International Journal of Scientific & Engineering Research Volume 2, Issue 5, May-2011 1
ISSN 2229-5518
Iatrogenic effects of Orthodontic treatment – Review on white spot lesions
Sangamesh B., Amitabh Kallury
Abstract— Demineralization is an inevitable side effect associated with fixed appliance orthodontic treatment, especially associated with poor oral hygiene. Fixed orthodontic appliances create several retentive areas for the accumulation of bacterial plaque. The acidic byproducts of these bacteria are responsible for the subsequent enamel demineralization and formation of white spot lesions (W SL), causing caries therefore leading to poor esthetics, patient dissatisfaction and legal complications. This highlights the need for assessing the saliva, oral hygiene status and caries rate before beginning of treatment and initiating preventive measures. Orthodontists must take up active responsibility to educate the patients about the importance of maintaining good dietary compliance and excellent oral hygiene regime. Depending on the oral environment, W SL can develop into cavities, stay stable for a long time, or heal to a certain extent. Thus, the prevention of W SL is crucial to prevent tooth decay as well as minimize tooth discoloration that could compromise the treatment results.
Index Terms— Iatrogenic effects, W hite spot lesion, Incidence, Orthodontic Treatment, Duration, Oral hygiene, Etiology, Prevention, Fixed Appliances, Demineralization.
—————————— • ——————————
hite spot lesion (WSL) is a common iatrogenic effect seen in patients undergoing orthodontic treatment with fixed appliances (Fig.1).1,2 Individuals with ma-
locclusions often have many plaque retention sites due to the
irregularities of their teeth (Fig.2). Orthodontic treatment with fixed appliances and complex loop designs further in- creases the risk for development of WSL due to the creation of additional retention sites on surfaces generally not sus- ceptible to caries.2 Hence a strong co-relation exists between oral hygiene and caries incidence in orthodontic patients as compared to in non orthodontic individuals.3 Despite inten- sive efforts to educate patients about effective oral hygiene procedures, WSL associated with fixed orthodontic ap- pliances remains a significant clinical problem (Fig.3). This clinical problem has increased since the advent of directly bonded orthodontic brackets.4 Appearance of these spots after the completion of orthodontic treatment can lead to patient dissatisfaction and legal complication.5 The formation of WSL after completion of orthodontic therapy is discouraging to a speciality whose goal is to improve esthetics in the dento-facial region. Orthodontists should be proactive and take active responsibility to prevent the devel- opment of WSL by educating their patients about the impor- tance of maintaining an excellent dietary compliance and oral hygiene regime. Oral hygiene regime must include topi- cal fluoride agents such as fluoridated toothpaste, fluoride-
————————————————
Sangamesh B
• Assistant Professor, Dept. of Orthodontics, SDM College of Dental
Sciences, Dharwad. Email: bsangamesh@gmail.com
Amitabh Kallury
• Professor and Head, Dept. of Orthodontics, Peoples Dental Academy, Bhopal. Email: dr.amitabhkallury@gmail.com
containing mouth rinse, gel and varnish to prevent or mi- nimize the formation of WSL during orthodontic treatment.6
The term white spot lesion was defined as ‘the first sign of a caries lesion on enamel that can be detected with the naked eye’.7
The WSL has also been defined as ‘subsurface enamel porosi- ty from carious demineralization’ that presents itself as ‘a milky white opacity when located on smooth surfaces’.8
CLASSIFICATION OF WHITE LESIONS ON ENAMEL White discolorations of enamel can be classified as dental fluorosis, opacities, or WSL.6 A set of criteria has been devel- oped to differentiate between fluorosis and opacities.9 Fluo- rosis (Fig.4) is a white/yellowish lesion that is not well de- fined, blends with normal enamel, and has symmetrical dis- tribution in the mouth. Nonfluoride opacities have a more defined shape, are well differentiated from surrounding enamel, often located in the middle of the tooth, and ran- domly distributed (Fig.5).
