The research paper published by IJSER journal is about Impact of Curzate (fungicide) on Hematological Parameters of Oreochromis mossambicus 1
ISSN 2229-5518
Bhavika Desai and Pragna Parikh
Abstract: Curzate, a fungicide, is currently registered for commercial use in over 50 countries on more than 15 crops, creates serious threat to the environment as well as target and non-target organisms like aquatic and land dwelling animals. The present investigation was carried out to study the impact of the fungicide on the hematological parameters of fresh water fish Oreochromis mossam bicus. Adult fish of nearly similar weight (25 ± 1.9 g) and length (15.5 ± 1.2cm) were exposed to two sub lethal concentration i.e. 4.9 mg/l and 2.45 mg/l of Curzate for a period of 21 days. The hematological analysis showed significant reduction in red blood cells (RBCs) count, hemoglobin (Hb) value, packed cell volume (PCV) and mean corpuscular hemoglobin concentration (MCHC), while total white blood cells (WBCs) count, mean corpuscular volume (MCV) and mean corpuscul ar hemoglobin (MCH) were significantly increased in the treated groups as compared the control group. The present study shows that Curzate causes alterations in hematological parameters leading to physiological dysfunctions thus validating the toxic effect of the fungicide on the fish.
Key words: fungicides, haematology, Blood indices and Oreochromis mossambicus
![]()
1 INTRODUCTION
Agricultural pesticides are indispensable in contemporary agriculture. They are beneficial by providing reliable, persistent and relatively complete control against harmful pests with less cost and effort [1]. Due to injudicious and indiscriminate use of these agrochemicals such as fertilizers, pesticides, insecticides and fungicides to boost crop production with the sole aim of getting more yield, water bodies like ponds, lakes, river and low lying water areas are continuously getting polluted. Normally these pesticides reach the aquatic environment through surface run off, sediment transport from treated soil and direct application as spray to water bodies to control the inhabiting pests [2]. 1
These chemicals may be directly toxic, deteriorate the water quality by changing its physico-chemical nature and cause ecological imbalance leading to health hazards to different types of aquatic organisms in general and fishes in particular [3]. In extreme cases there are records of catastrophic mortality of the entire aquatic biota [2].![]()
1
The use of agrochemicals in the field has the potential to change the aquatic medium, affecting the tolerance limit of aquatic fauna and flora, as well as creating danger to the ecosystem. Ayoola (2008) has reported that water pollution by pesticides is a serious problem to all aquatic fauna and flora and to a considerable extent even man. These agrochemicals adversely affect the non-target organisms, especially fish which are one of the most widely distributed organisms in an aquatic environment and being susceptible to environmental contamination may reflect the extent of the biological effects of environmental pollution in waters [5].
Blood analysis is crucial in many fields of
ichthyological research and fish farming and in the area of
toxicology and environmental monitoring as possible indicator of physiological or pathological changes in fishery management and diseases investigation [6]. Haematological indices are very important parameters for the evaluation of fish physiological status. The changes depend on fish species, age, the cycle of the sexual maturity of spawners, and diseases [7; 8 and 9]. In warm-blooded animals, changes in the blood parameters, which occur because of injuries or infections of some tissues or organs, can be used to determine and confirm the dysfunction or
Author, Bhavika Desai, a research scholar, Department of
Zoology, The M.S.University of Baroda, Vadodara-390002. Ph:
09979366015
E-mail: bhavu1611@yahoo.co.in
2 Co- Author, Pragna Parikh, Associate professor, Department of
Zoology, The M.S.University of Baroda, Vadodara-390002. Ph:
09825329148
E-mail: php59@yahoo.co.in
injuries of the latter i.e. organs or tissues. However in fish,
these parameters are more related to the response of the
whole organism, i.e. to the effect on fish survival, reproduction and growth.
A vast amount of scientific information is available
on the pesticide toxicity on fishes but limited information is available on the effect of these pesticides, in minute concentration, on the physiology of haemopoietic system,
IJSER © 2012 http://www.ijser.org
The research paper published by IJSER journal is about Impact of Curzate (fungicide) on Hematological Parameters of Oreochromis mossambicus 2
ISSN 2229-5518
thought to be most sensitive indicator towards
environmental pollutants. Therefore, the present study was undertaken to assess and contribute to knowledge on the haematological changes in fresh water fish, Oreochromis mossambicus at different concentration of Curzate.
