1 stage i.e. the bud stage was avoided. The total betalain content of each flower was estimated following the methods of Janna and Khairul et al.,
2006.

International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 1257
ISSN 2229-5518
mirabilis jalapa
Sujata Mahapatra* and Bhaskar Padhy**
ABSTRACT
The betacyanin pigments were extracted from the Mirabilis jalapa (dark pink ) flowers, of Nyctaginaceae family, available in Berhampur University campus of south eastern Odisha, using aquash acidified methanol (0.01 % Hcl).The extracted pigments were exposed to different pH. (1,4,7 ,10) at different temperature (4ºC,25ºC,35 ºC & 45
ºC) under dark conditions. The result showed that increase in pH, temperature or exposure to light destruct the betacyanin pigments. The antioxidant activity of methanolic extract of the test flower and the standard antioxidant ascorbic acid was assessed on the basis of the radical scavenging effect of the stable 2, 2- diphenyl-1-picryl hydrazyl (DPPH) free radical activity according to the method described by (Sadhu et al., 2003).
Key words: Betacyanin, pH, stability, temperature ,antioxidant ,DPPH. ascorbic acid, methanolic extra ct
Mirabilis jalapa belongs to the family Nyctaginaceae. It is a large herbaceous plant grown in the gardens throughout south-eastern Odisha. This plant is 50-100 cm high. Mirabilis jalapa can be grown very easily from flower seed. It needs warm soil to germinate. It germinates in just 10-14 days. The flower seeds should be kept moist. It grows best in full sun, but can tolerate light shade if necessary.
Author - Sujata Mahapatra
Lecturer in Botany, Sc.College
Hinjilicut,Ganjam,Odisha,India,
sujata.mini@rediffmail.com
It needs to be watered regularly and should ideally have a fertile soil. Four O' Clock plants bloom all summer long. They have antifungal, antimicrobial, antiviral, antispasmodic and antibacterial properties (Dimayuga, 1998; Yang, 2001).
The betalain are yellow-to-red nitrogen- containing compounds, derived from the amino acid tyrosine. It comprises the largest group of water soluble pigments in the plant kingdom, only in the order Caryophyllalles giving the natural colour yellow, magenta or dark pink to the flower, fruits and coloured leaves. They are included in
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 1258
ISSN 2229-5518
flavonoids or phenolic compounds (Malian-aubert et al, 2001).They are synthesized in the plant cell cytoplast and actively transported across the tonoplast
into the vacuole, where they accumulate (leng et al,
2000).Betalains are classified into red (crimson)
betacyanins and yellow betaxanthins (Strack et al.,
2003).
Beside the colour attraction, they are also beneficial to health by having antioxidants, antimicrobial and pharmacological properties. They are found to be a perfect alternative of the synthetic food colourant and synthetic dye. The intensity and stability of flower colour depends on the concentration of the batacyanin. Which in turn depends on various factors like structure, pigment concentration, pH., temperature, light intensity, co-pigment, metal ions, enzymes, oxygen , ascorbic acid, sugar etc. (lee,2002, Dimayuga
,1998;Yang SW,2001).
This paper investigated the relation of flower colour to betalain conc. in different pH. level, at different temperature and its antioxidant properties .
Fresh blossomed flowers were collected in
the early morning and placed in clear domed trays overnight under refrigerated condition. After washing them thoroughly in distilled water, they were dried by soaking them on blotting paper towel under shade in laboratory at 25ºC ± 2. When they were fully dried, they were homogenised into fine powder using a mortar and pestle, then stored in air tight bottles in cold at 4°C in the laboratory, and were used when required.
1 gm of fresh petals were taken & kept for 2/3 hours at room temperature mixed with 10ml of 0.01% Hcl (v/v) in Methanol (80%), in (1:1w/v) in darkness then mixed thoroughly with a clean mortar & pestle. The mixture then filtered through whatman 110 no. filter paper and the remaining solids were washed with
0.01% Hcl in methanol(80%) until a clear solution were obtained. The combined filtrates were dried using a rotary evaporator at 30 ºC. The aqaush concentrate was dissolved in 0.01% Hcl (V/v) in DW and the solution obtained were made up to 100 ml . & stored at 4 ºC, For further use.(method from a guide to
modern technique of plant analysis by J.B.Harborne).
