International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 154
ISSN 2229-5518
Hepatoprotective and Antioxidant Activity of
Zinnia Elegans Leaves Ethanolic Extract
Department of Biochemistry, Faculty of Agriculture, Cairo University, Giza, 12613, Egypt
Abstract — This study aimed to evaluate the hepatoprotective and antioxidant activity of Zinnia elegans leaves ethanolic extract comparing with silymarine, as standard in rats. The data revealed a highly amounts of phenolic compounds (2.6mg/g d.w of plant), which significantly reflected an antioxidant scavening activity (88%) at 250ppm.The hepatoprotection activity of Zinnia elegans leaves ethanolic extract (50, 100 and 125 mg/100g b.w) comparing with silymarine (0.2 g/kg b.w) against CCl4 toxicity when the Zinnia elegans leaves ethanolic extract improved the AST, ALT and recovered the activity of kidney function by decreasing the urea and creatinine content on the other hand, the administration of Zinnia elegans leaves ethanolic extract significantly suppress the oxidative stress via its direct scavenging against the reactive oxygen species under CCl4 stress. The results reported a decrease in the MDA, H2O2, NO accumulation and increase of GSH content. Finally the administration of Zinnia elegans leaves ethanolic extract have been improved the lipid profiles, LDL and HDL recovered also, the administration of Zinnia elegans leaves ethanolic extract significantly suppress the CCl4 toxicity via its activation of antioxidant enzymes (GST and SOD). The results showed that The Zinnia elegans leaves play an important role in the antioxidant hepatoprotective activity against CCl4 toxicity.
Index Terms— Zinnia elegans, hepatoprotective, antioxidant, scavening activity, oxidative stress and CCl4.
—————————— ——————————
he Zinnia elegans (syn. Z. violacea) is native to Mexico and Central America and now has world- wide importance as a garden plant. Tall, mid-sized,
and dwarf varieties of this species have been grown for decades, and flowers are available in a wide range of colors. Z. angustifolia (also known as Z. linearis) is less common in gardens, but is gaining in popularity. The plants have narrower foliage and smaller single flowers [1]. Flavonoids are major compounds in flowers and herb of zinnia such as flavonoids, glycosides, tannins, anthocyanins, saponins and phenols [2]. Through the supplement of phytochemicals, Zinnia elegans leaves damage induced may be useful as a hepatoprotective agent indeed retarded the liver injury by blocking the oxidative stress. Therefore, may be useful as a hepatoprotective agent against chemical-induced chronic liver fibrosis in vivo.
Carbon tetrachloride (CC14) is one of the most commonly used hepatotoxins for inducing liver injury in experimental animal studies [3]. Carbon tetrachloride was formerly used for metal degreasing and as a dry-cleaning fluid, fabric spotting fluid, fire-extinguisher fluid, grain fumigant and reaction CCl4 is a potent environmental hepatotoxin has been served as a model compound for study of hepatotoxicity and the cellular mechanisms behind oxidative damage and further was used to evaluate the therapeutic potential of drugs and dietary antioxidants [5]. Liver is prone to xenobiotic-induced injury because of its central role in xenobiotics metabolism, its portal location within the circulation, and its anatomic and physiologic structure [6]. CCl4 is known to induce reactive oxygen formation, and reduce antioxidant enzyme and antioxidant
substrates to induce oxidative stress that is an important factor in acute and chronic liver injury. The liver injury induced by CCl4 is resulted from free radicals and lipid peroxidation that cause hepatic cell damage. CCl4 requires bio activation by phase I cytochrome P450 system in liver to from reactive metabolic trichloromethyl radical (CCl•3) and proxy trichloromethyl radical (•OOCCl3).These free radicals can bird with polyunsaturated fatty acid (PUFA) to produce alkoxy (R•) and proxy radicals (ROO•), that, in turn, generate lipid peroxide, cause damage in cell mem- brane, change enzyme activity and finally induce hepatic injury or nicrosis [7]. Natural products such as flavonoids, terpenoids and steroids etc. have received considerable attention in recent years due to its diverse pharmacological properties including antioxidant activity [8]. This study aimed to prevent liver necrosis through the antioxidant activity of Zinnia elegans leaves ethanolic extract.
Zinnia elegans was collected in April 2012 from pharmacology farm.