2 MATERIALS AND METHODS
Fresh water fish Oreochromis mosssambicus (15 ± 2.6 cm and 24 ± 3 g) were obtained from a local pond of Baroda district and were acclimatized under laboratory condition. They were kept in glass aquaria containing 50 L of dechlorinated tap water.
30 tilapia fish were divided in 3 groups, 10 fish for each group:
Group 1 served as control without any treatment of fungicide.
Group 2 were treated with fungicide Curzate i.e. 4.9 mg/l
(LC 50 / 10).
Group 3 were treated with fungicide Curzate i.e. 2.45 mg/l
(LC 50 / 20).
Constant amount of the test chemical and test media were changed every 24 hours and the experiment lasted for 21 days. The fishes were fed once in a day throughout the duration of the sub-lethal toxicity tests.
Test organism was removed, from each tank for blood analysis. About 4 - 5ml of blood was collected from the caudal peduncle using separate heparinized disposable syringes containing 0.5mg ethylene diamine tetra acetic acid (EDTA) as anticoagulant; properly mixed and stored at
-20°C for haematological analysis. The blood was stored in -
4°C in deep freezer prior to analysis.
The red blood corpuscles (RBC) and White blood corpuscles (WBC) were counted using haemocytometer crystalline chamber using “Hayem’s” and “Turch’s” diluting fluid, respectively.
Haemoglobin Estimation (HB) and Pack Cell Volume
(PCV):
They were analyzed in NIHON KOHDEN
Automated Hematology Analyzer (Celtics α, Japan).
Mean Cell Haemoglobin Concentration (MCHC):
This refers to the percentage of
haemoglobin in 100 ml of red blood cell. This was calculated by dividing the haemoglobin content in g/dL by the PCV % of red blood according to the formulae:
MCHC = HB/PCV*1000 g/dL
Mean Corpuscular Volume (MCV):
The value of the corpuscular volume was calculated from the haematocrit value (PCV %) and the erythrocyte count (106/ µL) using the formula
MCV =PCV*1000/ RBCs fL
Mean Corpuscular Haemoglobin (MCH):
Mean corpuscular Haemoglobin concentration expresses the concentration of haemoglobin in unit volume of erythrocyte. It was calculated from the haemogobin value (HB) and from the erythrocyte count according to the following formulae
MCH = HB/RBCs pg
Leucocyte differential count:
Leucocyte differential count was done using Giemsa stain.
Statistical analysis was performed using Graph pad prism 5 software. The data was analyzed using two- way ANOVA test. Results were presented as mean ± SE. The significance was set as P<0.05, P<0.01 and P<0.001.
3 RESULTS AND DISCUSSION
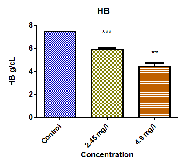
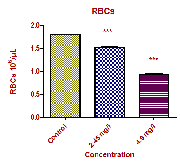
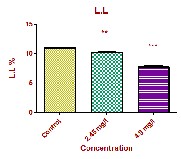
The changes of haematological parameters like, RBC, WBC, Hb, PCV, MCV, MCH and MCHC in the fish Oreochromis mossambicus both in control as well as sublethal concentrations of Curzate exposed after 21 days are shown in Table 1 and Fig: 1. The haematological analysis revealed a highly significant reduction in Red Blood Cell (RBCs) count from 1.807±0.006 106/µl in the control fish to 1.523±0.013 106/µl and 0.938±0.014 106/µl in the Low dose and High dose respectively. Also a significant decrease was recorded in hemoglobin (Hb) from
7.475±0.030 g/dl in control to 5.922±0.111 g/dl and
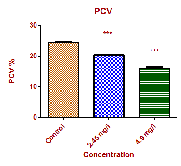
4.457±0.287 g/dl in low dose and high dose respectively. Haematocrit or PCV is essential in clinical haematology to
determine alterations in blood.