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 1259
ISSN 2229-5518
The flowers of different developmental stages were collected i.e. S-2, S-3, S-4 and S5. The S-
1 stage i.e. the bud stage was avoided. The total betalain content of each flower was estimated following the methods of Janna and Khairul et al.,
2006.
Figure –I (S-2, S-3 and S-4 stage of
Mirabilis jalapa
Total betacyanin content was determined by measuring the absorbance of the extract at 510nm by ph differential method using two buffer solutions one is highly acidic & other one a weak acid i.e. pH 1 and pH 4.5, using a UV-Vis spectro- photo meter. The standard solution used was cyandin-3-glucoside and
0.1% Hcl (V/v) in methanol solution (500 mg/ml).The
molecular wt. of cy-3-gl. i.e. (449.2 gm/mol).The
betacyanin was calculated using the following formulae-
A X mw X DF X 10³ / ∑ X L A= A max - A min.
{Mw=mol.wt , DF=dilution factor (0.2ml. sample
in 2 ml.=10)
{ For Cyanidin 3 glucoside ,∑=coefficient =L X CM¯ X MOL¯= 26900 }
The concentration of betacyanin was determined by applying the Beer-lambert Law. The spectra recorded in a spectrophotometer were measured at 25 ºC, against the solvent (d. w). The two buffer solution used are 1.86 gm kcl + 980 ml distilled water (pH is adjusted to 1 by adding conc.Hcl & the vol. was made up to 1 lt. by adding d.w), 54.43 gm. Of CH3CO2 Na.3H2 O in ~ 960 ml of distilled water (pH is adjusted to 4.5 by adding conc.Hcl & the vol. was made upto 1 lt by adding d.w ).
The mixture of buffer & samples were homogenised
& cetrifuged twice at 5000 rpm for 15 minutes. The supernatant was collected & its absorbance were measured at 510 nm by spectrophoto meter.
So total anthocyanin or betacyanin content (mg/lt) =
(A x mw x DF x 1000) /(∑ x l) .
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 1260
ISSN 2229-5518
For phytochemical screening the sample solutions of the test flowers were taken and tested by Benedict’s reagent for testing sugar, Fecl2 for phenol and tannins, for flavonoid test the shinoda’s test was done. The respective colour was observed by adding. the prescribed chemicals shown in the above methods
The effect of pH on colour stability was studied with 4 different buffer solutions at the same temperatures i.e strong acid (pH 1.0), weak acid (PH 4.0), neutral (pH
7.0) and base (pH 10.0) .Buffer solution of pH 1 is prepared by mixing 50 ml of 0.2 M KCl and 134 ml of
0.2 M HCl, buffer solution of pH 4.0 is prepared by mixing 847.0 ml of 0.1 m acetic acid and 153.0 ml of
0.1 m sodium acetate, buffer solution of pH 7.0 is prepared by mixing 100 ml of 0.1 M KH2PO4 and
58.2 ml of 0.1 M NaOH; buffer solution of pH 10.0 is prepared by mixing 100 ml of 0.025 M Na2B4O7-
10H2O and 36.6 ml of 0.1 M NaOH. pH of the solution was measured and adjusted to 1, 4, 7, 10 using 1N sodium hydroxicide solution or 10% citritic acid solution drop wise (a drop was measured as 1/20 of a ml.each time before use) .10 drops of each extract were added to each buffer solution and the colour of the sample solution was measured.
Samples were taken right after preparation i.e. (day 0)
and after day 1, 2, 4, 6, 8, 10, 15, 20, 25 and 30. It was centrifuged at 300 rpm (956 xg) for 5 minutes and
analysed using UV-visible spectrophotometer. The absorbance at 537, 573, 627 and 628 nm was observed for the extract at pH 1, 4, 7 and 10 respectively.(data shown in Fig.II)
Secondly aliquots of the sample were placed in 10 ml caped glass vials and kept in incubater for the study of effect of different temeperatures i.e (4ºC,25ºC,35ºC&45 ºC), on colour stability .data shown in figure-III
3 mg of ascorbic acid was dissolved in 3 ml of distilled water in 1:1 ratio. Then, it was diluted to
05, 10, 15, 20, and 25 μg/ml by adding distilled water.
2.6-2 Preparation of test sample:
Stock solutions of samples were prepared by dissolving 10 mg of dried hydro methanolic extract of the test flower in 10 ml of methanol to give concentration of 1mg/ml. Then different sample concentrations (05, 10, 25, 20 and 25 μg/ml) were prepared by diluting it in distilled water.