The leaves of Zinnia elegans were dried in an air oven at 50
ºC till complete dryness. The leaves were ground to fine
powder. The dried leaves of Z. elegans were mixed with ethanol (70%) under shaking for 2 days. The resulting ethanolic extract was filtered and subsequently concentrated with a rotary evaporator under low temperature (40 ºC) and reduced pressure.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 155
ISSN 2229-5518
Ferric chloride, Folin-Ciocalteu’s reagent, ethanol 2, 2- diphenyl-1-picrylhydrazyl (DPPH), kits reagents. All chemicals and reagents were purchased from Sigma Chemical Co. (London, Lab. Poole), England (Cairo branch). All other chemicals and solvents were analytical grade.
2.4.1 The qualtitative analysis of phytochemical analysis or secondary metabolites such as alkaloides, steroids, saponins, flavonoids, phenols and glycosides. The metabolites were determined according to Harborne methods [9].
2.4.2 The quantitative analyses of flavonoids and phenols.
Flavonoids contents have been determined according to
Markham method [10]. Total phenols were determined according to colorimetry method [11].
DPPH radical scavenging activity has been determined of extract according to Burits and Bucar method [12].
The Sprague Dawley albino male healthy rats were obtained from Research Institute of Ophthalmology; Giza, Egypt has been used in the present work. The animals were weighting (100 – 180g) and kept in an air conditioned animal room for two weeks before the present studies start. The animals were fed on basal diet composed according to Lane Peter and Pearson method [13]. After adaptation, 30 of those male rats were divided into 6 groups each group contained five rats as:
A. G1: Negative control: Fed on the basal diet.
B. G2: Positive control: Fed on the basal diet and injected
by (10% CCl4).
C. G3: Fed on the basal diet and injected by (10% CCl4)
and treated orally with silymarin (0.2 g/kg b. w).
D. G4: Fed on the basal diet and injected by (10% CCl4)
and treated orally with Zinnia elegans extract (50 mg/100g b. w).
E. G5: Fed on the basal diet and injected by (10% CCl4) and treated orally with Zinnia elegans extract (100 mg/100g b. w).
F. G6: Fed on the basal diet and injected by (10% CCl4) and treated orally with Zinnia elegans extract (125 mg/100g b. w).
At the end of the experiment, all the animals were subjected to over-night fasting before being scarified by decapitation.
The Blood samples were collected with heparin to obtain serum and plasma that were kept at -20ºC, for further analysis enzymatic.
AST and ALT activity were determined according to colorimetric method [14]. Urea and creatinine were determined according to A rapid and precise method [15,
16] respectively. Glutathione reduced and Glutathione-s-
transferase were determined according to colorimetric
method [17, 18] respectively. HDL-cholesterol and LDL –
cholesterol were determined according to colorimetric
method [19]. Hydrogen peroxide and Lipid peroxide assay were determined according to colorimetric method [20, 21] respectively. Superoxide dismutase activity and Nitric oxide were determined according to colorimetric method [22, 23] respectively.
All values were expressed as mean ± S.E and statistically analyzed for significance using ANOVA Duncan Test of Assistat program. P<0.05 was considered as statistically significant [24].
3.1.1 The qualitative analysis of secondary metabolites in Table (1) Show that Zinnia elegans contain of steroids, saponins, flavonoids, phenols and glycosides. The recorded data were in agreement with Athar [25] who found that saponins, phenolic and flavonoids in Z. elegans. The highly content of phenolic and flavonoids play important role as a natural antioxidant compounds in Z. elegans.
TABLE 1
PHYTOCHEMICAL ANALYSIS OF ZINNIA ELEGANS LEAVES
![]()
![]()
ETHANOLIC EXTRACT.
Phytochemical constituents | Z. elegans |
+Ve/-Ve | |
Alkaloids | -Ve |
Saponins | ++Ve |
Phenols | +Ve |
Steroids | +Ve |
Flavonoids | ++Ve |
Glycosides | +Ve |
![]()
+++ High ++ Medium + Low
3.1.2 The quantitative analysis of secondary metabolites such as flavonoids and phenols. The data are shown in Table (2) that Total flavonoids content and total phenols of Zinnia elegans leaves ethanolic extract reflected a highly content of phenols and flavonoids (2.60mg/g and 0.61mg/g) of dry weight respectively. The recorded data were in agreement with Samson [26] who found that the methanolic extract of E.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 156
ISSN 2229-5518
Alba showed the highest content of phenolic compounds and flavonoids.
