International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 202
ISSN 2229-5518
Herb “Asparagus curillus” Effecting Matrix Metallo- proteinases Plasma Levels in Patients with Coro- nary Artery Disease
Yogesh Bansal, Manveen Kaur Lall
Abstract— Asparagus species has been well known for their antioxidant, anti inflammatory and anti tumor activity. However anti atherosclerotic activity of Asparagus curillus extract has not been studied.The study group included 63 persons with angio-graphically verified CAD and 21 persons as healthy control. Circulating MMPs, TIMP-1, C-reactive protein and interleukins concentrations were measured by ELISA. Leucocyte subtype counts were deter- mined in whole blood. Differences in continuous variables b/w were tested by ANOVA and correlations were analyzed by linear regression method. MMPs and TIMP-1 concentration (markers of CAD) appeared to be differentially regulated by Asparagus curillus extract. The plasma concentration of MMP-2 was increased in patients (Stable and Unstable CAD) having AC root extract as dietary supplement. No significant increase in plasma concentra- tion of TIMP-1 was observed in patients (Stable and Unstable CAD) having AC root extract as dietary supplement as compare to control group ( p < 0.05 or 0.001). Therefore AC root extract as dietary supplement showed a direct correlation with plasma concentration of MMP-2, MMP-3 and TIMP-1.MMPs and TIMP-1 concentration (markers of CAD) appeared to be differentially regulated by Asparagus curillus extract.
Index Terms— Asparagus, Metalloproteinase, Coronary artory disease, Anti inflammatory effect.
—————————— ——————————
1 INTRODUCTION
atrix metalloproteinase (MMPs) are proteolytic, zinc dependent enzymes involved in degradation of ex- tracellular matrix like collagen, proteoglycan and elastin (Coker et al 1999 and Visse at al 2003). These MMPs are tightly regulated at several possible levels including transcription, zymogen activation, MMP interaction and inhibition by TIMP (Tissue inhibitor of metalloproteinase -1) (Creemers et al 2001 and Noji et al 2001). MMPs have been involved in many cellu- lar processes, such as smooth muscle cell migration, release of growth factor, embryonic development, wound healing, angi- ogenesis and ECM degradation, processes that may have dif- ferent effects on cardiovascular disease (Visse et al 2003 and Sang-Beom 2012). It is reported that the plasma concentration of metalloproteinase (MMP) like MMP-2, MMP-3, MMP-7 provides valuable information in patients with Coronary Ar- tery Disease (CAD) and concentrations of MMP-2 and MMP-9 (both gelatinases) are beneficial in non-viral heart disease (Westermann et al 2011, Wilson et al 2002 and Yamazaki et al
2004).
Serum containing growth factors and inflammatory molecules,
both modulates the secretion of various MMPs and TIMPs from fibroblast, smooth muscle and endothelial cells. MMPs are mostly activated by cysteine-switch (Tyagi et al 1993) and thereby promoting atherogenesis, plaque rapture and throm- bosis. In coronary heart disease (CHD), serum concentration
————————————————
• Yogesh Bansal( Ph.D.),Department of Biochemistry, D.A.V. © Dental
College and Hospital,Yamuna Nagar, Haryana, India, PH +91
9466671001. E- mail:yogeshbansalsaysu@gmail.com.
• Co-Author –Manveen Kaur Lall (M.D),Department of Physiology, Sudha
Rustagi college of Dental Sciences and Research, Faridabad , India, PH
+91 9910022612. E-mail: drmanveenk@gmail.com.
may reflect MMPs activity within the vessel wall (Bergman et al 2007 and Cheung et al 2000). A few studies have analyzed serum or plasma MMPs-2, 3 & 7 concentrations in relation to CAD. Patients with premature stable CAD, the plasma concen- tration of MMP-2 and MMP-3 were decreased; however MMP-
2 mediates (by negative feedback mechanism) chemokine cleavage that has an important role in cardiac inflammation (George et al 2005). Concentration of MMP-2 in stable CAD and unstable CAD is conflicting, it has been shown that serum MMP-2 is increased in patient with stable angina compared with control. In other MMPs like 7 & 9 are produced in vul- nerable region of atherosclerotic plaque and MMP-7 concen- tration is increased in patients with stable and unstable CAD (Nilsson et al 2006).