DPPH solution was prepared by dissolving 3.4 mg of
DPPH in 3 ml methanol.
5 ml of freshly prepared DPPH solution was mixed with 5 ml of the extract solution and standard solution
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 1261
ISSN 2229-5518
(05, 10, 25, 20, and 25 μg/ml) thoroughly. These solution mixtures were kept in the dark for 30 min. The change in the Purple colour of DPPH to yellow indicated the effectiveness of scavenging activity of the extract. The absorbance of the combination was measured at 517 nm using UV-Visible Spectrophotometer and ascorbic acid was served as a positive control. The antioxidant activity of methanolic extract and the standard antioxidant ascorbic acid was assessed on the basis of the radical scavenging effect of the stable 2, 2- diphenyl-1-
picrylhydrazyl (DPPH) free radical activity according
All the data were analysed and graphically presented using Microsoft excel. The data were computed to obtain the average and standard error (± S.E.) with the significant level at 95% and were subjected to ANOVA to detect significant differences among seasons and among treatments (P < 0.05) and significant difference between means were detected following the method of (Gomez and Gomez, 1984
The spectral analysis showed that In Mirabilis
to the method described by(Sadhu et al., 2003). Lower
jalapa, the A
max
were at 535-537 nm i.e. the blue-
absorbance of the reaction mixture indicated higher free radical scavenging activity. The percentage of scavenging was denoted by using the formulae given below:
% DPPH radical-scavenging =
(A control -A test Sample) / A control X 100
The treatments like DPPH were applied in pigment extract of Mirabilis jalapa flowers to verify their antioxidant activity.
green region, showing the presence of betacyanins
(J.B Harborne, 1984).
Here, maximum pigments are in S4 (230mg/gm) and minimum are in S-2(130mg/gm).The total betalain content was in the following order → S-4 > S-3 > S-5
> S-2.Data in Figure-II![]()
The effect of pH on stability of the petal extracts of flowers of dark pink (Mirabilis jalapa) was studied with different buffers at the same temperatures, strong acid (pH 1-3), weak acid (pH 4-6), natural effluent (pH 6-7) and base (pH 10.0). The extracts produced pinkish red solutions at pH 1.0 to 5.0 range, while at pH from 5.5 to 10, the extracts produced brown to
yellowish-brown solutions. Beyond pH 10, the solution was yellow or colourless. (Shown in Figure III and Table I).
Betacyanains are not as susceptible to hydrolytic cleavage as the anthocyanins studied in Clitorea ternatea ,Rosa indica, Hibiscus rosasinensis. Betacyanains are relatively stable over the broad pH
range from 3 to 7 (Jackman & Smith, 1996). Betalains
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 1262
ISSN 2229-5518
present in Mirabilis jalapa also stable in pH range from 3-7 supporting the view of Jackman and Smith,
1996 and Attoe & von Elbe, 1981. So, these were applied to low acidity foods as food colourants. Below pH 3.5, the Amax shifted towards lower wavelength
and above pH 7, it changed towards upper wavelength
It was found that the optimum pH range for betalain stability in Mirabilis jalapa was at pH 5-6 confirming the view given by Von Elbe and Amundson, 1985; However, as the anthocyanins are less stable at pH values above 3 (Stintzing & Carle, 2004) makes
the betacyanin to give reddish colour shades to low acid foods. Moreover, Betacyanains can effectively be stabilised by adding ascorbic acid, which on the other
hand, impairs anthocyanin stability (Shenoy, 1993).![]()
Hence, application of betacyanains instead of anthocyanins for colouring foods with high ascorbic acid contents may be possible. (Herbach et al., 2006).