TABLE 2
TOTAL AMOUNT OF PLANT PHENOLS AND TOTAL FLAVONOIDS COMPOUNDS OF ZINNIA ELEGANS LEAVES ETHANOLIC EXTRACT.
![]()
![]()
Total flavonoids Total phenols
100
80
60
40
20
0
Activity
Plant![]()
Zinnia elegans
mg/g d. w of plant
mg/g d. w of plant![]()
leaves 0.61± 0.61 2.60± 0.34
Values are mean if ± SD of three parallel measurements.
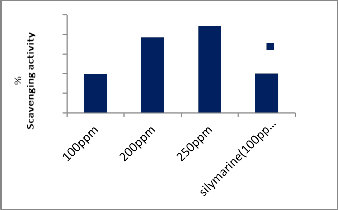
The data are shown in Table (3) and Fig (1) that The administration of different concentration of Zinnia elegans leaves ethanolic extract (100, 200 and 250 ppm) significantly reflected a highly free radical scaveniging activity (39.83, 77.03, 88.63%) respectively compared to that of silymarine (100ppm) was 40.20%, and this means that Zinnia elegans showed a highly scavenging activity of DPPH radicals, this due to the synergistic effect of antioxidant compound content in the extract. The results are in agreement with Prakash [27] who revealed that the selected plants would exert several beneficial effects by virtue of their antioxidant activity and could be harnessed as drug formulation and reported that Zinnia elegans leaves ethanolic extract significantly inhibited the activity of DPPH radicals.
TABLE 3
.ZINNIA ELEGANS LEAVES ETHANOLIC EXTRACT SCAVENGING ACTIVITY OF DPPH.
![]()
![]()
Samples Conc. (ppm) Scavenging activity (%)
100 39.83±1.21c
Fig.1. Effects of Different concentrations of Zinnia elegans leaves ethanolic
extract on scavenging activity of DPPH.
3.3.1 Liver function of the experimental animals.
The data are shown in Table (4) and Fig. (2) That the
adminstration of different concentration of Zinnia elegans
leaves ethanolic extract (50, 100 and 125mg/100g b.w) as
well as, standard drug (silymarin) significantly recovered
the activity of the AST and ALT enzymes against positive control. The lowest contents have been recorded (21.67 and
28.33 U/L of AST and ALT respectively) with 125 mg/100g b.w of Zinnia elegans leaves ethanolic extract against positive control (118.3 and 114.3 U/L of AST and ALT
respectively) these results may be due to the direct antioxidant scavenging activity of the Zinnia elegans extract phenols and flavonoids compounds. These data were in agreement with Murugaian [28] who reported that the improvement of ALT and AST activity with administration of Zinnia elegans extract and it could preserve the normal functional status of the liver.
TABLE. 4.
![]()
LIVER FUNCTION OF THE EXPERIMENTAL ANIMALS.
Zinnia elegans leaves
200 77.03±1.09b
Treatments![]()
U/L %
U/L %![]()
250 88.63±1.04a
Silymarin 100 40.20±0.62e![]()
LSD 5% 11.24
Results are mean ± SD of three parallel measurements.Values with the same letter are not significantly different at P<0.05.
NC Positive control (CCL4) Silymarine (0.2g/kg b.w)
50mg/100g b.w
100mg/100g b.w
125mg/100g b.w
LSD 5% b.w
41.67±2.08c
118.3±2.08a
100
284
35.67±3.05ef
114.3±3.05b
100
320![]()
Each value represents the mean of 5 rats (Mean ± SE).
The same letters in each column represents the insignificant difference at
P<0.05.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 157
ISSN 2229-5518
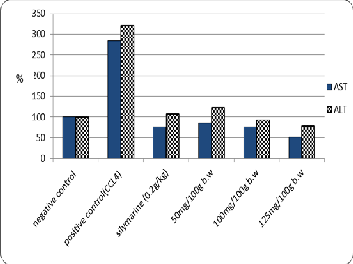
TABLE 5.
