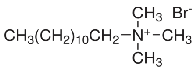International Journal of Scientific & Engineering Research, Volume 3, Issue 7, July-2012 1
ISSN 2229-5518
Experimental Study of Anionic and cationic surfactants effects on reduce of IFT and wet- tability alteration in carbonate rock
Elyas Golabi1. Fakhry Seyedeyn Azad2. Sayed Shahabuddin Ayatollahi3. Sayed Nooroldine Hosseini1. Mjid
Dastanian1.
Abstract: The oil recovery by water flooding from carbonate reservoir is not effective because of the capillary forces in naturally fractured oil-wet carbonate formations. Alkaline/surfactant solution is recommended to enhance the spontaneous imbibition between fractures and matrix by both the wettability alteration and ultra-low interfacial tensions. In this work, the effects of anionic surfactant (SDBS), and a cationic surfactant (C12TAB), on the interfacial tension (IFT) between oil and water and wettability alteration of limestone core samples were investigated. The understudied oil and limestone core were obtained from Aghajari reservoir, in the south -western part of Iran. The experimental results showed that the minimum amount of IFT achieved are using 0.5 wt% SDBS with 0.6 mol/lit NaOH, 1.5 wt% Na2CO3
and 5wt% NaCl at 70 oC. At 0.4 wt% and 70 oC, C
12TAB mostly altered the wettability toward water wet.
Key words: Fracture Carbonate Reservoir, Wettability Alteration, Surfactant, Oil-Wet, Water-Wet.
1 INTRODUCTION

The oil recovery methods generally used are pressure dep- letion and water flooding. The residual oil left after water flooding in fractured oil-wet reservoir has been reported to be very high [Chen et al., 2000]. This has been mostly due to the preferential flow of water, as the non-wetting fluid, in the fractures leaving most of the oil saturated Matrices completely unswept. The water imbibition into the Matric- es is hindered by the negative capillary pressure in the Ma- trices After a conventional water flooding process, the re- sidual oil in the reservoir is trapped as a discontinuous phase in form of oil drops by capillary forces [Dosher et al.,
1976]. On average, water flooding leaves approximately two third of the original oil in place (OOIP) as residual oil which is the target of further EOR recovery [Wardlaw,
1996].
Surfactants flooding system aims at producing the residual oil after secondary oil recovery with water flooding or gas
injection. Creation of ultra- low interfacial tension and wet- tability alteration are the most important mechanisms for oil recovery by surfactant flooding. Establishment of posi- tive capillary pressure of reservoir rock and displacement of oil in the porous media by surfactant flooding have been reported [Golabi et al., 2012, Yu, 2009, Hatiboglu et al.,
2007, Somasundaran et al., 2006, Babadagli, 2003, Standnes et al., 2003, Graue et al, 2002, Touhami et al., 2001, Babu et al., 1986, Xie et al, 2005, Austad et al., 2003,, and Rama- krishnan, 1983]. Many flooding systems, especially surfac- tant-enhanced alkaline systems have been investigated [Ramakrishnan et al., and Almalik et al.]. It has been re- ported that the alkaline addition into many flooding sys- tems plays an important role in reduction of interfacial ten- sion. On the other hand, the alkaline can react strongly
with the rock in the layer. So, a great quantity of alkaline can be consumed, and the layer can also be destroyed si- multaneously. Therefore, a high potential of achieving maximum oil recovery exist, with using a properly de- signed surfactant formulation.
Liu et al. performed a study on using alkaline/surfactant flooding for Western Canadian heavy oil reservoir. They used Na2CO3 and NaOH as alkalines and alkyl sulfate, al- pha olefin sulfonate, linear alkyl benzene sulfonate, alkyl benzene sulfonate and alkyl ether sulfate as surfactants. Their experimental results showed that the dynamic inter- facial tension of oil/water could be lowered by using a combination of Na2CO3 and NaOH solutions at low concen- trations of the surfactant.
Spontaneous imbibition tests in oil-wet dolomite cores
were carried out by Hirasaki and Zhang. No spontaneous imbibition was observed with brine for 8 months, while
with alkaline/surfactant solution, spontaneous imbibition was initiated within an hour. However, no conclusion was made toward what factor and to what extent contributed mostly to the observed oil recovery. Nedjhioui et al. inves- tigated the effect of using a combination of two anionic sur- factants (SDS and Marlon ARL), on water solubility of bio- polymer (Xanthan gum) and alkaline (NaOH) at various concentrations by measuring their electrical conductivity, as well as surface and interfacial tensions. They concluded that the mixture of different compounds has important ef- fects on the conductivity and interfacial tension of these systems and the crude oil/water system. Lorenz and Peru reported that interfacial tension was decreased by adding sodium hydroxide, sodium carbonate and sodium carbo- nate alkaline compounds. The amount of decrease was de-
Corresponding Author: E-mail addresses: elyas.golabi@iauo.ac.ir (Elyas Golabi)
1. Department of Petroleum Engineering, Omidiyeh Branch, Islamic Azad University, Omidiyeh, Iran.
2. Department of Chemical Engineering, University of Isfahan, Isfahan, Iran.
3. EOR Research Center, School of Chemical and Petroleum Engineering, Shiraz University, Shiraz, Iran.
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Experimental Study of Anionic and cationic surfactants effects on reduce of IFT and wet-tability alteration in carbonate rock 2
ISSN 2229-5518
clining going from sodium hydroxide to sodium carbonate alkaline compounds.
Mohanty investigated wettability alteration and imbibition of West Texas oil reservoir. He used anionic surfactants Alfotera, and cationic surfactant DTAB. His results showed that anionic surfactants could reduce IFT more than the cationic surfactant but in wettability alteration, the cationic surfactant was more powerful than the anionic surfactants. He also showed that the cationic surfactant was more ap- propriate than the anionic surfactants for oil recovery at the core after 48 hours.
It was reported that thermal and miscible tertiary recovery
techniques were not effective in fractured carbonate reser- voirs [Spinler et al.]. Regarding to special properties of