Here the maximum stability was at 4ºC and least at 35
ºC.The colour degradation was in the order of 4ºC >
45ºC >25 ºC >35 ºC. Some studies reported that the rate of betalain degradation increases with increasing temperatures (Saguy et al., 1978; Havlı´kova´ et al.,
1983; Garcı´a Barrera et al., 1998). Thermal degradation of betacyanin in betanin solutions as well as in red beet and purple pitaya juices was reported, which follows the first-order kinetic reaction (Von Elbe et al., 1974; Saguy et al., 1978; Saguy, 1979; Herbach et al., 2004b). During heat processing, betanin may be degraded by isomerisation, decarboxylation or cleavage (by heats or acids), resulting in a gradual reduction of red colour and eventually the appearance of a light brown colour (Huang & von Elbe, 1985).Brown colour shifted to yellow colour, when dehydrogenation of betanin
occurs. It leads to form neobetanin. Further, cleavage
of betanin and isobetanin, generates the bright yellow betalamic acid and the colourless glycoside (cyclo- Dopa-5-O-glycoside).It can also be induced by bases (Schwartz & von Elbe, 1983; Schliemann et al., 1999). Herbach et al. (2004) proposed that while betanin colour is maintained upon C15-isomerisation or decarboxylation,C17-decarboxylation causes a hypsochromic shift of the absorption peak from 538 to
505 nm, resulting in an orange-red colour. Betanin may be regenerated from their primary degradation products, when the extracts are kept for some time under temperature below 10 °C and pH around 5.0 (Huang & von Elbe, 1985, 1987). Betanin regeneration, which consists in a partial resynthesis of betanin from its hydrolysis products, involves a condensation of the amine group of cyclo-Dopa-5-O- glycoside with the aldehyde group of betalamic acid. Betanin is rapidly formed when both compounds are
mixed in solution (Huang & von Elbe, 1985). The
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 1263
ISSN 2229-5518
activation energy for betacyanin degradation decreased with pH. This did not impair pigment application to most foods undergoing ordinary thermal treatment. For example, it lost less than 10% when the extract was acidified to pH 4 at 80°C for 5 mins
(Vaillant et al, 2005). Havlikova et al, 1983 reported![]()
that high temperature shifted the optimum pH for betacyanin stability towards pH 6. However, anaerobic condition favours betalain stability at lower pH (Von Elbe, 1985).
2.7-3 The antioxidant effect
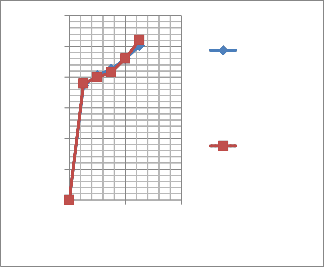
The decrease in absorbance of DPPH radical was caused by antioxidants, because of the progress of the reaction between antioxidant molecules and radical, which results in the scavenging of the radical by hydrogen donation. Data is mentioned in Figure-V.
The present results suggest that the tested plant extracts have moderate to potent antioxidant activity. A high correlation was demonstrated between colour concentration and antioxidant capacities. It was observed that flower pigments with high antioxidant properties tend to be brightly coloured data in Figure IV. The Mirabilis jalapa showed fast scavenging activity than the other flowers like Clitorea,Rosa indica and Hibiscus rosasinensis (Vankar and Srivastava, 2010).
It has been determined that the antioxidant effect of plant products is mainly due to radical scavenging activity of phenolic compounds such as flavonoids, polyphenols and tannins (Mishra and Srivastava et. al.,
2007). The antioxidant activity of phenolic compounds is mainly due to their oxidation reduction
properties, which can play an important role in
adsorbing and neutralising free radicals, reducing singlet and triplet oxygen, or decomposing peroxides (Harborne, 1984).The blue coloured flowers showed fast scavenging activity within 5 min than red coloured flowers in 20 min. This may be due to the structure of flavinnium ion. Blue anthocyanins like Malvidin, Delphidin, Petunidin contain higher number of methoxy groups than in red coloured anthocyanins like Cyanidin, pelargodin and peonidin which have more of hydroxyl groups (Andersen and Jordheim,
2006).
For flavonoids, the major factors that determine the radical-scavenging capability [Sahidi et.al,1992; Bors et.al,2002] are:
(i) The ortho-dihydroxy structure on the B ring, which has the best electron-donating properties and confers higher stability to the radical form and participates in electron delocalization.
(ii) The 2, 3-double bond with a 4-oxo function in the C ring, which is responsible for electron delocalisation from the B ring.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 1264
ISSN 2229-5518
(iii) The 3- and 5-hydroxyl groups with the 4-oxo function in A and C rings, which are essential for maximum radical scavenging potential.
(iv)The 3-hydroxyl group is important for antioxidant activity. The 3-glycosylation reduces their activity when compared with corresponding aglycones.