The screening of plants extracts and natural products for anti
CAD activity has revealed the potential of higher plants as a source of new anti CAD agents. Herbal plant “Asparagus curil- lus” (AC) belongs to Liliaceae family. This plant is a great source of Vit-B6 ,Ca, Mg, Zn, Vit-A, Vit- C, Vit-E , Vit-K, Thia- mine, Riboflavine and Ph, Fe, K, Mn, Se and Cr as trace ele- ments (Jagmohan et al 2010). Recently, compounds like spiro- tanol, furostanol glycosides, saponines, glucopyranoside and oligofurostanosides are isolated from AC (Sharma et al 1982 and 1993) . Nutritional studies have shown that six spears of Asparagus species contain 135µg of folate i.e. almost half of RDI (Recommended daily intake) and 20µg of K+. Another report on folate showed its key role in homocysteine metabo- lism (Biomolecule implicated in heart disease).The root extract of AC possesses antioxidant and anti-inflammatory properties. Although, above herb has many useful claims. But the mecha- nisms of its medicinal effects are not understood.
So, the objectives of this study are to evaluate the plasma con-
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 203
ISSN 2229-5518
centrations MMPs like 2,3 & 7 together with their inhibitor
TIMP-1 in patients with CAD and in patients of CAD adminis- tered with AC root extract whether as these concentrations correlates with those of CRP, cytokines and immune cells.
2 MATERIALS AND METHODS
2.1 Botanical Saterial
The dried roots of Asparagus curillus (BSD 112754) were col- lected from forest region of Gupt kashi (Uttarakhand, INDIA) and identified in Botanical Survey of INDIA, Dehradun.
2.2 Extraction of Plant Material
This herbal plant material taken from for the study was under refrigerated condition till use. The sample was prepared by grinding equal amount of fresh roots in water (wt. /vol.) in pre chilled pestle and mortar. The extract thus obtained was provided to the patients for oral administration (20µl/Kg/Day) for three months.
2.3 Subjects and Clinical Course
We studied a total of 63 persons (38 males and 25 females) with angiographically verified CAD and 21 persons as healthy controls. The patients with stable CAD had effort-related an- gina without any worsening of symptoms in the past 6 months. Exclusion criteria were age >60 years diabetes, sever heart failure, immunologic disorder, cancer, surgery and treatment of immunosuppressive or anti-inflammatory agents. Blood was obtained by venipuncture in the morning after 12 h fast. The research protocol was approved by the local ethics committee “DAV © Dental College and Hospital”.
2.4 Collection of Blood Sample and Estimations of
MMPs
Blood samples were collected in Vacutainer Tubes with or without EDTA and centrifuged within 15 min to separate plasma/serum. Total serum cholesterol, high density lipopro- tein (HDL) cholesterol and serum triglyceride were deter- mined by Roche Diagnostics Kits used in Roche Modular P-
900. Plasma CRP was determined by highly sensitive latex- enhanced turbidimetric immunoassay (Roche Diagnostics). The MMPs and TIMP-1 were measured with the Biotrak MMP-2, MMP-3, MMP-7 and TIMP-1 human ELISA system (Amersham Biosciences).We determined the distribution of peripheral blood mononuclear cells by Beckton Dickinson FACS cell sorter located in P.G.I.M.E.R., Chandigarh, INDIA. Data were analyzed by using CELL Quest software (Becton Dickinson).
3 RESULTS
Mean age of study patients was 67±13 years. Thirty eight pa- tients were males excluding healthy control i.e. (61%). All the patients were receiving beta blockers digioxin, calcium antag- onists and nitrates. In addition, all the patients with UCAD were receiving angiotensin converting enzymes inhibitors or
low molecular wt. heparin.
Serum TG, cholesterol, LDL and HDL levels were very high in CAD and UCAD patients as compare to healthy control (Data shown in Table 1). However, values of above parameters were significantly decreased in CAD patients with AC extract (CAD-AC) and UCAD patients with AC extract (UCAD-AC) as compare to patients without AC root extract as dietary sup- plement.
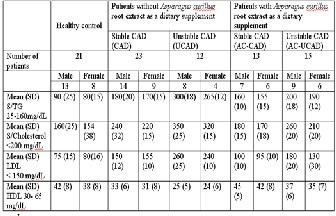
TABLE 1- Comparison between Lipid profile of Patients (CAD and UCAD) with and without root extract of Asparagus curillus as dietary supplement.