Red blood cell mass as measured by packed cell
volume (PCV) and Hemoglobin content (Hb) of exposed fish groups showed a progressive decrease parallel to the increasing concentration of the fungicide. Wahbi et al., (2004) and Zaki et al., (2008) attributed the decrease in the
IJSER © 2012 http://www.ijser.org
The research paper published by IJSER journal is about Impact of Curzate (fungicide) on Hematological Parameters of Oreochromis mossambicus 3
ISSN 2229-5518
RBC to heamolytic crisis that results in severe anemia in
fish exposed to heavy metals and herbicide respectively. Furthermore, the reduction of RBC also leads to development of hypoxic condition which in turn leads to increase in destruction of RBC or decrease in rate of formation of RBC due to non availability of Hb content in cellular medium (Chen, et al., 2004). The damage of toxicant on erythrocyte may be secondary, resulting from a primary action of toxicant on erythropoietic tissues on which there exist a failure in red cell production and or due to increase in the erythrocyte destruction. These results are in affirmative agreement with that investigated by Wahbi, et al., (2004).
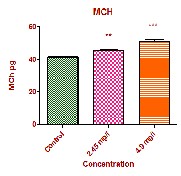
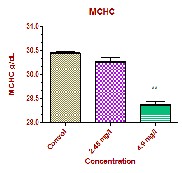
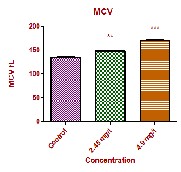
The values of MCV in the experimental groups showed significant increase (p<0.01), MCH values showed significant increase at high dose (p<0.001) and at low dose (P<0.01) respectively. MCHC values showed insignificant decrease at low dose and a significant decrease at high dose (p<0.01). The MCV, MCH and MCHC values are completely dependent upon the factors of PCV, RBC count and haemoglobin concentration. In the present study, the PCV, RBC and hemoglobin concentration is completely altered. So indirectly the values of MCV, MCH and MCHC were affected. In the present study the decreased PCV values with increased MCV and MCH associated with decreased MCHC values could probably due to stress induced by the fungicide and confirms the occurrence of haemolytic anemia in experimental fish which exaggerates further disturbances in haemopoietic activities of fish. Similar finding were also observed by a number of studies in different fish [12, 13, 14 and 15].
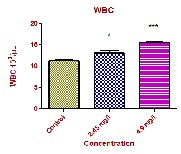
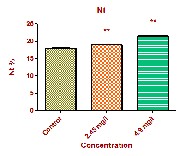
Total WBCs count was significantly increased from
11.31 ± 0.184 103 /µL in control fish to 13.09 ± 0.657 103 /µL
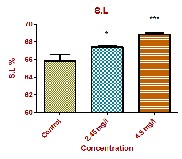
and 15.48 ± 0.213 103 /µL at low dose and high dose respectively. Associated with the increase in total WBC count was a noticeable percentage increase in small lymphocytes (S.L) and neutrophils (Nt). WBCs are important cells in the immune system, because of their main defensive function. The WBC will respond immediately to the change in medium due to xenobiotic transformation [16]. During exposure period of curzate the WBC counts got enhanced, indicating that the fish can develop a defensive mechanism to overcome the toxic stress. Our studies are in agreement with Lovell and Jantrarotai,(1991); Nanda, (1997); Wahbi, (1998); Hymavathi and Rao, (2000); Lebelo, et al.,(2001); Hassen, (2002) and Joshi, et al., (2002). .
Examination of Giemsa stained blood
smears of control fish showed well developed erythrocytes and neutrophil (Nt) with bilobed nucleus, (fig: 2 A) while examination of Giemsa stained blood smears of treated fish
showed increased number of lymphocytes and neutrophils
with associated morphological alterations similar to clinical features of neutrophilia and lymphocytosis. It is indicative of compensatory and defensive reaction to the toxicant in a dose dependent manner. (Fig: 2 B and C).
The measurement of hematological parameters, which are used in this study, has provided valuable information which can contribute to the applied and basic research needs of aquatic toxicologists in the assessment of fish health and in monitoring stress responses. The present study suggests that the perturbations in the blood indices are a defense reaction against curzate toxicity. Whether these changes reflects compensatory mechanisms in the fish or biochemical results of the toxic action of the fungicide remains to be elucidated. Further biochemical and histomorphometry studies are required and will help in understanding the metabolic alterations.
4 ACKNOWLEDGEMENTS
The authors are grateful to the Department of the Zoology, The Maharaja Sayajirao University of Baroda for providing the facilities for carrying out the present work.