Preliminary phytochemical screening revealed the presence of flavonoids, phenols, tannins and reducing
sugars in the studied test flower, showing its involvement in medicinal fields. The anti-bacterial nature of the test flower, will be confirmed by the antimicrobial test which is needed to be done later. Screening and proper evaluation of the test flowers could offer possible alternatives that may be both sustainable and environmentally acceptable
250
200
150
100
Mirabilis…
50
0
S-2 S-3 S-4 S-5
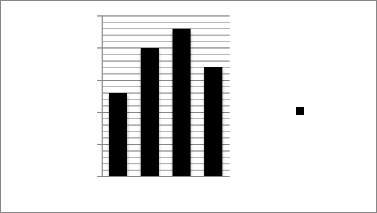
Figure II Total pigment content in different developmental stages in Mirabilis jalapa
(S2 -early developmetal stage,S3 -petal partly opened but fully developed
S4 -fully opened flower ,S5 -petals at the senescence stage )

Figure-III (a) showing effect of pH on colour of the extracts of Mirabilis jalapa (Before 30 days)
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 1265
ISSN 2229-5518

Figure - III (b) showing effect of pH on colour of the extracts of Mirabilis jalapa
(after 30 days)
Table-I
Effect of pH on colour stability of the petal extract of Mirabilis jalapa flower
% of residual colours in diff.days at diff. pH in Mirabilis jalapa flower
Day | pH-1 | pH-4 | pH-7 | pH-10 |
zero | 75 | 90 | 80 | 65 |
1st day | 74 | 89 | 77 | 63 |
2nd day | 73 | 86 | 75 | 60 |
4th day | 71 | 85 | 70 | 58 |
6th day | 71 | 84 | 68 | 55 |
8th day | 65 | 84.2 | 65 | 50 |
10th day | 58 | 83 | 63 | 48 |
15th day | 56 | 80 | 64 | 46 |
20thday | 50 | 75 | 62 | 42 |
25thday | 45 | 70 | 60 | 38 |
30thday | 43 | 68 | 59 | 35 |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 1266
ISSN 2229-5518
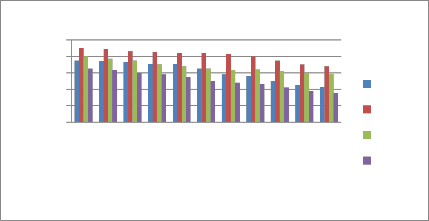
Figure-III Effect of pH on colour stability of the petal extract of Mirabilis jal apa flower
100
80
60
40
20
0
% of residual colours in diff. days at diff.pH in Mirabilis jalapa
pH-1 pH-4 pH-7 pH-10
Days
120
100
80
60
40
20
0
0 5 10 15
Temperature at
45°C
Temperature at
35°C
Temperature at
25°C
Temperature at
4°C
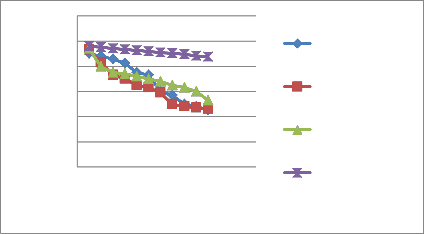
Figure IV Effect of different temperature on colour stability of M.jalapa floral extract
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 1267
ISSN 2229-5518
[3] Bagchi D, C.K. Sen, M. Bagchi, and
. M. Atalay, (1990).“Anti-angiogenic, antioxidant, and
60
50
40
30
20
10
0
0 20 40
% of scavenging activity Ascorbic acid
% of scavenging activity Mirabilis jalapa
anticarcinogenic properties of anthocyanin
in aqueous solution. J. Phytochem. 29:1097-1102.
[4] Bagchi D., Garg A., Krohn R.L., Bagchi M., Bagchi B.J., Balmoori J., (1998) .
General Pharmacology:30(5), 771–776.
[5] Bors W.; Michel C.( 2002). Chemistry of the antioxidant effect of polyphenols.
Figure- V Scavenging activity of the M.jalapa
[1] Attoe & von Elbe, 1981; Cai et al.,
(1998 )Von Elbe, J.H. (1975).Stability of betalains as food colors.
Food Technology, 5: 42–44.
[2] Azeredo H. M. C. (2008). Metal-pigment Betalains – a review 2369,Institute of Food Science and Technology,
International Journal of Food Science
and Technology 2009,(44: 2365– 237)
Ann. N. Y. Acad. Sci.: 957: 57-69.
