However, in the case of carbon and low alloy steels the nitrogen content needed to be restricted since the same is not desired. Hence to understand the influencing parameter in the processing of different steels, this study was undertaken.
International Journal of Scientific & Engineering Research Volume 2, Issue 10, October-2011 1
ISSN 2229-5518
How to make N2 listen to you in steel making!
P R Sureshkumar, D R Pawar and V Krishnamoorthy
Stainless steel consists of 18% Cr and 8% Ni. But to reduce the cost, Nitrogen content is increased to a desired level to compensate Ni. Though nitrogen is lost due to aging and causes cracking, this steel is popular for use-and- throw materials. The nitrided manganese addition had been a must for ensuring nitrogen ppm in some of the 200 series stainless steel. Without adding nitrided manganese, normally we can achieve the nitrogen level up to 500ppm. Further the nitrogen level, may be another 700ppm, is increased through the addition of nitrided manganese. However, the cost of nitrided manganese is very high in comparison with other ingredients. The cost of nitrided manganese alone was substantial and was around 10% of the total cost of the steel. So the necessity to find an alternative method to increase nitrogen level was very crucial.
However, in the case of carbon and low alloy steels the nitrogen content needed to be restricted since the same is not desired. Hence to understand the influencing parameter in the processing of different steels, this study was undertaken.![]()
Authors
1. P R Sureshkumar, email: pr_sureshkumar@yahoo.com
Mob: +91 9745402552
2. D R Pawar, email: drpawar@mukand.com
Mob: +91 9987835318
3. V Krishnamoorthy, email: vkmagus@gmail.com
Mob: +420 725 650 193
Numerous sources of nitrogen exist during the melting, ladle processing and casting operations of steel. Sources of nitrogen in oxygen steelmaking include hot metal, the scrap, impurity nitrogen in oxygen and the nitrogen used as a stirring gas.
Nitrogen pickup from the atmosphere can occur during oxygen re-blows in which case the furnace fills up with air, which is then entrained into the metal when the oxygen blow restarts. Also during the tapping of steel, air bubbles are entrained into the steel where the tap stream enters the bath in the ladle. Other sources may include atmosphere (through ladle slag), coke (carburizers) and various ferro- alloys. Ladle additions often contain moisture. To get an impression of the sources of nitrogen during the melting process, the amount of nitrogen present in each of the feed materials typically used in the EAF is shown in Table 1.
Table 1: Nitrogen content of feed materials used in EAF
steelmaking [1]
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 10, October-2011 2
ISSN 2229-5518
While steel is liquid, the nitrogen exists in the solution. However, solidification of steel may result in following nitrogen-related phenomena: formation of blowholes; precipitation of one or more nitride compounds.
The presence of significant quantities of other elements in liquid iron affects the solubility of nitrogen. More importantly, the presence of dissolved sulfur and oxygen limit the absorption of nitrogen because they are surface- active elements.
The effect of nitrogen on steel properties can be either detrimental or beneficial, depending on the presence of other alloying elements in it, the form and quantity of nitrogen present, and the required behavior of the particular steel product.
In general, however, most steel products require that nitrogen be kept to a minimum. High nitrogen content may result in inconsistent mechanical properties in hot-rolled products, embrittlement of the heat affected zone (HAZ) of welded steels, and poor cold formability. In particular, nitrogen can result in strain ageing and reduced ductility of cold-rolled and annealed steels.
Figure 1 shows that the strength of LCAK (Low Carbon Aluminium Killed) steels decreases slightly and then increases with increasing nitrogen. Conversely, the elongation decreases and the r-value (measure of thermal resistance) increases with increasing nitrogen. The r-value is the average ratio of the width to thickness strain of strip tensile specimens tested in various orientations, and is inversely proportional to formability. Hence, high nitrogen content leads to poor formability of LCAK steels, even after annealing.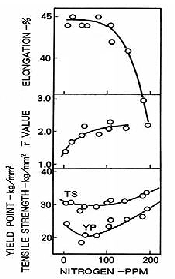
Figure 1: Effect of nitrogen on yield strength, tensile strength, r-value and elongation of LCAK steel in the annealed condition [1]
The effect of nitrogen on mechanical properties is the result of interstitial solid solution strengthening by the free nitrogen; precipitation strengthening by aluminum and other nitrides; and grain refinement due to the presence of nitride precipitates.