Orthodontic patients have significantly more WSL than non- orthodontic patients and these WSL may present esthetic problems years after treatment.3,10 A recent review of litera- ture11 showed variations ranging from 2% to 97%, for WSL prevalence associated with orthodontic treatment3,10,12-17. This high prevalence is attributed to the difficulties in performing oral hygiene procedures on bonded dental arches along with long-time accumulation and easier retention of bacterial pla- que on tooth surfaces around fixed orthodontic ap- pliances.15,18 The variation in WSL prevalence among studies could be attributed to differences in the number of teeth ex-
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 5, May-2011 2
ISSN 2229-5518
amined, the methods and the standardizations in examina- tions, the location of the study sample (cultural differences), time era of the study, age at the start of treatment, treatment duration, and materials (banding vs bonding).19 In general, the prevalence of WSL in patients after orthodontic treatment varies from 15% to 85%13, with most studies reporting 50% to
70%.10,15-17,20-22 It is reported that any tooth in the mouth can be affected by the process with the common ones being max- illary lateral incisors, maxillary canines, and mandibular premolars.15 The incidence was highest in the labio-gingival area of the maxillary lateral incisors (Fig.6) and lowest in the maxillary posterior segment. The reported incidence and prevalence of WSL between males and females have been found to be inconclusive.10,11,14 No significant differences between the right and left sides of the maxilla and mandible were noted.10,14
WSL can occur on any tooth surface in the oral cavity where the plaque is allowed to develop and remain for a period of time (Fig.7). The naturally occurring self cleansing mechan- isms of the oral musculature and saliva are limited by the irregular surface of brackets, bands, and wires.23 The compo- sition of the bacterial flora of the plaque shows a rapid shift following the placement of orthodontic appliances. Patients undergoing treatment with fixed orthodontic appliances have a rapid increase in the volume of dental plaque (with a lower pH) than that in non-orthodontic patients.24,25 The le- vels of acidogenic bacteria, especially Streptococcus mutans and lactobacillus, are significantly elevated.26 Both S. mutans and lactobacilli are often associated with caries development. Streptococcus mutans colonize over the retentive areas of orthodontic appliances and surrounding enamel surfaces. Lactobacillus is responsible for the progression of the carious lesion. Their presence in large numbers is indicative of the necessary condition for dental caries to exist.27 However, the association between caries and bacteria is not straightfor- ward. The prediction of caries development based on bac- terial counts is uncertain and of minor clinical significance.28
S. mutans and lactobacilli produce organic acids in the pres- ence of fermentable carbohydrates and this is responsible for lowering the pH. Sucrose plays an important role in plaque formation inducing the formation of a cariogenic plaque.29
There is a direct relationship between plaque pH and total plaque fluoride. Total plaque fluoride levels are low in areas of low pH. The lowest pH (as low as 4) during resting and fermenting conditions was observed in the plaque of the bonded upper incisors.30 After bonding, resting pH is lo- wered. In the patient with good oral hygiene, fluoride is able to prevent lesions to develop by increasing remineralization and inhibiting demineralization. With poor oral hygiene, plaque builds up around the appliance and the resting pH may reach the limit of the fluoride effect at pH 4.5. During an acid attack, caries and even erosions develop.29 Carious de- calcification occurs when the pH drops below the threshold for remineralization and creates an alteration in the appear- ance of the enamel surface which is visualized as WSL.25,31
Such lesions have been clinically noticed within a short span
of 4 weeks2. If these are not treated, they progress to a cavi-
tated carious lesion.32 WSL makes the affected area softer than the surrounding sound enamel, making the tooth more prone to caries33. There is about 10% reduction in the mineral content of enamel in these incipient carious lesions. This leads to their increased abrasion in vivo.34 This makes the affected teeth more susceptible to enamel loss while debond- ing.35 Fast developing white spots may remineralize almost completely within a few weeks of the removal of the cari- ogenic challenge. However, lesions that develop slowly take a longer period to remineralize.36 Micro-leakage around or- thodontic brackets can be another cause for the formation of WSL(Fig.8). 37 The teeth expand and contract when they are heated and cooled by the ingestion of hot or cold foods38. The linear thermal coefficient of expansion of enamel, ceram- ic/metal brackets and the adhesive systems do not match.39
This repeated expansion and contraction at different coeffi- cients results in fluids being sucked in and pushed out at the margins of the bracket. In comparison with ceramic brackets, the metal brackets are associated with more micro-leakage (Fig.9).40 Metal brackets contract and expand more than ce- ramic brackets, enamel, or the adhesive systems, producing microgaps between the bracket and the adhesive system causing leakage of oral fluids and bacteria beneath the brackets, leading to the formation of WSL.41
Formation of WSL is primarily due to the subsurface demi- neralization resulting in porosities and a change in the opti- cal properties. If the surface of porous enamel remains intact, there is a possibility of arrest/remineralization of the lesion due to the buffering action of the saliva. If the pH of plaque remains low for a prolonged period of time, the environment becomes conducive for long periods of demineralization with short periods of remineralization, resulting if frank ca- rious lesions. Risk factors for the development of incipient caries during orthodontic treatment are young age (preado- lescents), number of poor oral hygiene citations during treatment, unfavorable clinical outcome score, white ethnic group, and inadequate oral hygiene at the initial pretreat- ment examination.42
Factors such as the patient’s medical history, dental
history, medication history, diet; salivary flow rate, levels of calcium, phosphate, and bicarbonate in saliva, fluoride levels and genetic susceptibility also play an important role.23,43,44
There is a poor correlation between length of treatment time and the incidence of number of white spot formations.14
Studies have shown that decalcification is a significant risk during fixed orthodontic treatment.3,10,14,1745 Current evi- dence suggests that topical fluoride treatment (TFT) is bene- ficial in preventing the development of WSL during ortho- dontic treatment.46 When topical fluoride is applied on the tooth surface (enamel/dentin), a calcium fluoride-like ma- terial (CaF2) builds up in plaque, or in incipient lesions which acts as a reservoir and releases fluoride ions when the pH is lowered during a caries attack.47 Implementing a good oral hygiene regimen including proper tooth brushing with a fluoridated dentifrice is the most important prophylactic
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 5, May-2011 3
ISSN 2229-5518
measure to prevent the occurrence of WSL in orthodontic patients. Fluoride concentrations of less than 0.05% are bene- ficial in reduction of the carious lesions.48 Evidence suggests that reduced demineralization and enhanced remineraliza- tion can occur with a toothpaste containing 5000ppm fluo- ride.49,50
When fluoride ions are incorporated into the sur- face of enamel, it forms a fluoroapatite crystal structure that has lower solubility in the oral environment compared with hydroxyapatite. Fluoroapatite helps in reducing tooth decay by remineralization of small decalcified areas and reduction in the formation of new lesions.51 In addition, stannous fluo- ride may have a plaque-inhibiting effect by interfering with the adsorption of plaque bacteria to the enamel surface.52,53
Atoms of tin in stannous products block the passage of su-
crose into bacterial cells and thus inhibit acid production. The use of a fluoridated antiplaque dentifrice may reduce enamel demineralization around brackets more than the use of a fluoridated dentifrice alone.54 Enamel dissolution occurs rapidly around orthodontic brackets even during regular use of a fluoride dentifrice31. Thus, supplemental sources of fluo- ride are suggested.