such reservoirs, surfactant flooding is a promising method for enhanced oil recovery. Alkaline surfactant solutions are used to recover oil from these types of reservoirs by en- hancing the imbibition of water between fracture and the matrix by both wettability alteration and interfacial tension reduction. Aghajari reservoir is one of these types of reser- voirs. Thus, chemical flooding seems to be a suitable me- thod for increasing oil recovery from the reservoir if the surfactant formulation for the oil recovery is properly de- signed and the formulation is properly controlled This work is committed to quantify the effects of the anionic and cationic surfactants on the oil-water IFT reduction in the presence of alkaline as well as the wettability alteration of the reservoir rock from the Aghajari, oil reservoir.
2. EXPERIMENTAL
2.1. Materials The anionic surfactant, cationic surfactant and alkaline used in this study were sodium dodecylben- zene sulfonate (CH3(CH2)11C6H4SO3Na) with molecular
weight 347.48 gr/mol, Critical Micelle Concentration (CMC) at 25 0C is 0.526 wt% and grade ≥ 80% (the structure is shown in Fig. 1). Cationic surfactant C12TAB (C12N(CH3)3Br) with molecular weight 308.34 gr/mol, Crit- ical Micelle Concentration (CMC) at 25 0C is 0.418 wt% and grade ≥ 98% (The structure is shown in Fig. 2). These sur- factants were obtained from Sigma-Aldrich Company. NaOH and Na2CO3 were purchased from Merck Company. The oil and core samples that were used in this study were obtained from Aghajari reservoir that was located in south west of Iran. The oil properties and composition and reser- voir brine composition are given in table 1 to 2. Core data regarding porosity, permeability and compressibility are presented in table 4.
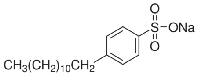
Fig 1: Chemical structure of SDBS
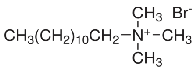
Fig 2: Chemical structure of C12TAB
Table 1: Oil properties