5 REFERENCE
[1] E. Lawrence, and T. Isioma, “Acute toxic effects of Endosulfan and Diazinon pesticides on adult amphibians (Bufo regularis),” Journal of Environmental Chemistry and Ecotoxicology, vol. 2, no. 5, pp. 73-78, 2010.
[2] S.B. Kumari, and M.C. Subisha, “Haematological
responses in a freshwater fish Oreochromis mossambicus exposed to chlorpyrifos,” The Ekol., vol.
10, no. 1-2, pp. 83-88, 2010.
[3] M.J. Hemmer, B.L. Hemmer, C.J. Bawman, K.J. Croll, L.C. Formar, D. Marcovich, M.D. Hoglund, and N.D. Denslow, “Effect of pnonylphenol, methoxychlor and endosulfan on vitellogenin induction and expression in sheephead minnow (Cyprinodon variegates),” Environ Toxicol Chem., vol. 20, pp. 336-343, 2001.
[4] S.O. Ayoola, “Histopathological Effects of Glyphsate on
Juvenile catfish (Clarias gariepinus),” American- Eurasian Journal of Agricultural and Environmental Science, vol. 4, no. 3, pp. 362-367, 2008.
[5] J. Pant, H. Tewari, and T.S. Gill, “Effects of aldicarb on the blood and tissues of a freshwater fish. Bull. Environ.” Contam. Toxicol., vol. 38, pp. 36-41, 1987.
[6] O.B. Adedeji, V.O. Taiwo, and S.A. Agbee,
“Comparative haematology of five Nigerian freshwater fish species,” Nig. Vet. Journal, vol. 21, pp. 75-84, 2000.
IJSER © 2012 http://www.ijser.org
The research paper published by IJSER journal is about Impact of Curzate (fungicide) on Hematological Parameters of Oreochromis mossambicus 4
ISSN 2229-5518
[7] L.D. Zhitrineva, T.G. Poltavceva, O.A. Rudnickoja,
“Atlas of normal and pathological cells in 23256526 the blood of fish.” Rostov-on-Don., pp. 112, 1989.
[8] N.A Golovina, “Morpho-Functional Characteristics of the blood of fish as objects of aquaculture,” pp. 53, 1996. (Doctorial thesis. Moscow)
[9] V. Luskova, “Annual cycles and normal values of
haematological parameters in fishers.” Acta Sc. Nat.
Brno., vol. 31, No. 5, pp. 70, 1997.
[10] O.M. Wahbi, S.M. Shyma, and A.Y. El-Dakar, “Effect
of pulp and paper industrial effluent on some blood parameters, gonads and flesh proteins in experimentally exposed striped seabream Lithognathus mormyrus.” Egyptian journal of Aquatic research., vol. 30, No. A, pp.
25-42, 2004.
[11]. M.S. Zaki, O.M. Fawzi, and El-Jackey, J., “Pathological and biochemical studies in Tilapia nilotica infected with Saprolegnia parasitica and treated with potassium permanganate.” American-Eurasiun J. Agric. & Environ. Sci.,vol. 3, No. 5, pp. 677-680, 2008.
[12] X. Chen, D. Yin, S. Hu, and Hou, “Immunotoxicity of penta chlorophenol on macrophage immunity and IgM secretion of the crucian carp (Carassius auratus),” J. Bull. Environ. Contam and Toxicol., vol. 73, pp. 153-160, 2004.
[13] A. Murad, and S. Mustafa, “Blood parameters of
catfish, heteropneuttstes fassilis (bloch) parasitized by metacercaria of Diplostomum species.” J. Fish Dis., vol.
9, pp. 295-302, 1988.
[14] V.K. Garg, S.K. Garg and S.K. Tyabi, “Manganese induced Hematological and Biochemical anomalies in H. fossils,” J. Environ. Biol., vol. 10 no. 4, pp. 349-353,
1989.
[15] A. Laurent, J.J. Durussel, J. Dufaux, C. Penhonet, A.L.
Bailly, M. Bonneau, and J. Merland, “Effect of contrast media on blood rheology comparison in human, pigs
and sheep.” J. Card. Vas. Intervent. Radiol. ,vol. 22, pp.
62-66, 1999.
[16] J. Drastichova, Z. Svobodova, V. Luskova, and J.
Machova, “Effect of cadmium on haematological indices of common carp Cyprinus Carpio,” J. Bull. Environ. Contam and Toxicol., vol. 72, no. 4, pp. 725-732, 2004.