Fluoridated mouth rinses containing 0.05% sodium
fluoride used daily have been shown to significantly reduce lesion formation beneath bands. Chemical agents such as chlorhexidine or benzydamine used in the form of mouth rinses or oral sprays are useful adjuncts in plaque and in- flammation control.55 These mouth rinses have been com- bined with antibacterial agents such as chlorhexidene, triclo- san, or zinc to improve their cariostatic effect.33 When pa- tients have been noncompliant with other oral hygiene regi- mens, chlorhexidine mouthwashes might be beneficial in preventing white spot caries lesions as an intensive, short- term regimen. Chlorhexidine mouthwash used as a comple- ment to fluoride therapy has demonstrated demineraliza- tion-inhibiting tendencies in patients with fixed orthodontic appliances.56 The main goal of antimicrobial therapy is to achieve a shift from an ecologically unfavorable to an ecolog- ically favorable biofilm.57 Patients are instructed to use chlorhexidine rinse (available in nonalcohol formulations for patients with xerostomia or saliva dysfunction) for 30 seconds once a day, preferably before bedtime, because sali- va flow diminishes overnight and the concentration of the drug in the oral cavity remains high until morning.58 A 14- day regimen is usually recommended.58 While these prod- ucts provide the patient with increased caries protection, patient compliance is mandatory. A fluoride mouth rinse will work best if it is used regularly by the patient. Studies have showed that less than 15% of orthodontic patients rinsed daily as instructed but patients who were more com- pliant had fewer WSL.15
An in-office application of a high concentration of fluoride in the form of a varnish can be beneficial for the less compliant patients. It eliminates the need for patient cooper- ation that is required with fluoride rinses. The American Dental Association’s Council on Scientific Affairs recom- mends application of ‘in-office fluoride varnish at six-month intervals for moderate and high-risk patients’. Although varnish application is associated with the temporary discolo-
ration of the teeth and gingival tissue, it has been reported that the application of a fluoride varnish resulted in a 44.3% reduction in enamel demineralization in orthodontic pa- tients.59 Acid-resistant coatings of calcium fluoride or tita- nium fluoride on the enamel surface and the use of fluoride in combination with different antimicrobials have been sug- gested to improve the cariostatic effect of fluoride at low pH.60 Varnish forms of the other antibacterial solutions such as benzydamine, triclosan, and xylitol could be helpful for suppressing levels of oral mutans or the other microbes for long periods, when used before the placement of fixed or- thodontic appliances. In contrast to one-time topical applica- tion in high doses, a long-term, low-dose fluoride availability might increase the caries-resistant fluorapatite concentration in enamel, helping the prevention and reduction of demine- ralization.48,51
Unfortunately, preventive and chemoprophylactic products, such as high-fluoride toothpaste or gel, fluoride varnish, and chlorhexidine rinse, gel, or varnish, are rarely prescribed by orthodontists. It was reported that 95% of or- thodontists provide oral hygiene instructions, while only
52% prescribe fluoride mouth rinse61.
