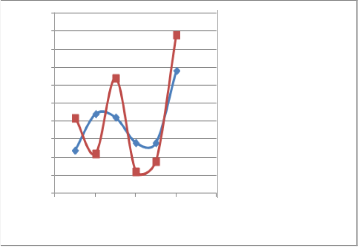
Fig. (9) Effect of additive (B) on the RON s of both naphtha samples using Zeltex 101 C instruments.
IJSER © 2015
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 321
ISSN 2229-5518
Addition of additive (B):
Six blends from additive (B) components prepared with
different motor octane number as shown below:
Additive (B) Blends | ||||||
Blend No. | B1 | B2 | B3 | B4 | B5 | B6 |
MON | 300 | 240 | 220 | 400 | 310 | 175 |
Then these blends added to both naphtha samples with the
using OKM – 2 instruments. The results are show in table (15).
specified percentages, and octane number measured for all
using OKM – 2 instruments. The results are show in table (15).
Table (15) effect of additive (B) on ONS of both naphtha samples using 0 KM – 2 instruments.
Additive (B) | ONS of both naphtha sample | |||||
Additive (B) | Naphtha from Taq-Taq | Naphtha from khormala | ||||
Additive (B) | RON | MON | AKI | RON | MON | AKI |
B1 , 12.28 %V | 94.2 | 71.3 | 82.7 | 95.1 | 83 | 89.1 |
B2 , 15 %V | 95.2 | 68.1 | 81.6 | 94.1 | 82.1 | 88.1 |
B3 , 16.7 %V | 95.1 | 72.6 | 83.8 | 96.2 | 84.7 | 90.4 |
B4 , 23 %V | 94.4 | 67.5 | 80.9 | 93.6 | 77.5 | 85.5 |
B5 , 10.9 %V | 94.4 | 68 | 81.2 | 93.9 | 78.6 | 86.2 |
B6 , 20.63 %V | 96.4 | 85.5 | 90.9 | 97.4 | 86.6 | 92.0 |
While addition of additive (B) and measuring ONS using zeltex are shown in table (16).
Table (16) effect of additive (B) on ONS of naphtha samples using zeltex instrument.
Additive (B) | ONS of both naphtha sample | |||||
Additive (B) | Naphtha from Taq-Taq | Naphtha from khormala | ||||
Additive (B) | RON | MON | AKI | RON | MON | AKI |
B1 | 75.9 | 70.9 | 73.4 | 78.9 | 71.7 | 75.3 |
B2 | 76.4 | 71.6 | 73.9 | 78.2 | 72.7 | 75.4 |
B3 | 78.9 | 72 | 75.5 | 80.6 | 72.7 | 76.7 |
B4 | 75.9 | 70.6 | 73.2 | 80.3 | 72.6 | 76.4 |
B5 | 75.5 | 70.5 | 73 | 76.8 | 71.1 | 73.9 |
B6 | 80.4 | 73.5 | 77 | 84.1 | 76.3 | 80.2 |
Zeltex 101C instrument measures octane number via near –
infrared (NIR) transmission spectroscopy , the instrument contains a patented sold – state optical system comprising 14 near – infrared emitting diodes with narrow band pass filters, a silicon detector system, and fully integrated micro processor , this instrument operates in the short NIR, from 800 to 1100 nm wave , the instrument is factory calibrated to predict octane number from the absorption spectra of the fuels being tested , this prediction is accomplished through the use of a
multivariate regression of the form : [18],[19]
Octane number = K° + K1 ( OD1) + K2 (OD2) ……..…. + K14 (OD14) + K15 (Ta ) where K° is a bias term, K1 through K15 are slope coefficients, OD1 through OD14 are the absorbencies measured at each of the 14 wave length , and Ta is the ambient temperature .