P<0.05compared to control. All values are ex- pressed as mean± SD
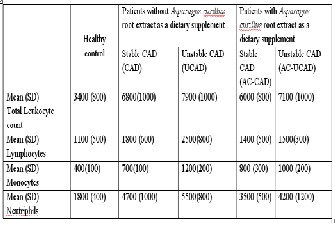
Table 2- Basic characteristics of DLC (Differential leukocyte count) in patients with and without Asparagus curillus root ex- tract as dietary supplement
Values are expressed as mean ± SD, *p<0.05.
Patients with UCAD had higher total leukocyte counts than stable CAD and control (Table 2). This difference was account- ed for by higher monocytes and neutrophilic granulocyte counts. On comparing UCAD and UCAD-AC, the level of total leukocyte was significantly reduced. On the other hand, CAD and CAD-AC, the reduction in monocytes was not very signif- icant. So it has been inferred from the table 2 that total leuko- cyte count and neutrophil count were reduced significantly in patients that were administrated with AC extract.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 204
ISSN 2229-5518
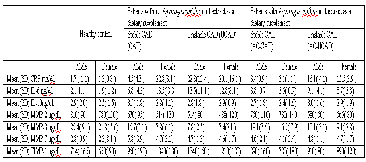
Table 3 Plasma concentration of MMPs and other components in CAD and UCAD patients with and without Asparagus curillus root extract as dietary suppliment
ANOVA comparing all groups. Adjusted for age, smoking and body mass index.Compared with pa- tients with stable CAD: p < 0.01, p < 0.02 and p
< 0.01.
Plasma concentrations of MMPs and TIMP-1 are shown in table 3. Matrix MMP-2 plasma levels were significantly corre- lated with age (data not shown) whereas mean MMP-2 con- centration in healthy patients were 145±20 ng /mL. Plasma concentration of MMP-2 &7 were significantly decreased in patients with UCAD compared with stable CAD and healthy control. But the patients of CAD-AC and UCAD-AC have higher values of MMP-2 & 7 (Data shown in table 3). MMP-3 level showed 2.5 times decreased in UCAD-AC (male) and 3.5 times decrease in UCAD (male) as compare to control but less significant decrease was being observed in UCAD-AC and CAD-AC female patients (shown in table 3). MMP-7 concen- trations were increased by 2, 2.36 and 1.67 times in CAD (male), CAD (female) and UCAD (male and female both) pa- tients respectively as compared to control patients. It was also being observed from table 3 that TIMP-1 concentration was very high in patients with UCAD (male and female) that was followed by stable CAD (male and female), UCAD-AC (male and female) and CAD-AC (male and female) patients respec- tively.
IL-6 is responsible for stimulating acute phase protein as well
as neutrophils in the bone marrow. As from table 3, concentra- tion of IL-6 and counts of neutrophils were correlated very comfortably. From table 3, it was also observed that concentra- tion of IL-6 was high in stable CAD (male and female) patients as compare to control, UCAD, stable CAD-AC and UCAD-AC patients. CRP is a general marker of inflammation and infec- tion. Its concentration in human serum is usually lower than
10 mg/L slightly increased with aging. Higher values are found in inflammation and viral infection and from table 3 it was very much clear that CRP values were 14 times higher in UCAD patients which was followed by 10 times increase in UCAD-AC patients as compare to control, whereas this in- crease of CRP concentration was not much pronounced in sta-
ble CAD and stable CAD-AC patients (about 2 to 3 times in-
crease as compare to control).
4 DISCUSSION
MMPs are best known as proteases responsible for the degra- dation and remodeling of extracellular matrix protein in both physiological and pathological condition, including various cardiac pathologies (Wilson et al 2002). However, the discov- ery of the intracellular localization (Wang et al 2002) and func- tion of MMP-2 to proteolyze troponin I, myosin light chain-I and α- actinin during myocardial oxidative stress injury chal- lenged the canonical notion of extracellular only action of this enzyme (Sawieki et al 2005 and Sung et al 2007). In present study, we showed and analyzed that AC root extract have a significant effect on plasma concentration several MMPs and TIMP-1 in patients with stable and unstable CAD. It was also inferred from the above experiments that in AC administered patients (CAD and UCAD) and in without AC administered patients (CAD and UCAD) have significant difference in plasma concentration between the group of MMP-2, 3, 7 and TIMP-1. Importantly, the plasma concentration of MMP-7 were significantly increased in CAD patients, But this increase was not much pronounced in patients of CAD-AC. Previously it has been considered that MMP-7 might be a marker of ath- erosclerosis because it was increased equally in all patients with stable and unstable CAD (Nilsson et al 2006). Our study proves that AC root extract has intracellular effect on the MMP-7 activity. It might be possible that AC root extract have some compounds that cause different pattern of enzymatic modulation in CAD patients.