Xylitol, a polyol (a type of carbohydrate) that does
not act as a metabolizing substrate for Streptococcus mutans, can be used as a low-calorie sugar substitute to prevent ca- ries.62 Xylitol has been used as a caries preventive agent in form of gum and mints. It is noncariogenic and appears to have antimicrobial properties that help to inhibit S mutans attachment to the teeth. The salivary pH remains stable as there is no metabolism by bacteria, and the environment does not favor acidogenic bacteria.63 Additionally, the con- sumption of chewing gum and mints has been demonstrated to result in increased production of stimulated saliva con- taining more calcium and phosphate ionic concentrations when compared with non-stimulated saliva.63 The systematic use of xylitol chewing gum can significantly reduce the risk of caries compared with gums that contain sorbitol and su- crose.64 Chewing xylitol gum thrice a day for 5 minutes has shown positive results.65 However, long-term clinical trials with a standardized methodology are needed. Moderate and high-risk adult patients are recommended to chew 2 pieces of xylitol gum for 10minutes at least, 3 to 5 times a day.66
Therapeutically, 6gm/day of xylitol is recommended for
adults.67 However, xylitol can cause diarrhea if the recom- mended doses are exceeded.63
Enamel demineralization might be prevented by the application of products containing casein phosphopeptides- amorphous calcium phosphate (CPP-ACP). CPP-ACP is a nanocluster that binds calcium and phosphate ions in an amorphous form. CPP-ACP has been shown to adhere to the bacterial wall of microorganisms and tooth surfaces.68,69
When an intraoral acid attack occurs, the calcium and phos- phate ions are released to produce a supersaturated concen- tration of ions in the saliva, which then precipitates a cal- cium-phosphate compound onto the exposed tooth surface.69
However, there is insufficient clinical trial evidence to make a recommendation regarding its long-term effectiveness.63
The prolonged duration of orthodontic treatment
places the patient at an increased caries risk. This risk can be
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 5, May-2011 4
ISSN 2229-5518
minimized by a continuous fluoride release from the bond- ing system around the bracket base. The introduction of fluo- ride-releasing adhesive systems, resin composites, and glass ionomer cements for bracket bonding offered a means of fluoride delivery adjacent to bracket-enamel interface inde- pendent of patient cooperation. However, the ability of these materials to reduce decalcification clinically remains equi- vocal.70 Glass ionomer cements(GIC) do not provide com- plete caries protection under loose bands or in areas of miss- ing/dissolved cement.71 However the shear bond strength (SBS) of GIC was not adequate for bonding brackets. In an attempt to increase the bond strength of GIC’s, resin particles were added to their formulation to create resin modified Glass Ionomer (RMGI) bonding systems. These adhesives release fluoride like conventional GIC’s and can also be used successfully to bond orthodontic brackets because of their relatively higher SBS.72-83 Additionally, in vivo studies have shown no significant differences in bracket failure rates be- tween the RMGI’s and composite adhesives.84 Because of the recent improvements in the fluoride-releasing capabilities and the SBS of RMGI, it has been suggested that these adhe- sives should be used more widely in bonding orthodontic brackets in the future.85 However a recent study concluded that it is impossible to make recommendations on the use of fluoride-containing orthodontic adhesives during fixed or- thodontic treatment.86 The authors found sufficient evidence to suggest that GIC is more effective than composite resin in preventing white spot formation, but further research is re- quired to determine the effectiveness of the various fluoride- containing orthodontic adhesives.
A fluoride releasing antibacterial bonding agent has been developed by combining the physical advantages of dental adhesive technology and antibacterial effect. The anti- bacterial activity of 12-methacryloyloxydodecyl-pyridinium bromide (MDPB) incorporated in the antibacterial adhesive systems demonstrated inhibition of caries formation, espe- cially along the enamel margins.87 Incorporating MDPB into self-etching primer and adhesive resin has demonstrated in vitro antibacterial activity, bonding ability, cytotoxicity, and pulpal response. It was confirmed that MDPB-containing primer has got antibacterial effects in vivo when used in an- imal models.88,89
Other fluoride-release mechanisms like fluoride- re-
leasing elastic ligature and power chains have been tried. Research has shown that fluoride-releasing elastomeric liga- tures were effective in reducing plaque accumulation and decalcification around the brackets.16,90,91 However, later in- vestigations reported that fluoride-releasing elastomeric liga- tures did not reduce the amount disclosed plaque around the brackets.92 Research has shown that the fluoride release was high in the first week but decreased significantly in the sub- sequent weeks.93
Finally, use of argon laser to cure composite resins
has demonstrated its ability to alter the enamel, rendering it less susceptible to demineralization. It was also shown that combining laser irradiation with fluoride treatment can have a synergistic effect on acid resistance preventing formation of WSL and dental caries.94 Research has shown that exposing the teeth to an argon laser for 60 seconds at the time of ap-
pliance placement reduced lesion depth by 91.4% and lesion area by 94.6% when compared with untreated control teeth.95
The authors recommend the following measures to pre- vent WSL in orthodontic patients:
1. Educate and motivate the patients at every visit to
maintain optimal oral hygiene around the ap- pliances to obtain the full effect of fluoride.29
2. Daily brushing with fluoride toothpaste (1500ppm
or more) twice a day.56 Use of
interdental brushes to remove plaque around the
brackets.
3. Daily use of a fluoride mouth rinse (0.05% NaF).29,31,55
4. Performing oral prophylaxis (scaling) and reinforcing
instructions at each appointment in non-compliant patients.
5. Use of chlorhexidine mouth rinse at night for 2 weeks in patients with poor oral hygiene.
6. Use of topical fluoride in the form of solutions, var-
nishes, or gels around the brackets of
non-compliant/ high risk patients at 6 months interval.
7. Cementing the bands with good quality GIC.
WSL on the enamel surface adjacent to fixed orthodontic appliances is an important and prevalent iatrogenic effect of orthodontic therapy. The components of the appliance and the bonding materials create stagnation areas for plaque ac- cumulation and bacterial colonization. The subsequent acid production by the acidogenic bacteria leads to enamel decal- cification. The orthodontist must educate the patient regard- ing the importance of maintaining good oral hygiene and dietary regime. Fluoride is the most important agent to pre- vent decalcification and restrict lesions from progressing. Oral hygiene regime must include topical fluoride agents such as fluoridated toothpaste, fluoride-containing mouth rinse, gel and varnish to prevent or minimize the formation of WSL during orthodontic treatment.