Hardness is the resistance of a material to surface indentation. The hardness increases linearly with increasing nitrogen content. Nitrogen absorbed during steelmaking results in interstitial solid solution strengthening and grain refinement, both of which increase hardness.
This property of N2 leads to a specialized heat treatment process called “Case Nitriding” where the surface of a component is preferentially enriched with N2 to increase its hardness while retaining a soft core to give it a combination of a material with strength and hardness simultaneously.
Strain ageing occurs in steels containing interstitial atoms, predominantly nitrogen, after they have been plastically deformed. After deformation, the nitrogen segregates to dislocations causing discontinuous yielding when further deformed. Not only does strain ageing result in increased hardness and strength, as well as reduced ductility and toughness, but it may also result in the appearance of 'fluting' or 'stretcher strains' on the surface of deformed material.
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 10, October-2011 3
ISSN 2229-5518
The ability of a material to withstand impact loading is commonly known as toughness. It is sometimes quantified by measuring the amount of energy that is absorbed by a test piece of known dimensions prior to fracture. It is further analyzed by determining the fracture mechanism upon impact over a range of temperatures. As temperature is decreased, the fracture type will change from fibrous/ductile to crystalline/brittle. This arbitrary temperature is termed the 'ductile-to-brittle' transition temperature. The lower the transition temperature the better the impact properties, since failure by ductile fracture may be less catastrophic than that through brittle failure. Figure 2 demonstrates that as free nitrogen increases, the transition temperature increases, and therefore toughness decreases. This is attributed to solid solution strengthening.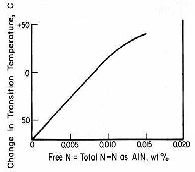
Figure 2: Effect of free nitrogen on impact properties [1]
Conversely, limited amounts of nitrogen present as precipitates have a beneficial effect on impact properties. Nitrides of aluminum, vanadium, niobium and titanium result in the formation of fine-grained ferrite. Further, the smaller the grain size the lower the transition temperature, hence improved toughness. Therefore, it is necessary to carefully control, not only the nitrogen content, but also the form in which it exists, in order to optimize impact properties.
Nitrogen is known to affect the toughness of the heat- affected zone (HAZ) of welded steel. This is important, since the weld metal should not be a point of weakness in a welded structure. This loss in toughness is often referred to as HAZ embrittlement. It is thought this occurs when the nitrides present in the HAZ are dissociated as a result of the elevated temperatures that exist during welding. The absence of precipitates results in grains of larger diameter. Also, the metal cools quickly producing low toughness martensite or bainite, which contain high levels of free nitrogen further exacerbating the loss of toughness. Using
lower heat input and several passes to prevent dissociation of the nitrides may prevent this.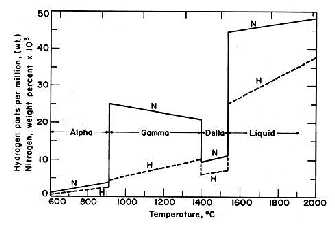
Figure 3: Solubility of nitrogen in iron for temperatures of
600-2000°C. [2]
It can be observed that the solubility of nitrogen in iron increases with the temperature in liquid metal phase. This prompted us to conduct the experiment at a very high temperature like 1650°C. In the first company, the facilities were 30T Arc furnace, LRF and 100mm2 billet caster and the second company had 40T UHP, Converter, LRF/VD/VOD and Bloom caster.
As per the solubility graph shown in fig 3, the nitrogen solubility in liquid iron drastically goes up beyond 1575°C.
In the first company, there was a problem of high nitrogen content in alloy steels which was detrimental as far as the final quality was concerned. When the problem became very severe, it was decided to conduct a study.
Here once the hot metal was ready, the temperature of hot metal had been maintained around 1600°C which is the lifting temperature, till the caster was ready. As this temperature was well ahead of the critical temperature mentioned above, the pickup of nitrogen was very high. Due to this many heats had been finishing with nitrogen content beyond 100ppm which was the upper specification limit. So to avoid this situation, experiments were planned to maintain the temperature of the molten metal well below the critical temperature till the clearance of the caster was given. Only after the caster was ready the temperature was raised to the lifting temperature. More number of controlled experiments proved that the new method can ensure the nitrogen level well below 100ppm.