The instrument can store up to 10 calibration equations and is factory calibrated for RON, MON, and AKI of blended gasoline. The results obtained by zeltex 101C measurements in this research have no significant differences between
measurements before and after additive addition this means that
the existing calibration mode of the instrument is not suitable
for these additives (A) and (B) and it needs factory calibration to predict octane numbers from the absorption spectra of the fuels being tested, because the empirical rules of octane number dependence on the structure of alkenes are amended [20]. ON decreases with the number of CH2 groups and increases with the number of CH3 groups, the number of adjacent CH2 groups has the highest influence; ON decreases with the separation between branches; it increases with the more central position of branches and with their bulkiness, Ethyl group causes apparently contradictory effects: if it increases the number of CH2 groups, ON decreases; if not, ON increases.
The use of structured features of alkanes i.e. the size of the
molecule, the number of branches , the position of branches , the separation between them, the type of branches , and the type of the branched structure enables a more thorough understanding of the relation between the structure of alkane and their physicochemical properties (20).
The composition of each additive package used in this research
is different from the other, so it is necessary to factory
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 322
ISSN 2229-5518
calibration of this zeltex 101C instrument to be suitable to predict octane number from the absorption spectra of the blends being tested.
While the principle of the operation of OMK – 2 instruments is based on the test of hydrocarbon fuel under high temperature, which causes the oxidation reaction (combustion), followed by
the release of heat. As a result of the measurement of the
temperature characteristic of the oxidation reaction we establish an unambiguous relationship with the parameters of the oxidation reaction with knock resistance of the tested gasoline. The low octane number is indicated also by higher reaction temperature and lower time of reaction period as show in the figures below.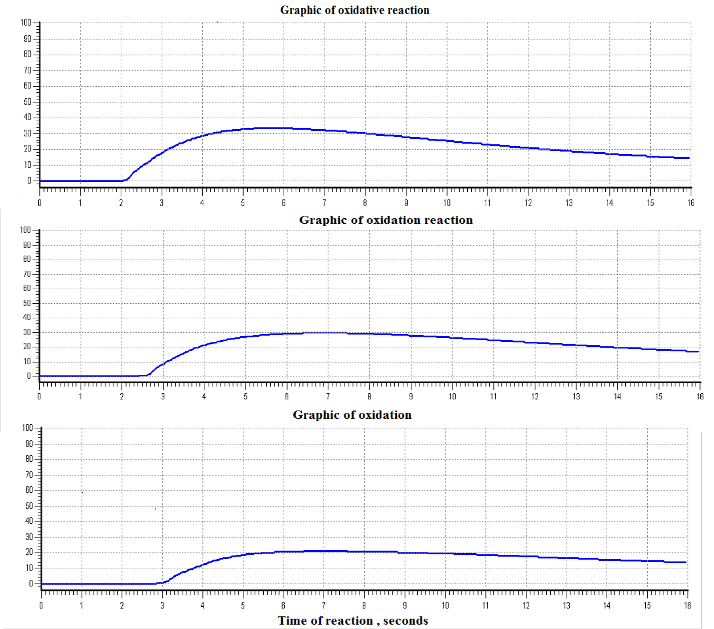
Shatox instrument operates by measuring the sample's dielectric properties and comparing the results to stored parameters in its internal program of typical known chemical compounds widely used in fuel production Extremely sensitive to changes in these dielectric properties , the octane meter is able to detect subtle differences in the chemical makeup of the fuel sample and therefore become valuable tool for determining the octane , cetane as well as many other parameters for typical gasoline , diesel fuel and other petroleum products.
Since different fuel blends react differently, all shatox octane meters employ additional modes providing the ability to make corrections quickly and easily.
The disadvantage is that the octane meter is not compatible with Bio – fuels and ethanol fuel blends.
1- Fractionation of gas condensate showed that it contains some kerosene and gas oil fractions, which must be removed prior to blend with additives.
2- The instruments must be factory calibrated for RON, MON, and AKI of blended gasoline samples with additives.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 3, March-2015 323
ISSN 2229-5518
3- The instrument OMK - 2 is based on the oxidation reactions following by the release of heat, so it gives better results.