KIDNEYS FUNCTION OF THE EXPERIMENTAL ANIMALS
![]()
Treatments
![]()
Negative
mg/dl
Urea
![]()
%
mg/dl
Creatinine
%
Fig.2.The Effects of different concentrations of zinnia elegans leaves Ethanolic extract on AST and ALT activity of experimental CCl4- intoxicated male albino rats
3.3.2 Kidney functions of the experimental animals.
The data are shown in Table (5) and Fig. (3) That the
adminstration of different concentration of Zinnia elegans
control
NC
Positive control (CCL4)
Silymarine
(0.2g/kg b.w)
50mg/100g b.w
100mg/100g b.w
125mg/100g b.w
![]()
LSD 5%
25.33±2.30de
62.33±5.85a
30.33±0.57cd
30.33±4.04cd
32±2.00c
28±0.00c
3.09
100
246
120
120
126
111
0. 7±0. 1b
1.66±0.28a
0.63±0.05b
0.76±0.15b
0.73±0.23b
0.6±0.00b
0.26
100
237
90
109
104
86
leaves ethanolic extract (50, 100 and 125mg/100g b.w) as well as, standard drug (silymarin) significantly improved of the kidneys function against positive control. The lowest contents have been recorded (28 and 0.6 mg/dl of urea and creatinine respectively) with 125 mg/100g b.w against positive control (62.33 and 1.66 mg/dl of urea and creatinine respectively) these results may be due to the direct antioxidant effects of phenols and flavonoids. These data were in agreement with Abdul jalal [29] who found that a significant improvement of urea and creatinine activity with administration of Zinnia elegans ethanolic extract is due to the presence of phenols and flavonoids which might offer antioxidant activity and recover the kidney function activity.
3.3.3 Lipoprotein profile of the experimental animals
The data are shown in Table (6) and Fig. (4) That the
adminstration of different concentration of Zinnia elegans
leaves ethanolic extract (50, 100 and 125 mg/100g b.w) as
well as, standard drug (silymarin) significantly increased
the level of HDL (60 mg/dl with 50 mg/100g) and
decreased the level of LDL (83.33 mg/dl with 125 mg/100g of Zinnia elegans leaves ethanolic extract) against positive control (22.67 and 146 mg/dl with HDL and LDL respectively) these results may be due to the direct antioxidant effects of phenols and flavonoids. These data were in agreement with Wang [30] who found that a significant improvement of LDL and HDL content with administration of TNJ juice appears to protect the liver
Each value represents the mean of 5 rats (Mean ± SE).
The same letters in each column represents the insignificant difference at P<0.05.
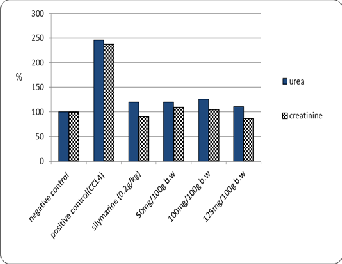
Fig.3.The Effects of different concentrations of zinnia elegans leaves Ethanolic extract on urea and creatinine of experimental CCl4- intoxicated male albino rats.
TABLE (6).
LIPOPROTEIN PROFILE OF THE EXPERIMENTAL ANIMALS.
from chronic exogenous CCl4 exposures via phenols and
flavonoids compounds.![]()
Treatments
HDL
LDL
mg/dl
% mg/dl %
![]()
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 158
![]()
ISSN 2229-5518
Negative control
NC
Positive control (CCL4) Silymarine (0.2g/kg b.w)
50mg/100g b.w
90.33±5.50a
100
86.67±4.72e
100
liver damage via its protection activity against the accumulation of MDA under CCl4 stress that induce reactive oxygen formation and reduce antioxidant enzymes and antioxidant substrates to induce oxidative stress. These data were in agreement with Lizby [31] who found that a significant increase of SOD, GST and GSH activities and a significant reduced MDA activity with administration of Zinnia elegans ethanolic extract reduce the accumulation of MDA when suppress the peroxidation of poly unsaturated fatty acids moderated MDA.
100mg/100g b.w
125mg/100g b.w
LSD 5%
![]()
Each value represents the mean of 5 rats (Mean ± SE).
The same letters in each column represents the insignificant difference at
P<0.05.