In contrast to MMP-7, Plasma MMP-2 was significantly de-
creased in the patients with stable CAD and was even lower in the group with CAD. Although, we analyzed that AC root extract administered patients (UCAD and CAD) has higher values of plasma MMP-2 concentration as compare to stable and unstable CAD. Reports on MMP-2 inhibition with ONO-
4817 prevented ischemia/ reperfusion-induced titin degrada- tion (Ali et al 2010) and improved the recovery of myocardial contractile function. Titin degradation (largest mammalian myofilamentous protein ) was also reduced in hearts from MMP-2 knockout mice subjected to ischemia/re perfusion in vivo compared with wild-type control (Ali et al 2010 and Fu- kuda et al 2008).
Whereas, it has been observed that MMP-2 (in relation to plasma concentration in CAD patients) has given conflicting results (Chow et al 2007). The most significant finding by George et al, 2005 in patients with serum MMP-2 levels above
352 ng /ml are at a significant risk of death, hospitalization for
heart failure, or either of these 2 end points. Low MMP-2 plasma levels are associated with intracranial location of cere-
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 205
ISSN 2229-5518
bral atherosclerosis, suggesting that MMP-2 may play a role in
the development of ICAS (Sang-Beom Jeon et al 2012). The MMP-2 concentration was also correlated with the number of NK cells (natural killer cells) in CAD patient (Gao et al 2003). The number of NK cells is lower and NK activity is impaired in patients with choronic immunologic disease and decreased NK cells function has been associated with atherosclerosis in elderly human (Flodstrom et al 2001). Indeed, we found that AC root extract causes decrease concentration of MMP-2 and also causes inhibition of plasma MMP-2 in CAD and UCAD patient and this could also be associated with low number of NK cells in patients (CAD-AC and UCAD-AC).
Lubos et al demonstrated that high serum TIMP-1 is a risk
predictor for future cardiovascular death. The assay used in present study recognizes the total TIMP-1 content, i.e., free TIMP-1 and TIMP-1 complexed with MMPs (Ikonamidis et al
2005). Thus, increased plasma TIMP-1 in patients with stable and unstable CAD could reflect increased matrix-degrading activity with accumulation of MMP-TIMP-1 complex in plas- ma. The plasma concentration of TIMP-1 correlated strongly with makers of inflammation, such as IL-6 and CRP, in pa- tients with manifest CAD (Sang-Beom et al 2012 and Wiernicki et al 2011) ). As the concentration of IL-6 from stable CAD to UCAD patients and stable CAD-AC to UCAD-AC were in- creased, the counts of neutrophils were also increased up to same extant but this significant increase was not pronounced in CAD-AC and UCAD-AC patients. This interleukin is as anti-inflammatory cytokine and produced by monocytes and lymphocytes (some extant) (Schulze et al 2009). It is released by cytotoxic T-cell to inhibit the action of NK cells during the immune response to vital infection (Bruunsgaard et al 2001). In the present study, the concentration of TIMP-1 in patients (CAD and UCAD) with AC root extract was significantly de- creased. These results clearly indicated the improvement in the patients suffering with CAD and UCAD.
5 CONCLUSION
AC root extract may contain some anti inflammatory com- pounds that effect on plasma concentration of MMPs and TIMPs very significantly in CAD and UCAD patients. Pro- spective studies are required to demonstrate whether the
compounds in AC work in synergism or individually.
ACKNOWLEDGMENT
The author is grateful to Post Graduate Institute of Medical Education and Research, Chandigarh, INDIA for facility for ELISA estimation and also thankful for the Medicine depart- ment DAV (c) Dental College and Hospital, Yamuna Nagar, INDIA .
REFERENCES
1. M. A. M. Ali, W. J. Cho, B. R. Hudson, Z.
Kaassisi, H. Granzier, R. Schulz, “Titin
is a target of matrix metalloproteinase-2 “implication in myocardial ischemia/ reperfusion injury,” Circulation, vol.
122, pp. 2039-2047, 2010.