[6] Brouillard R and Dangles O (1993). Flavonoids and flower colour. In: The flavonoids Advances in research since 1986 (ed. J.B. Harborne) pp 565-586. Chapman and Hall, London.
[7] Brouillard R.( 1982). Chemical structure of anthocyanins, Academic Press, New York, pp. 1-40.
[8] Brouillard R. (1988). Flavonoids and flower colour. In: Harborne JB,ed.
The flavonoids Advances in research since 1980. pp 525-538. London:Chapman and Hall.
[9] Castellar R., J.M. Obon, M. Alacid and J.A.
Fernadez-Lopez,
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 1268
ISSN 2229-5518
(2003) ,Color properties and stability of
Betacyanins from Opuntia : J. Agric. Food Chem. 51 pp. 2772–2776.
[10] Clinton S. K., (1998), Lycopene: Chemistry, biology, and implications for human health and disease. Nutr. Rev: 35–51.
[11] Dakora F D (1995). Plant Flavonoids: Biological Molecules for Useful Exploitation. Aust. J. Plant Physiol. 22: 87-99.
[12] Dangelmayr B, Stotz G, Spribille R,
Forkmann G.( 1983). Relationship between
[16] Hagen CW Jr. (1966) .The differentiation of pigmentation in flower parts. II.
Changes in pigments during development of buds in
Impatiens balsamina genotype ll HHPrPr.
American Journal of Botany 53: 54-60
[17] Harborne J. B., (1984), Phytochemical Methods, Chapman and Hall, London, 2nd Edition, Chapters 1, 2 and 5.
[18] Harborne, J.B.(1958),“Spectral methods of
flower development, anthocyanin accumulation and activity characterizing anthocyanin.
Enzymes involved in flavonoid biosynthesis in Matthiola incana R. Br.Zeitschrift fuX r Naturforschung 38c: 551-555.
[13] Dimayuga , R E(1998). Antimicrobial activity of medicinal plants from Baja California
Sur/Mexico. Pharmaceutical Biol 36: 33-43.
”Biochem. J., 70: pp. 22-28.
[19] Hatano,T.,H.Kagawa,T.Yasuhar and T.Okuda,(1988). Two new flavonoids and constituents in licorice root: their relative astringency and radical scavenging effects Chem. Pharm. Bull., 36: 1090-2097 health perspective New york: Marcel dekker., 1996
[14] Furtado P., P. Figueiredo, H. C. D. Neves, and F. Pin[a20(]1H9a9v3l)ı.´Pkhoovtao´c,heLm., iMcaıl´kaonvda´th, eKrm. &al Kdyzelgirnakd,ation of
anthocyanidins,
J. Photochem. Photobiol. A 75: 113-118
[15] Goodwin.T.W, (1965).Chemistry and
Biochemistry of Plant Pigments,
Academic Press, London, Chapters 3, 4,8 and 9.
V. (1983). Heat stability of betacyanins.
Zeitschrift fu¨r Lebensmittel-Untersuchung und
–Forschung,177: 247–250.
[21] Herbach K.M., Stintzing, F.C. & Carle, R. (2004). Impact of thermal treatment on colour and pigment pattern of red beet (Beta vulgaris L.) preparations. Journal of Food Science, 69:
C491–C498
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 1269
ISSN 2229-5518
[22] Herbach K.M., Stintzing, F.C. & Carle, R. (2004). Thermal degradation of betacyanins in juices from purple pitaya (Hylocereus polyrhizus [Weber] Britton and Rose) monitored by high-performance liquid chromatography-tandem mass spectrometric analyses. European Food Research and Technology, 219: 377–385.
[23] Herbach K.M., Stintzing, F.C. & Carle, R. (2006). Betalain stability and degradation – structural and chromatic aspects.
Journal of Food Science, 71:R41–R50
[24] Huang A.S. & von Elbe, J.H. (1985). Kinetics of the degradation and regeneration of betanine. Journal of Food Science, 50:115–
1120.