[1] Øgaard B., Bishara S, Duschner H: Enamel effects during bonding— debonding and treatment with fixed appliances, in Graber T, Eliades T, Athanasiou A, eds: Risk Management in Orthodontics. Experts’ Guide to Malpractice. Quintessence, 2004, pp 19-46.
[2] Ogaard B, Rølla G, Arends J. Orthodontic appliances and enamel
demineralization. Part 1. Lesion development. Am J Orthod Dentofacial
Orthop 1988;94:68-73.
[3] Zachrisson BU, Zachrisson S: Caries incidence and oral hygiene dur- ing orthodontic treatment. Scand J Dent Res 1971;79:394-401.
[4] Zachrisson BU: A post treatment evaluation of direct bonding in or- thodontics. Am J Orthod Dentofacial Orthop 1977 ; 71:173-189.
[5] Machen D E Legal aspects of orthodontic practice: risk management concepts. Am J Orthod Dentofacial Orthop 1991,100: 93-94.
[6] Bishara SE, Ostby AW. White Spot Lesions: Formation, Preven- tion,and Treatment. Semin Orthod 2008;14:174-182.
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 5, May-2011 5
ISSN 2229-5518
[7] Fejerskov O, Nyvad B, Kidd EAM. Clinical and histological manife- stations of dental caries. In: Fejerskov O, Kidd EAM, editors Dental caries: the disease and its clinical management. Copenhagen, Den- mark: Blackwell Munksgaard; 2003.pp.71-99.
[8] Summitt JB, Robbins JW, Schwartz RS: Fundamentals of Operative
Dentistry: A Contemporary Approach, 3rd ed. Hanover Park, IL, Quin- tessence Publishing, 2006, Chapter 1,pp 2-4.
[9] Russell AL: The differential diagnosis of fluoride and nonfluoride
enamel opacities. J Public Health Dent 1961;21:143-146.
[10] Ogaard B. Prevalence of white spot lesions in 19-year-olds: a study on untreated and orthodontically treated persons 5 years after treatment. Am J Orthod Dentofacial Orthop 1989;96:423-7.
[11] Boersma JG, van der Veen MH, Lagerweij MD, Bokhout B, Prahl-Andersen B. Caries prevalence measured with QLF after treatment with fixed orthodontic appliances: influencing factors. Caries Res
2005;39:41-7.
[12] Ogaard B, Larsson E, Henriksson T, Birkhed D, Bishara SE. Ef- fects of combined application of antimicrobial and fluoride varnishes in orthodontic patients. Am J Orthod Dentofacial Orthop 2001;120:28-35. [13] Mitchell L. Decalcification during orthodontic treatment with fixed appliances. Br J Orthod 1992;19:199-205.
[14] Gorelick L, Geiger AM, Gwinnett AJ. Incidence of white spot forma- tion after bonding and banding. Am J Orthod 1982;81:93-8.
[15] Geiger AM, Gorelick L, Gwinnett AJ, Griswold PG. The effect of a fluoride program on white spot formation during orthodontic treatment. Am J Orthod Dentofacial Orthop 1988;93:29-37.
[16] Banks PA, Chadwick SM, Asher-McDade C, Wright JL. Fluoride releasing elastomerics: a prospective controlled clinical trial. Eur J Orthod
2000;22:401-7.
[17] A°rtun J, Brobakken B. Prevalence of carious white spots after ortho- dontic treatment with multibonded appliances. Eur J Orthod 1986;8:229-
34.
[18] Zachrisson BU. Fluoride application procedures in orthodontic prac- tice, current concepts. Angle Orthod 1975;45:72-81.
[19] Joshua A. Chapman, W. Eugene Roberts, George J. Eckert, Ka- therine S. Kula, and Carlos Gonza´ lez-Cabezas Risk factors for inci- dence and severity of white spot lesions during treatment with fixed ortho- dontic appliances Am J Orthod Dentofacial Orthop 2010;138:188-94. [20] Mizrahi E. Surface distribution of enamel opacities following ortho- dontic treatment. Am J Orthod 1983;84:323-31.
[21] Marcusson A, Norevall LI, Persson M. White spot reduction when
using glass ionomer cement for bonding in orthodontics: a longitudinal and comparative study. Eur J Orthod 1997;19:233-42.
[22] Basdra EK, Huber H, Komposch G. Fluoride release from orthodon- tic bonding agents alters enamel surface and inhibits enamel demineraliza- tion in vitro. Am J Orthod Dentofacial Orthop 1996;109:466-72.
[23] Mount GJ, Hume WR. Preservation and restoration of tooth struc- ture. 2nd Edition. Queensland, Australia: Knowledge Books and Software; 2005;61-82.
[24] Chatterjee R, Kleinberg I. Effect of orthodontic band placement on the chemical composition human incisor plaque. Arch Oral Biol 1979;
24:97-100.
[25] Gwinnett JA, Ceen F. Plaque distribution on bonded brackets: a scanning electron microscope study. Am J Orthod 1979;75:667-677.