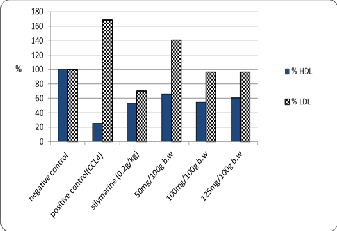
Fig. 4. The Effects of different concentrations of zinnia elegans leaves Ethanolic extract on HDL and LDL-cholesterol of experimental CCl4- intoxicated male albino rats.
3.3.4 Liver enzymes such as MDA, GSH as well as the activity of GST and SOD of the experimental animals.
The data are shown in Table (7) and Fig. (5). That the adminstration of different concentration of Zinnia elegans leaves ethanolic extract (50, 100 and 125 mg/100g b.w) as well as, standard drug (silymarin) significantly improved of
Superoxide dismutase (SOD), Glutathion-S-Transferase (GST), Glutathione reduced (GSH) and lipid peroxide (MDA) activities. The highest activity of SOD, GST and GSH (618.3, 6.66 U/g and 11.3 mg/g respectively) and the lowest activity of MDA (4.66 nmol/g) with 100 mg/100g of Zinnia elegans leaves ethanolic extract and against positive control (458.3, 3.33 U/g, 3 mg/g and 18.6 nmol/g of SOD, GST, GSH and MDA respectively),The hepatoprotective activity and antioxidant potential of Zinnia elegans ethanolic extract was investigated against CCl4 induced
TABLE.7.
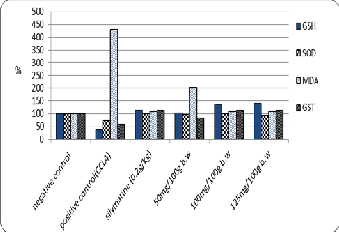
Fig. 5. The Effects of different concentrations of zinnia elegans leaves Ethanolic extract on MDA, SOD, GSH, and GST of experimental CCl4- intoxicated male albino rats.
3.3.5 Liver oxidation Nitric oxide and Hydrogen peroxide of the experimental animals.
The data are shown in Table (8) and Fig. (6) That the adminstration of different concentration of Zinnia elegans leaves ethanolic extract (50, 100 and 125 mg/100g b.w) as well as, standard drug (silymarin) significantly reduced the NO and H2O2 contents (9.3 and 39.6 µM/L respectively) with 125 mg/100g b.w against positive control (44.0 and
157.3 µM/L of NO and H2O2 respectively) these results
may be due to the direct antioxidant effects of phenols and
flavonoids. The hepatoprotective activity and antioxidant
potential of Zinnia elegans ethanolic extract was
investigated against CCl4 induced liver damage via its protection activity against the accumulation of NO and H2O2 under CCl4 stress. These data were in agreement with Bahmanzadeh [32] who found that Nitric oxide and Hydrogen peroxide were increased as a result of oxidative stress and the improvement of NO and H2O2 content with administration of Zinnia elegans ethanolic extract reduce the accumulation of NO and H2O2 under CCl4 stress.![]()
LIVER LIPID PEROXIDATION, GLUTATHIONE REDUCED AS WELL AS THE ACTIVITY OF GLUTATHION -S-TRANSFERASE AND SUPEROXIDE DISMUTASE OF THE EXPERIMENTAL ANIMALS.
Treatments
SOD
GST
GSH
MDA
![]()
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 159
![]()
ISSN 2229-5518
Negative control
NC Positive control (CCL4) Silymarine (0.2g/kg b.w)
50mg/100g b.w
100mg/100g b.w
![]()
U/g
623.3±2.88a
458.3±2.88d
617.7±2.51a
594±0.00b
618.3±2.88a
%
100
74
99
95
99
U/g
6.00±0.00b
3.33±0.57e
6.66±0.57a
5.00±0.00cs
6.66±0.57ab
%
100
56
111
83
111
mg/g
8.00±1.00bc
3.00±0.00d
9.00±0.00b
8.33±0.57bc
11.30±0.00a
%
100
38
113
104
138
nmol/g
4.33±0.57f
18.67±1.52b
4.66±0.57ef
8.66±1.15c
4.66±0.57ef
%
100
431
108
200
108
125mg/100g b.w
575±19.00c
92 6.66±0.57ab
111
11.33±1.52a
142
4.66±0.57ef
108
![]()
LSD 5%
14.80
0.90
1.50
1.75
Each value represents the mean of 5 rats (Mean ± SE).