As was previously mentioned the problem at the second company was two pronged. In SS the N2 needed to be
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 10, October-2011 4
ISSN 2229-5518
introduced in an economic way while in alloy steel the same N2 had to be kept at levels less than 100ppm, preferably at 80 ppm.
Though the intermediate state of the stainless steel had a very high value of N2 (in excess of 1500 ppm) when the steel leaves the converter stage, the subsequent VOD process resulted this N2 to reach an equilibrium value of around 400 ppm.
In second company, normally during stainless steel making, after the oxygen blowing is over, a process called VCD (Vacuum Carbon Decarburization) is done. After this process the steel temperature is not less than 1650°C. This is followed by lime addition and heavy ferro-alloy addition including the costly nitrided manganese and the temperature will be around 1520°C. The lifting temperature being around 1550°C, normally the temperature is never raised beyond 1550°C after this. So it was planned to conduct an experiment to bottom purging the steel with nitrogen immediately after lime addition when the bath temperature was around 1650°C.
During the experiment, the bottom purging was continued till the temperature was down to 1550°C. Then it was found that at this point the nitrogen level was around 800ppm as against 400ppm in the normal case. Further the temperature was raised to 1650°C and ferro-alloys were added. Then the temperature was brought down to desired level through nitrogen bottom purging. It was found that the nitrogen level reached the desired level all by itself. This new procedure was repeated and confirmed that the desired nitrogen levels could be achieved through nitrogen bottom purging alone at temperatures beyond 1575°C.
So the effect of temperature was harnessed and the N2 value was increased just with cheaper method of purging with N2. It was also observed that the pick up of N2 was faster if the Si level was kept low.
In alloy steel the process was much more vigorous and the challenge huger. Any steelmaker would know that it is easy to introduce something into steel but in certain cases almost impossible to remove it from steel. So in order to control N2 all avenues of ingress into steel had to be stopped. This was one place where the old saying “Prevention is better than cure” held true.
There were no real issues in side the EAF as pick up of N2 in oxidizing condition is never favorable. We had to ensure that no cold air enters the furnace.
The process of controlling the N2 starts from the moment the steel leaves the arc furnace.
1. In ladle - As oxidizing conditions are preferred to control N2 the first step that was taken was to remove the addition of Al in ladle. Any deoxidation that was needed was carried out with Ferro alloys and carbon.
2. At Ladle Furnace – The addition of Al is delayed to
the extent possible as it was observed that the N2
pick up was steady and a danger, the moment the
steel is killed with Al. The addition of Al was done just before the heat reached the stage of going for vacuum degassing.
3. At ladle Furnace – The addition of petroleum coke was found to have a huge influence on nitrogen
pick up and petroleum coke was replaced with graphite fines. This improved the situation significantly.
4. At ladle Furnace – The addition of calcium silicide was changed. The old method of injecting the
powder with a carrier gas of N2 was replaced with cored wire. Of course the use of cored wire is now the NORM in all steel plants but at that time (circa
1993) the technology was still new.
5. On caster – All the best efforts could still be lost on
the caster if the killed steel is exposed to
atmosphere. So first an external ring of argon was provided around the shroud creating an artificial inert atmosphere and later specially designed shrouds which had slots through which Argon could be distributed, were used.
All these steps together when implemented with regular consistency could bring down the N2 from a level of 110 ppm to below 85 ppm.
This experiment was continued for four more campaigns and found that the results were consistent. Thus this procedure was confirmed as a standard then onwards for making Nitrogen steel.
Nitrogen in steel can be raised or lowered to the desired level through steps mentioned above.
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 10, October-2011 5
ISSN 2229-5518
The authors express their gratitude to the colleagues Mr.
[1]
Ashok Patil, Mr. VK Mohanan, Mr. Thomas Johnson Thomas, Mr. T P Suryanarayanan, Mr. S M Bhabhale and Mr. P S Hule. The authors thank Mr. T K Gosh for his guidance out of his practical experience. The authors also express their special thanks to Dr. M R Suresh for giving the initial shape for this paper.
http://www.keytometals.com/page.aspx?ID=CheckArticle
&site=kts&NM=204
[2]
http://www.keytometals.com/page.aspx?ID=CheckArticle
&site=kts&NM=202
IJSER © 2011 http://www.ijser.org