[26] Fournier A, Payant L, Bouchin R. Adherence of Streptococcus mu- tans to orthodontic brackets. Am J Orthod Dentofacial Orthop
1998;114:414-417.
[27] Klock B, Krasse B. A comparision between different methods of pre- diction of caries activity. Scand J Dent Res 1979;87:129-139.
[28] Hausen H, Seppä L, Fejerskov O: Can caries be predicted?,in Thyl- strup A, Fejerskov O, eds: Textbook of Clinical Cariology. Copenha-
gen, Munksgaard, 1994, pp 393-411.
[29] Øgaard B. White Spot Lesions During Orthodontic Treatment: Me- chanisms and Fluoride Preventive Aspects Semin Orthod 2008;14:183-
193.
[30] Arneberg P, Giertsen E, Emberland H, et al: Intra-oral variations in total plaque fluoride related to plaque pH. A study in orthodontic pa- tients. Caries Res 31:451-456, 1997.
[31] O’Reilly MM, Featherstone JD. Demineralization and remineraliza- tion around orthodontic appliances: an in vitro study. Am J Orthod Den- tofacial Orthop 1987; 92:33-40.
[32] Mitchell L. An investigation into the effect of fluoride releasing adhe- sive on the prevalence of enamel surface changes associated with directly bonded orthodontic attachments. Br J Orthod 1992;19:207-214.
[33] Ogaard B: Oral microbiological changes, long-term enamel alterations due to decalcification, and caries prophylactic aspects, in Bratley WA, Eliades T (eds): Orthodontic Materials: Scientific and Clinical As- pects. Stutgard, Thieme, 2001, pp 127.
[34] Linton JL. Quantitative measurements of remineralization of incipient caries. Am J Orthod Dentofacial Orthop 1996;110:590-597.
[35] Tufekci E, Mirrill TE, Pintado MR, et al. Enamel loss associated with orthodontic adhesive removal on teeth with white spot lesions: an in vitro study. Am J Orthod Dentofacial Orthop 2004;125:733-740.
[36] Ogaard B, ten Bosch J: Regression of white spot enamel lesions. A new optical method for quantitative longitudinal evaluation in vivo. Am J Orthod Dentofacial Orthop 1991;106:238-242.
[37] James JW, Miller BH, English JD, et al. Effects of high speed curing devices on shear bond strength and microleakage of orthodontic brackets. Am J Orthod Dentofacial Orthop 2003; 123:555-561.
[38] Gladwin M, Bagby M: Clinical Aspects of Dental Materials Theory, Practice and Cases. Baltimore, MD, Lippincott Williams & Wilkins,
2004.
[39] Van Noort R: Introduction to Dental Materials. 1st ed. London, UK, Mosby, 1994, pp 53-54.
[40] Arhun N, Arman A, Cehreli SB, et al. Microleakage beneath metal
and ceramic brackets bonded with a conventional and an antibacterial adhe- sive system. Angle Orthod 2006;76:1028-1034.
[41] Arhun N and Arman A Effects of Orthodontic Mechanics on Tooth
Enamel: A Review Semin Orthod 2007;13:281-291.
[42] Chapman et al Risk factors for incidence and severity of white spot lesions during treatment with fixed orthodontic appliances Am J Orthod Dentofacial Orthop 2010;138:188-94.
[43] Chalmers JM. Minimal intervention dentistry: strategies for the new caries challenge in our older patients. JCDA 2006;72:325-31.
[44] Papas AS, Joshi A, MacDonald SL, Maravelis-Splagounias L, Pretara-Spanedda P, Curro FA. Caries prevalence in xerostomic individ- uals. J Can Dent Assoc 1993;59:171-179.
[45] Ingervall B. The influence of orthodontic appliances on caries frequen-
cy. Odontol Revy 1962;13:175-90.
[46] Suri L, Huang G, English JD Jr, Owen S, Nah HD, Riolo ML, Shroff B, Southard TE and Turpin DL. Topical fluoride treatment (Read- ers’ Forum, Ask Us). Am J Orthod Dentofacial Orthop 2009,135:561-
63.
[47] Øgaard B: Effects of fluoride on caries development and progression in vivo. J Dent Res 69(Spec Issue):813-819,1990.
[48] Margolis HC, Mareno EC, Murphy BJ. Effect of low levels of fluo- ride in solution on enamel demineralization in vitro. J Dent Res1986;65:23-29.
[49] Baysan A, Lynch E, Ellwood R, Davies R, Petersson L, Borsboom
P. Reversal of primary root caries using dentifrices containing 5000 and
1100 ppm fluoride. Caries Res 2001;35:41-6.
[50] Schirrmeister JF, Gebrande JP, Altenburger MJ, Mo¨nting JS,
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 5, May-2011 6
ISSN 2229-5518
Hellwig E. Effect of dentifrice containing 5000 ppm fluoride on non- cavitated fissure carious lesions in vivo after 2 weeks. Am J Dent
2007;20:212-6.
[51] Mellberg JR, Mallon DE. Acceleration of remineralization in vitro by sodium mono fluoriphosphate and sodium fluoride. J Dent Res 1984;
63:1130-1155.