The same letters in each column represents the insignificant difference at P<0.05.
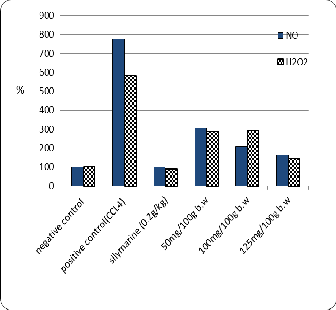
TABLE 8.
LIVER OXIDATION; NITRIC OXIDE AND HYDROGEN PEROXIDE OF THE EXPERIMENTAL ANIMALS.
![]()
![]()
Treatments
![]()
Negative control
NC Positive control (CCL4) Silymarine (0.2g/kg b.w)
50mg/100g b.w
100mg/100g b.w
125mg/100g b.w
NO
µM/L
5.66±0.57f
44.0±3.46b
%
100
777
H2O2
µM/L
27.00±2.64f
157.3±2.30a
%
100
583
LSD 5%
![]()
Each value represents the mean of 5 rats (Mean ± SE).
The same letters in each column represents the insignificant difference at
P<0.05. Fig. 6.The Effects of different concentrations of zinnia elegans leaves
Ethanolic extract on H2O2 and NO levels of experimental CCl4-
intoxicated male albino rats.
In the present study, the effect of Zinnia elegans leaves was evaluated in vitro and in vivo as therapy in experimental CCl4-intoxicated albino rats. The results revealed a high content of total phenols, significantly increase of DPPH, improvement of AST, ALT, NO, H2O2, LDL, MDA, urea, creatinine, GST, SOD and HDL by Zinnia elegans leaves extract. The results showed that the Zinnia elgans leaves have a hepatoprotective activities against CCl4 induced toixicity on rats. Zinnia elegans leaves damage induced may be useful as a hepatoprotective agent indeed retarded the liver injury by blocking the oxidative stress.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 160
ISSN 2229-5518
[1] Fanelli FL, Dole JM, Fonteno WC, Harden BT.
Optimizing Postharvest Life of Cut Zinnias.
Department of Horti. Culture. Sci., North Carolina
State University, Raleigh 2014; 2: 7695-7609.
[2] Yamaguchi M, Terahara N, Shizkuishi K.
Acetaylatedanthocyanins in Zinnia elegansflowers.
Phytochemistry, 1990; 20 (4): 2269-2270.
[3] Temitope MO, Damilola SA, Chukwuemelie ZU.
Antioxidant capacity of Moringa oleifera seed oil
against CCl4-induced hepatocellular lipid
peroxidation in wistar albino rats. Euro J Exp. Bio.
2014; 4(1): 514-518
[4] DeShon ND. Carbon Tetrachloride. Kirk-Othmer
Encyclopedia of Chemical Technology. Vol. 5, (3rded),
John Wiley and Sons, New York, 1979, pp. 704 – 714.
[5] Basu S. Carbon tetrachloride-induced lipid peroxidation: eicosanoid formation and their regulation by antioxidant nutrients. Toxicology, 2003; 189(1-2): 113–
127.
[6] Jones A, Dolak Robert L, Waller D, Eric A, Glende Jr,
Recknagel Richard O. Liver Cell Calcium Homeostasis in Carbon Tetrachloride Liver Cell Injury. J Biochem. Toxicology, 2006; 3(4): 329-342.
[7] Weber WD, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: Carbon tetrachloride as a toxicological model. Critical reviews
in toxicology, 2003; 33(2): 105-136.
[8] Chen YR, Zweier JL. Cardiac mitochondria and reactive
oxygen species generation. Circulation Research, 2014;
114: 524–537
[9] Harborne JB. "Phytochemical Methods", 2nd edn.
Chapman and Hall, London, 1984
[10] Markham KR. "Techniques of Flavonoid Identification
". Academic Press, London, 1982, Vol. 2, pp. 427-468.
[11] Singleton VL, Rossi JAJ. Colorimetry of total phenolics
with phosphomolybdic-phosphotungstic acid
reagents. Am J Enol Viticult, 1965; 16: 144-158.
[12] Burits M, Bucar F. Antioxidant activity of Nigella
sativaessential oil. Phytotheraphy Res., 2000; 14: 323–
328.