2. G. Bergers, R. Brekken, G. McMahon, Vu.
GH, “Matrix metalloproteinase-9 triggers
the angiogenic switch during carcinogene- sis,” Nature cell biology, vol. 2, pp.
737-744, 2000.
3. M. R. Bergman, J. R. Teerlink, R. Li. L.
Mahimkar, B. Q. Zhu, A. Nguyen, A. Dahi, J. S. Karliner, D. H. Lovett, “Cardiac ma- trix metalloproteinase-2 expression inde- pendently induces marked ventricular re- modeling and systolic dysfunction,” Am J Physiol Heart Circ Physiol, vol. 292, pp. H1847–H1860, 2007.
4. H. Bruungaard, A. N. Pedersen, M. Schroll,
P. Skinhoj, B. K. Pedersen, “Decreased natural killer cell activity is associated with atherosclerosis in elderly humans”, Exp. Gerontol. vol. 37, pp. 127-136, 2001.
5. P. Y. Cheung, G. Sawicki, M. Wozniak, W.
Wang, M. W. Radomski, R. Schulz, “Matrix metalloproteinase-2 contributes to ische- mia-reperfusion injury in the heart, ” Circulation, vol. 101, pp. 1833–1839,
2000.
6. A. K. Chow, J. Cena, A. F. El-Yazbi, B. D.
Crawford, A. Holt, W. J. Cho, E. E. Dan- iel, R. Schulz, “Caveolin-1 inhibits ma- trix metalloproteinase-2 activity in the heart,” J. Mol. Cell. Cardiol. vol. 42, pp. 896 –901, 2007.
7. M. L. Coker, M. A. Doscher, C. V. Thomas,
Z. S. Galis, F. G. Spinale, “Matrix metal- loproteinase synthesis and expression in isolated LV myocyte preparations,” Am. J. Physiol. vol. 277, pp. H777–H787,1999.
8. E. E. Creemers, J. P. Cleutjens, J. F.
Smits, M. J. Daemen, “Matrix metallopro-
teinase inhibition after myocardial in- farction: a new approach to prevent heart failure?,” Circ. Res. vol. 89, pp. 201–210
, 2001.
9. M. Flodstrom, A. N. Pedersen, M. Schroll, P. Skinhoj, B. K. Pedersen, “Decreased natural killer cell activity is associated with atherosclerosis in elderly humans,” Exp. Gerontol. vol. 37, pp. 127-136,
2001.
10. N. Fukuda, H. L. Granzier, S. Ishiwata, S.
Kurihara, “Physiological functions of the giant elastic protein titin in mammalian striated muscle,” J. Physiol. Sci. vol.
58, 151–159, 2008.
11. C.Q. Gao, G. Sawicki, W. L. Suarez-Pinzon, T. Csont, M. Wozniak, P. Ferdinandy, R. Schulz, “Matrix metalloproteinase-2 medi- ates cytokine-induced myocardial contrac- tile dysfunction,” Cardiovasc. Res. vol.
57, pp. 426–433, 2003.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 206
ISSN 2229-5518
12. J. George, S. Patal, D. Wexler, A. Roth, D. Sheps, G. Keren, “Circulating matrix metalloproteinase-2 but not matrix metal- loproteinase-3, matrix metalloproteinase-
9, or tissue inhibitor of metalloprotein-
ase -1 predicts outcome in patients with congestive heart failure,” Am. Heart J. vol. 150, pp. 484-487, 2005.
13. S. N. Jagmohan, S. Pramod, J. N. P. Geeta,
S. M. R. Mohan, “Trace Elements Contents in Asparagus curillus,” Biol. Trace Elem. Res. vol. 136, pp. 364–371, 2010 .
14. J. A. Kwan, C.J. Schulze, W. Wang, H. Le-
on, M. Sariahmetoglu, M. Sung, J. Sawicka, D. E. Sims, G. Sawicki, R. Schulz, “Matrix metalloproteinase-2 (MMP-
2) is present in the nucleus of cardiac
myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro” FASEB J. vol. 18, pp. 690–692, 2004.
15. E. Lubos, R. Schnabel, H. J. Rupprecht, C. Bickel, C. M. Messow, S. Prigge, “Prog- nostic value of tissue inhibitor of metal- loproteinase-1 for cardiovascular death among patients with cardiovascular dis- ease, result from the atherogene study,” Eur. Heart J. vol. 27, pp. 150-156, 2006.