Viscosity (cp) | API | Compressibility (Psi-1) | Density at 25 oC (g/ml) | Asphaltene (wt%) | Oil volume Factor (RB/STB) | Acid number (mg KOH/g oil) |
0.570 | 34.3 | 2.81×10-5 | 0.845 | 0.25 | 1.4 | 1.16 |


2.2. Interfacial tension measurement
The prepared anionic solution with reservoir brine con- tained anionic surfactant at 0.5 wt% (CMC concentration), NaOH at 0 to 0.6 mol/lit and Na2CO3 at 0.5, 1 and 1.5 wt%,
Corresponding Author: E-mail addresses: elyas.golabi@iauo.ac.ir (Elyas Golabi)
1. Department of Petroleum Engineering, Omidiyeh Branch, Islamic Azad University, Omidiyeh, Iran.
2. Department of Chemical Engineering, University of Isfahan, Isfahan, Iran.
3. EOR Research Center, School of Chemical and Petroleum Engineering, Shiraz University, Shiraz, Iran.
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Experimental Study of Anionic and cationic surfactants effects on reduce of IFT and wet-tability alteration in carbonate rock 3
ISSN 2229-5518
while the cationic solution with reservoir brine at 0.1 to 0.5 wt%.
In order to investigate equilibrium phase behavior of oil and surfactant-brine aqueous solution, the prepared solu- tions were mixed with the reservoir oil in a test tube in 1:1 volume ratio. The test tube was shaken and then left for at least five days to achieve phase equilibrium. The IFT be- tween the top oil layer and the bottom brine-surfactant so- lution layer and then was measured using tensiometer SITE 100 model.
Table 4: core data
2.3. Wettability alteration
In order to study of the effects of these two types of surfac-
tants at CMC concentration and different temperature on wettability alteration, the limestone core adapt to parts with dimensions 3.25 × 5 cm. Firstly, they were saturated with the reservoir brine and after that were saturated with oil sample. Each segment was first immersed in brine- surfactant solutions inside the experimental cell and then oil bubble was injected through an orifice at the bottom cell.
In addition, the wettability alteration was verified by com- paring the contact angle between the oil drop and the rock after aging the rock at the specified temperature and con- centrations of the surfactants. The contact angle was meas- ured by imaging system (Figure 3).
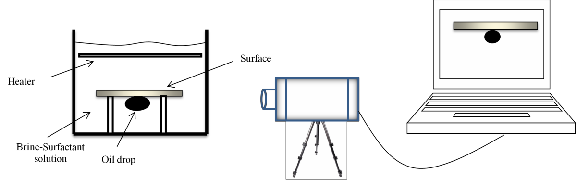
Fig 3: Experimental cell and imaging system for contact angle measurement
Table2: Composition of Oil
Corresponding Author: E-mail addresses: elyas.golabi@iauo.ac.ir (Elyas Golabi)
1. Department of Petroleum Engineering, Omidiyeh Branch, Islamic Azad University, Omidiyeh, Iran.
2. Department of Chemical Engineering, University of Isfahan, Isfahan, Iran.
3. EOR Research Center, School of Chemical and Petroleum Engineering, Shiraz University, Shiraz, Iran.
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Experimental Study of Anionic and cationic surfactants effects on reduce of IFT and wet-tability alteration in carbonate rock 4
ISSN 2229-5518



Component | Mol Fraction |
H2S | 0.13 |
N2 | 1.13 |
CO2 | 3.55 |
CH4 | 3.20 |
C2H6 | 2.08 |
C3H8 | 6.00 |
i-C4H10 | 1.84 |
n-C4H10 | 4.74 |
i-C5H12 | 3.43 |
n-C5H12 | 6.41 |
C6H14 | 12.27 |
C7+ | 55.22 |