[52] Øgaard B, Gjermo P, Rolla G. Plaque-inhibiting effect in orthodontic patients of a dentifrice containing stannous fluoride. Am J Orthod
1980;78:266-272.
[53] Boyd RL, Chun YS. Eighteen-month evaluation of the effects of a
0.4% stannous fluoride gel on gingivitis in orthodontic patients. Am J Orthod Dentofacial Orthop 1994;105:35-41.
[54] de Moura MS, de Melo Simplicio AH, Cury JA. In-vivo effects of
fluoridated antiplaque dentifrice and bonding material on enamel deminera- lization adjacent to orthodontic appliances. Am J Orthod Dentofacial Orthop 2006;130:357-363.
[55] Zachrisson BU. Cause and prevention of injuries to teeth and sup- porting structures during orthodontic treatment. Am J Orthod
1976;69:285-300.
[56] Derks A, Katsaros C, Frencken JE, Van’t Hof MA, Kuijpers- Jagtman AM. Caries-inhibiting effect on preventive measures during or- thodontic treatment with fixed appliances: a systematic review. Caries Res
2004;38:413-20.
[57] Marsh PD. Are dental diseases examples of ecological catastrophes?
Microbiology 2003;149:279-94.
[58] Emilson C, Linquist B,Wennerholm K. Recolonization of human tooth surfaces by streptococcus mutans after suppression by chlorhexidine treatment. J Dent Res 1987; 66:1503-8.
[59] Vivaldi-Rodrigues G, Demito CF, Bowman SJ, et al. The effective- ness of a fluoride varnish in preventing the development of white spot le- sions. World J Orthod2006; 7:138-144.
[60] Buyukyilmaz T, Øgaard B. Caries-preventive effects of fluoride- releasing materials. Adv Dent Res1995 ;9:377-383.
[61] Derks A, Kuijpers-Jagtman AM, Frencken JE, Van’t Hof MA, Katsaros C. Caries preventive measures used in orthodontic practice: an evidence-based decision? Am J Orthod Dentofacial Orthop
2007;132:165-70.
[62] Scheinin A, Ma¨kinen KK, Ylitalo K. Turku sugar studies: Final report on the effect of sucrose, fructose and xylitol diets on the caries inci- dence in man. Acta Odonto Scan 1976;34 (4):179-216.
[63] Sandra Guzma´n-Armstrong,Jane Chalmers, John J. Warren. White spot lesions: Prevention and Treatment (Readers’ Forum, Ask Us) Am J Orthod Dentofacial Orthop 2010;138:690-696.
[64] Makinen KK, Bennett CA, Hujoel PP, Isotupa KP, Pape HR, Ma- kinen KK. Xylitol chewing gums and caries rates: a 40-month cohort study. J Dent Res 1995;74:1904-13.
[65] Zimmer S, Robke FJ, Roulet JF. Caries prevention with fluoride
varnish in a socially deprived community. Community Dent Oral Epi- demiol 1999;27:103-8.
[66] Isokangas P, Alanen P, Tiesko J, Makinen KK. Xylitol chewing gum in caries prevention: a field study in children. J Am Dent Assoc
1984;117:315-20.
[67] Dawes C, Macpherson LM. Effects of nine different chewinggums and lozenges on salivary flow rate and pH. Caries Res 1992;26:176-82.
[68] Aimutis W. Bioactive properties of milk proteins with particularfocus on anticariogenesis. J Nutr 2004;134:989S-95S.
[69] Tung MS, Eichmiller FC. Dental applications of amorphous calcium phosphates. J Clin Dent 1999;10:1-6.
[70] Turner PJ. The clinical evaluation of a fluoride containing orthodontic bonding material. Br J Orthod 1993;201:307-313.
[71] Rezk-Lega F, Ogaard B, Arends J. An in vivo study on the merits of
two glass ionomers for the cementation of orthodontic bands. Am J Orthod
Dentofacial Orthop1991;99:162-167.
[72] Diaz-Arnold AM, Holmes DC, Wistrom DW, et al. Shortterm fluoride release/uptake of glass ionomer restoratives. Dent Mater
1995;11:96-101.
[73] Forsten L. Resin-modified glass ionomer cements: fluoride release and uptake. Acta Odontol Scand1995; 53:222-225.
[74] Forss H. Release of fluoride and other elements from light-cured glass ionomers in neutral and acidic conditions. J Dent Res 1993;72:1257-1262. [75] Forsten L. Fluoride release of glass ionomers. J Esthet Dent 1994;
6:216-222.
[76] McCourt JW, Cooley RL, Barnwell S. Bond strength of light-cure fluoride-releasing base-liners as orthodontic bracket adhesives. Am J Or- thod Dentofacial Orthop 1991;100: 47-52.
[77] Komori A, Ishikawa H. Evaluation of a resin-reinforced glass iono- mer cement for use as an orthodontic bonding agent. Angle Orthod
1997;67:189-195.
[78] Rix D, Foley TF, Mamandras A. Comparison of bond strength of three adhesives: composite resin, hybrid GIC, and glass-filled GIC. Am J Orthod Dentofacial Orthop 2001;119:36-42.