[13] Lane Peter WA, Pearson AE. Dietary
requirements.In:The laboratory animal principles and practice. Acedemic press Eds. London, New York,
1971, PP. 142
[14] Reitman S, Frankel SA colorimetric method for the
determination of serum glutamic oxaloacetic and
glutamic pyruvic transaminases. Anal J Clin. Path,
1957; 28: 56.
[15] Fawcett JK, Scott JE. A rapid and precise method for
the determination of urea. J Clin. Pathol, 1960; 13: 156-
159.
[16] Schirmeister J, Willmann H, Kiefer H. Plasma
creatinine as rough indicator of renal function. Dtsch
Med Wochenschr, 1964; 89: 1018-1023.
[17] Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin. Med, 1963 ; 61: 882-888.
[18] Habig WH, Pabst MJ, Jakoby WB. Glutathione S- transferases. The first step in Mercapturic acid. J Biol Chem, 1974; 249: 7130-7139.
[19] Wieland H, Seidel DA. simple specific method for precipitation of low density lipoproteins. J Lipid Res,
1983; 24(7): 904-909.
[20] Ortega E, Marcos S, Galban J. Fluorometric enzymatic
autoindicating biosensor for H2O2 determination
based on modified catalase. Biosensors &
bioelectronics, 2013; 41: 150-156.
[21] Satoh K. Serum lipid peroxide in cerebrovascular
disorders determined by a new colorimetric method.
Clin Chim Acta, 1978; 90(1): 37-43.
[22] Nishikimi M, Rao NA, Yagi K. The occurrence of
superoxide anion in the reaction of reduced phenazine
methosulfate and molecular oxygen. Biochem Biophys
Res Commun, 1972; 46: 849-854.
[23] Montgomery HAC, Dymock JE. The determination of
nitrite in water. Analyst. J Biol Chem, 1961; 86: 414-
416.
[24] Silva FAS. The ASSISTAT Software: statistical
assistance. In: International Conference on Computers in Agriculture, 6, Cancun, Anais. Cancun: American Society of Agricultural Engineers, 1996, pp. 294-298.
[25] Athar A. Studies on saponin from some indigenous
plants. Park Vet J, 1990; 10: 146-148.
[26] Samson Guenne, Nabere Ouattara, Adama Hilou,
Jeanne FM, Odile GN. Antioxidant, Enzyme Inhibition Activities and Polyphenol Contents of Three Asteraceae Species used in Burkina Faso Traditionally Medicine. Int J Pharm Sci, 2011; (3)5: 524-528.
[27] Prakash Veeru, Mishra Pankaj Kishor, Mishra
Meenakshi. Screening of medicinal plant extracts for antioxidant activity. J Medi Plants Res, 2009, 3(8) pp.
608-612.
[28] Murugaian P, Ramamurthy V, Karmegam N.
Hepatoprotective Activity of Wedelia calendulacea L.
against Acute Hepatotoxicity in Rats. Res J Agriculture and Biological Sci, 2008; 4(6): 685-87.
[29] Abdul jalal A, Selvakumar S, Nallathambi R, Jeevaprakash G, Dheivanai S, Senthilvelan S. Hepatoprotective Activity of Wedelia Chinensis
Against Carbon Tetrachloride Induced Liver Damage in Rats. Int j phytopharmacology. 2012; 3(2): 121-125.
[30] Wang MY, Anderson G, Nowicki D, Jensen J. Hepatic protection by noni fruit juice against CCl(4)-induced chronic liver damage in female SD rats. Plant Foods Hum Nutr, 2008; 63(3): 141-145.
[31] Lizby AM, Dhanyaraj D, Prathibhakumari PV, Prasad G. Hepatoprotective and Antioxidant Potential of Sphaeranthus Indicus [Linn] on Liver Damage in Wistar Rats. Int J Pharm Sci, 2012; 4(3): 222-225.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 161
ISSN 2229-5518
[32] Bahmanzadeh M, Abolhassani F, Amidi F, Ejtemaiemehr SH, Salehi M, Abbasi M. The effects of nitric oxide synthase inhibitor (L-NAME) on epididymal sperm count, motility, and morphology in varicocelized rat. DARU, 2008; 16(1) 23-28.
IJSER © 2015 http://www.ijser.org