[25] Huang A.S. & von Elbe, J.H. (1987). Effect of pH on the degradation and regeneration of betanine. Journal of Food Science, 52:1689–1693
[26] . Jackman R.L. and Smith, J.L. (1996). Anthocyanins and Betalains. In Natural Food Colorants, 2nd ed. (G.A.F. Hendry and J.D. Houghton, eds.) pp.244-309. Blackie and Son, Ltd., London
[27] Janna O.A., Khairul A., Maziah M., Mohd. Y., (2006). Flower pigment analysis of Melastoma malabathricum. African Journal of Biotechnology Vol. 5 (2) pp 170-174
[28] Justesen H, Andersen AS, Brandt K
(1997);Accumulation of Anthocyanins and
Flavones during Bud and Flower Development in Campanula isophylla Moretti. Ann of Bot,
79:355-360.
[29] Kim J.H. and K. Fujieda. (1991). Studies on the flower color variation in Hibiscus syriacus L.: Relation of flower colors to anthocyanin, pH and.co-pigmentation J. Kor. Soc. Hort. Sci.
32:247–255.
[30] Schwartz S.J. ,Von Elbe, J.H. (1983).
Identification of betanin degradation products. European Food Research and Tecxhnology,
176: 448–453.
[31] Shahidi F., Wanasundara P.K. (1992).
Phenolic antioxidants. Cri.t Rev. Food Sci. Nutr ., 32: 67-103.
[32] Strack, D., Vogt,T. , Schliemann,W.
(2003) .Recent advances in betalain research.
Phytochemistry, 62:247–269.
[33] Upadhyaya H., Panda, S.K., Dutta, B.K., (2008). Variation of physiological and ntioxidative responses in tea cultivars subjectedto elevated water stress
followed by rehydration recovery.
Acta Physiol. Plant. 30: 457–468.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 1270
ISSN 2229-5518
[34] Upadhye M., Dhiman A., Shriwaikar A. (2009),Antioxidant activity of aqueous extract of Holostemma Annulare(Roxb).
Adv Pharmacol Toxicology, 10 (1), 127–131.
[35] Vaillant F., Perez, A., Davila, I., Dornier, M., Reynes, M. (2005).Colourant and Antioxidant properties of red-purple pitahaya (H sp.) Fruits, 60, 1–10.
[36] Vankar, Padma S. and Srivastava, Jyoti (2010)"Evaluation of Anthocyanin Content in Re and Blue Flowers" International Journal of
Food Engineering: and Medicinal plants
.Vol.6: Iss. 4, A-7.
[37] Von Elbe J.H. (1975). Stability of betalaines as food colors.
Food Technology, 5: 42–44.
[38] Von Elbe J.H., Maing, I. & Amundson, C.H (1974), Colour stability of betanin.
Journal of Food Science, 39: 334–337. [39] Yang SW,2001. Three new phenolic compounds from a manipulated plant cell culture, Mirabilis jalapa. J Nat Prod 64: 313-17, 2001.
[40] Madhavi D.L., Deshpande S.S. & SulunkheD.K., (1996), Food antioxidants: toxicological and technological, health perspectives; New york: Marcel dekker.,
41] Madhujith T, Naczk M, Shahidi F. (2004).
Antioxidant activity of common beans
(Phaseolus vulgaris L.). J Food Lip 11:220–33.
[42]Mishra J., Srivastava R.K., Shukla S.V., Raghab C.S.,( 2007),Antioxidants in Aromatic and Medicinal plants. Science tech entrepreneur., 1–16.
[43]Rice E., Miller C. A. and Paganga G., (1997), Antioxidant properties of phenolic compounds. Trends Plant Sci. 2:152-159.
[44]Rice-Evans, C.A.Miller, N.J., Bolwell, P.G., Bramley, P.M.,Pridham, J.B., (1995), The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 22:
375-383.
[45]Saguy I. (1979). Thermostability of red beet pigments (betanine and vulgaxanthin-I): influence of pH and temperature ,Journal of Food Science,
44:1554–1555.
[46]Saguy I., Kopelman I.J.,Mizrahi S. Thermal kinetic degradation of betanin and betalamic acid. Journal of Agricultural and Food Chemistry, 26:
360–362
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 1271
ISSN 2229-5518
Author - Sujata Mahapatra
Lecturer in Botany, Sc.College Hinjilicut,Ganjam,Odisha,India,
Co-author - Prof. Bhaskar Padhy, Ph.D.,D.Sc. Division of Plant Physiology and Biochemistry,
Department of Botany, Berhampur university ,Bhanja vihar, Berhampur-760007,Odisha (india)![]()
IJSER © 2013 http://www.ijser.org