[79] Coups-Smith KS, Rossouw PE, Titley KC. Glass ionomer cements as luting agents for orthodontic brackets. Angle Orthod 2003; 73:436-444. [80] Bishara SE, VonWald L, Olsen ME, et al. Effect of time on the shear bond strength of glass ionomer and composite orthodontic adhesives. Am J Orthod Dentofacial Orthop1999; 116:616-620.
[81] Fricker JP. A 12-month clinical evaluation of a lightactivated glass polyalkenoate (ionomer) cement for the direct bonding of orthodontic brack- ets. Am J Orthod Dentofacial Orthop 1994;105:502-505.
[82] Silverman E, Cohen M, Demke R, et al. A new lightcured glass ionomer cement that bonds brackets to teeth without etching in the presence of saliva. Am J Orthod Dentofacial Orthop 1995;108:231-236.
[83] Bishara SE, Ostby AW, Laffoon J, et al. Shear bond strength com- parison of two adhesive systems following thermocyling: a new self-etch primer and resin-modified glass ionomer. Angle Orthod2007; 77:337-341. [84] Summers A, Kao E, Gilmore J, et al. Comparison of bond strength between a conventional resin adhesive and a resinmodified glass ionomer adhesive: an in vitro and in vivo study. Am J Orthod Dentofacial Orthop
2004;126:200-206.
[85] Eliades T. Orthodontic materials research and applications:Part 1. Current status and projected future developments in bonding and adhe- sives. Am J Orthod Dentofacial Orthop 2006;130:445-451.
[86] Rogers et al. Fluoride-containing orthodontic adhesives and decalcification in patients with fixed appliances: A systematic review Am J Orthod Dentofacial Orthop 2010;138:390.e1-390.e8.
[87] Han L, Edward C, Okamoto A, et al. A comparative study of fluo-
ride-releasing adhesive resin materials. Dent Mater2002;21:9-19.
[88] Imazato S, Torii M, Tsuchitani Y, et al. Incorporation of bacterial inhibitor into resin composite. J Dent Res 1994;73:1437-1443.
[89] Imazato S, Kinomoto Y, Tarumi H, et al. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial mo- nomer MDPB. Dent Mater 2003;19:313-319.
[90] Whitshire WA. In vitro and in vivo fluoride release from orthodontic elastomeric ligature ties. Am J Orthod Dentofacial Orthop
1999;115:288-292.
[91] Mattick CR, Mitchell L, Chadwick SM, Wright J. Fluoride releas- ing elastomeric modules reduce decalcification: a randomized controlled trial. J Orthod 2001;28:217-219.
[92] Benson PE, Shah AA, Campbell IF. Fluoride elastomers: Effects on disclosed plaque J Orthod 2004;31:41-46.
[93] Joseph VP,Grobler SR, Rossou PE. Fluoride release from orthodontic elastic chain. J Clin Orthod 1993;27:101-105.
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 5, May-2011 7
ISSN 222S-5518
[94] Hicks MJ, Flaitz CM, Westerman GH, et al. Enamel caries initia tion and progression following low jluence (energy) argon laser and fluoride treatment. J Clin Pediatr Dent 1995;20:9-13.
[95] Anderson AM, Kao E, Gladwin M, Benli 0, and Ngan P Ihe
effects of argon laser irradiation on enamel decalcification: An in vivo study.Am J Orthod Dentofacial Orthop 2002;122:251-9.
IJSER 2011 http/ lwww .qser.ora
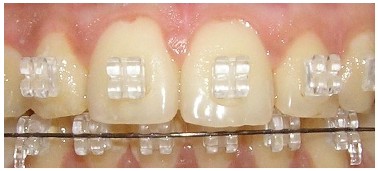
Fig.1 Cervical carious lesions observed after orthodontic treatment
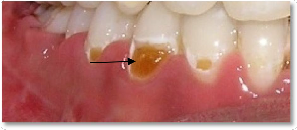
Top


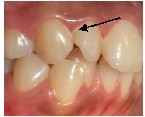
Fig.2 Bacterial plaque accumulations in areas of crowding in natural dentition. Top
Fig.3 Inaccessible areas created due to orthodontic appliances
Top
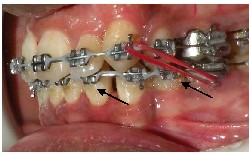
Fig.4 Fluorosis showing white and yellowish-brown areas
Top
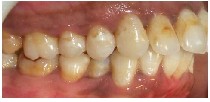
Fig.S Non specific white opacities on the distal tip of the left central incisor
Top
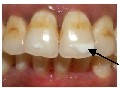
Fig.6 White spot lesions with lateral incisor
Top
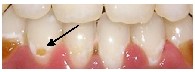
Fig.7a,b Bacterial plaque accumulations in cervical region of canine
Top 7a
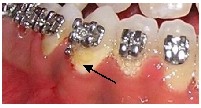
Top7b
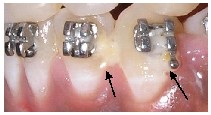
Fig.8 Arrows showing micro-leakage around the brackets
Top
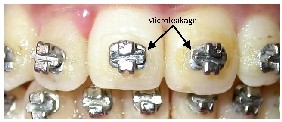
Fig.9 Least amount of micro-leakage around ceramic brackets
Top