[17] A. Naveed, P. Venkateshwarlu, and C. Janaiah,
“Impact of triazophos infestion on heamatological
parameters of cat fish Channa punctatus (Bloch).”
International journal of pharmacy & life sciences., vol. 1, No.
6, pp. 298-301, 2010.
[18] R.T. Lovell, and W. jantrarotai, “Subchronic toxicity of dietary aflatoxin B1 to channel catfish.” Journal of
Aquatic Animal Health., vol. 2, No. 4, pp.248-254, 1991. [19] P. Nanda, “Haematological changes in the common
Indian Catfish Heteropneustes fossilis under nickel stress.” J. Ecobiol., vol. 9, pp. 243-246, 1997.
[20] O.M. Wahbi, “Effect of tanning processing wastewater
in physiological characteristics of Solea sp.” Ph.D. Thesis, Faculty of Science, Alexandria University, 1998.
[21] V. Hymavathi, and M. Rao, “Effect of sublethal concentration of lead on the haematology and biochemical constituents of Channa punctatus,” J. Bull. Pure and Appl. Science, vol. 19, no. 9, pp. 1-5, 2000.
[22] S.L. Lebelo, D.K. Saunders, and T.G. Crawford,
“Observations on blood viscosity in striped bass, Morone saxtilis (Walbaum) Associated with fish Hatchery conditions.” Kansa Acad. Sci., vol. 104, pp. 183-
194, 2001.
[23] F.E.Z.M. Hassen, “Studies on diseases of fish caused by
Henneguya infestation,”2002. (Ph.D Thesis, Faculty of
Veterinary Medicine, Suez Canal University. Egypt.)
[24] P.K., Joshi, M. Bose, and D. Harish, “Haematological
changes in the blood of Clarias batrachus exposed to mercuric chloride, Ecotoxicol.” Environ. Monit., vol. 12, pp. 119-122, 2002.
IJSER © 2012 http://www.ijser.org
The research paper published by IJSER journal is about Impact of Curzate (fungicide) on Hematological Parameters of Oreochromis mossambicus 5
ISSN 2229-5518
IJSER © 2012 http://www.ijser.org
The research paper published by IJSER journal is about Impact of Curzate (fungicide) on Hematological Parameters of Oreochromis mossambicus 6
ISSN 2229-5518
Parameters | Concentration mg/l | ||
Parameters | Control (0 mg/l) | Low Dose (2.45 mg/l) | High dose (4.9 mg/l) |
RBCs 106 /µL | 1.807±0.006 | 1.523±0.013*** | 0.938±0.014*** |
HB g/dL | 7.475±0.030 | 5.922±0.111*** | 4.457±0.287** |
PCV (Htc) % | 24.50±0.063 | 20.47±0.069*** | 16.04±0.505*** |
MCV fL | 135.0±1.00 | 148.2±0.881** | 170.2±2.557*** |
MCHC g/dL | 30.45±0.028 | 30.27±0.088 | 29.37±0.074** |
MCH pg | 41.66±0.172 | 45.67±0.346** | 51.15±1.011*** |
Total WBC 103 /µL | 11.31 ± 0.184 | 13.09 ± 0.657* | 15.48 ± 0.213*** |
Small Lymphocytes % | 65.82 ± 0.745 | 67.42 ± 0.144* | 68.88 ± 0.170*** |
Large lymphocytes % | 11.03 ± 0.051 | 10.20 ± 0.152** | 7.798 ±0.170*** |
Neutrophils % | 18.02 ± 0.063 | 19.01 ± 0.129** | 21.46 ± 0.158** |
***Significant p < 0.001; **Significant p <0.01; *Significant p< 0.05; ± S E
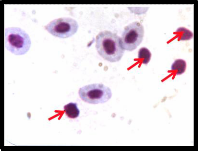
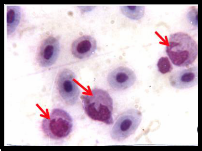
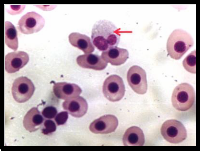
Fig 2 A shows well developed erythrocytes and neutrophil (↑) with bilobed nucleus, Fig 2 B and C
shows increased number of lymphocytes and neutrophils with associated morphological alteration (↑).

IJSER © 2012 http://www.ijser.org