4- The additive EPT alone can raise RON only 3-4 numbers up to 2.5% dosage and it is better to use it with higher octane number gasoline to reach the premium grade gasoline.
References:
1- Selah Aldan Neman, Fanner M.Saleem ."Evaluation and improvement of gasoline and naphtha cut of Tawke crude oil wells, Zakho ", J. OF petroleum and Gas Exploration Research, Vol.2 (4) PP.069 – 079, 2011.
2- Al – Hasan M." Effect of ethanol unleaded gasoline blends on engine performance and exhaust emission" Energy converse manage 2003; 44; 15 47 – 61
3- Russell M., Lacorte E, Ginebreda A, Barceló D." Simultaneous determination of methyl tert – butyl ether and its degrading products, other gasoline oxygenate and benzene, toluene, ethyl benzene, and xylenes in Catalonian. Ground water by purge – and – trap – gas chromatography – mass spectrometer. J chromatogram a 2003; 995: 171 – 84.
4- Seddon D. Reformalated gasoline, opportunities for new catalyst technology. Catal. Today 1992; 15: 1 – 21.
5- Tade MO , Tian Y . "Conversion inference for ETBE
reactive distillation ", Sep purif Technol 2000; 19: 85 – 91.
6- Nadim F, Zack P, Hang GE, Liu S. "United state experience with gasoline additives. Energy policy 2001; 29: 1 – 5.
7- US Environmental protection Agency. Impact of gasoline characteristics on fuel economy and its measurement, Report No. 76 – 10 JLB, office of mobile source Air pollution control, Emission control Technology Division. Technology Assessment and Evaluation Branch; 1976.
8- Wagner TO, Russum LW, "SAE Technical paper 730552:
1973.
9- Brown EC, Corner ES, Compton RA. Oil Gas J. 1975: 29 (9): 125 – 8.
10- CONCAWE. The rational utilization of fuels in private transport (RUFIT). Extrapolation to unleaded gasoline case. Report No. 8/80. Den Haag: 1980.
11- Pulkrabek WW. "Engineering fundamental of internal combustion engine". 1 st ed. Upper saddle River: prentice Hall:
1997.
12- Rafat Assi: "The Relation between gasoline quality, octane number and the environment" presented at: Jordan National workshop on lead phase – out 23 – 24 July 2008, Amman – Jordan.
13- Brudzewski K, Kesik Kolodziejczyk K, zborowska u, ulaczyk J, " Gasoline quality prediction using gas
chromatography and FTIR spectroscopy: an artificial intelligence approach. Fuel 2006; 85: 553 – 8.
14- Gisele Mendes, itelga G. Aleme, Paulo J.S Barberia. "Determination. Of octane number in gasoline by distillation
curves and partial least square regression" fuel 97 (2012) 131 –
136.
15- L .Guan , X.L.Feng , Z.C.Li , G.M.Lin Determination of octane number for clean gasoline using dielectric spectroscopy fuel 85 (2009) 1453 – 1459.
16- pre – sanction Reports, Kurdistan Refinery project, prepared by NRG – GLOBALL .LLC. 9th June 2011.
17- Abdul salam R. karim and Luqman O. hama salih " Fractionation and Evaluation of light petroleum products produced from gas condensate of khor – mhor fields in chemchemal , Kurdistan Region – Iraq" zanco , J . Of pure and applied sciences / salahaddin university – hawler, Vol.24, No.
2. p48.
18- abdulsalam R.K , Aziz A.M Loqman O.H. "Evaluation of Naphtha's produced from some Iraqi – Kurdistan crude oils using gas chromatography and determination of their clear (RON) by IR – spectroscopy ". IPCBEE, Vol.45 (2012).
19- http:// www.cie-eic.com / zeltex / eval.htm "Evaluation of an octane Analyzer" Reprinted from American laboratory news, August 1996 by Glenn N. Merberg.
20- Anton Perdih, franc perdih "chemical inter pertation of octane number" Acta chim. Slov. 2006, 53, 306 – 315.
IJSER © 2015 http://www.ijser.org