16. L. Nilsson, L. Jonasson, J. Nijm, A. Ham-
stem, P. Eriksson, “Increased plasma con- centration of matrix metalloproteinase-7 in patients with coronary artery disease,” Clinical chemistry, vol. 52, issue 8, pp.
1522-1527, 2006.
17. Y. Noji, K. Kajinami, M. A. Kawashiri, Y.
Todo, T. Horita, A. Nohara, “Circulating matrix metalloproteinases and their inhib- itors in premature coronary atherosclero- sis,” Clin. Chem. Lab Med. vol. 39, pp.
380-384, 2001.
18. J. Sang-Beom, C. Sail, C-K. Smi, C. Hyun-
Sook, “Biomarkers and location of athero- sclerosis: Matrix metalloproteinase-2 may be related to intracranial atherosclero- sis,” Atherosclerosis, vol. 223, pp. 442-
447, 2012.
19. G. Sawicki, H. Leon, J. Sawicka, M. Sari-
ahmetoglu, C. J. Schulze, P. G. Scott, D. Szczesna-Cordary, R. Schulz, “Degradation of myosin light chain in isolated rat hearts subjected to ischemia-reperfusion injury: a new intracellular target for ma- trix metalloproteinase-2,” Circulation, vol. 112, pp. 544–552, 2005.
20. H. Schuett, M. Luchtefeld, C. Grothusen, “How much is too much? Interleukin-6 and its signaling in atherosclerosis,” Thromb Haemost. vol. 112, pp. 544-552, 2009.
21. S. C. Sharma, O. P. Sati, R. Chand, “Ste- roidal saponins of Asparagus curillus,” Phytochem. vol. 21, issue 7, pp. 1711–
1714, 1982.
22. S. C. Sharma, H. C. Sharma, “Oligofurosta- nosides from Asparagus curillus leaves”. Phytochem. vol. 33, issue 3, pp. 683–686,
1993.
23. M. M. Sung, C.G. Schulz, W. Wang, C.
Sawicki, N. L. Bautista-Lopez, R. Schulz, “Matrix metalloproteinase-2 degrades the cytoskeletal protein alphaactinin in per- oxynitrite mediated myocardial injury” J. Mol. Cell Cardiol. vol. 43, pp. 429–436,
2007.
24. S. C. Tyagi, S. G. Kumar, S. J. Hass, H.
K. Reddy, D. J. Voelker, M. R. Hayden,
“Post transcriptional regulation of extra- cellular matrix metalloproteinase in human heart end stage failure secondary to is- chemic cardiomyopathy,” J. mol. cell car- diol. vol. 28, pp. 1415-1428,1996.
25. R. Visse, H. Nagase, “Matrix metallopro-
teinases and tissue inhibitors of metallo- proteinases: structure, function and bio- chemistry,” Circ. Res. vol. 92, pp. 827-
839, 2003.
26. W. Wang, C. J. Schulze, W. L. Suarez- Pinzon, J. R. Dyck, G. Sawicki, R. Schulz, “Intracellular action of matrix metalloproteinase-2 accounts for acute my- ocardial ischemia and reperfusion injury,” Circulation, vol. 106, pp. 1543–1549,
2002.
27. D. Westermann, K. Savvatis, D. Linder, C.
Zietsch, “Reduced degradation of chemokine
MCP-3by matrix metalloproteinase-2 exacer- bates myocardial inflammation in experi- mental viral cardiomyopathy,” Circulation, vol. 124, pp. 2082-2093, 2011.
28. I. Wiernicki, B. Millo, K. Safranow, “MMP-
9, homocystein and CRP circulating levels
are associated with intraluminal thrombus thickness of abdominal aortic aneurysms: new implication of the old biomarkers,” Dis. Markers, vol. 31, pp. 67-74, 2011.
29. E. M. Wilson, H. R. Gunasinghe, M.
L.Coker, “Plasma matrix metalloproteinase
and inhibitor profiles in patients with heart failure,” J. Card. Fail. vol. 8, pp.
390-398, 2002.
30. T. Yamazaki, J. D. Lee, H. Shimizu, “Cir-
culating matrix metalloproteinase-2 is el- evated in patients with congestive heart failure,” Euro. J. Heart Fail. vol. 6, ppp. 41-45, 2004.
IJSER © 2013 http://www.ijser.org