Table 3: Composition of Brine
Corresponding Author: E-mail addresses: elyas.golabi@iauo.ac.ir (Elyas Golabi)
1. Department of Petroleum Engineering, Omidiyeh Branch, Islamic Azad University, Omidiyeh, Iran.
2. Department of Chemical Engineering, University of Isfahan, Isfahan, Iran.
3. EOR Research Center, School of Chemical and Petroleum Engineering, Shiraz University, Shiraz, Iran.
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Experimental Study of Anionic and cationic surfactants effects on reduce of IFT and wet-tability alteration in carbonate rock 5
ISSN 2229-5518
3 RESULTS AND DISCUSSIONS
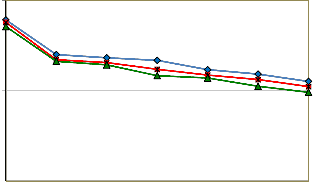
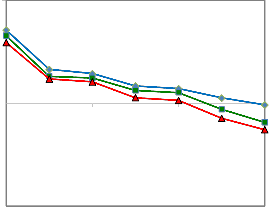
Interfacial tensions between the oil sample and brine- surfactant solution containing 0.5 wt% CMC concentration SDBS surfactant and 0.5 to 1.5 wt% Na2CO3 were measured at 30, 50, and 70 oC for various NaOH concentrations. The results obtained at 30, 50, and 70 oC are shown in Figures 4,
5, and 6 respectively.
Figure 4 indicates that by increasing NaOH concentration,
an initial dramatic decline occurs in IFT until 0.1 mol/liter
and after that reduction of IFT get slightly slope. The min-
imum amount of IFT was obtained at 1.5 wt% Na2CO3 and
0.6 mol/lit NaOH solution at 30 oC. The amount of IFT de-
creased more at 1.5 wt% of Na2CO3 compared to two other concentrations.
Although the trend of IFT reduction at 50 oC (Figure 5) is similar to the IFT behavior at 30 oC (Figure 4), the amount of IFT reduction is more at 50 oC compared to that at 30 oC for all concentrations of Na2CO3.
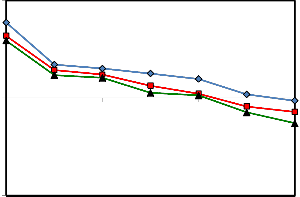
The same results of IFT reduction are shown in Figure 6 for
70 oC. This indicates that the optimum temperature for the
minimum IFT at the SDBS concentration of 0.5 wt% at the
mentioned conditions is 70 oC. As we can see from Figure 7,
tant.
10
1
0.1
T=30 C, Surfactant (SDBS) = 0.5%wt

0.5 %wt Na2CO3  1 %wt Na2CO3
1 %wt Na2CO3
0 0.2 0.4 0.6
NaOH concentration (mol/lit)
the higher concentration of Na2CO3, causes the lower amount of IFT.
Figures 4, 5, and 6 indicate that at 1.5 wt% Na2CO3 the min- imum amount of IFT was obtained at lower concentration of NaOH (0.6 mol/lit) compared with the amount of IFTs obtained at 0.5 wt% of Na2CO3. This suggests that NaOH is more effective than Na2CO3 to reduce IFT, as minimum amount of IFT can be achieved at a lower concentration of NaOH solution when the concentrations of Na2CO3 are rel- atively high.
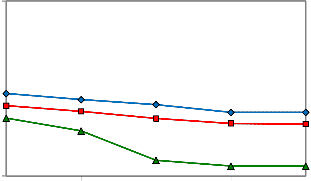
The effect of cationic C12TAB surfactant concentration on the oil-brine IFT was also examined at the temperatures of
30, 50 and 70 oC (Figure 8). By increasing surfactant concen- tration, an initial decrease of IFT to its minimum followed by slight increase was observed. The minimum amount of IFT for this surfactant was obtained for 0.4 wt% surfactant at 70 oC. As can we see at each temperature with 0.4 wt% (CMC concentration) the minimum value of IFT were ob- tained and after that change of IFT relatively stayed stable. Operating temperature of 70 oC was found to be more ap- propriate in comparison with temperatures of 30 and 50 oC, however it doesn’t mean this is definitely the optimum temperature for surfactant as the experiment was not car- ried out at higher temperatures because of reservoir tem- perature is around of 70 oC.
According to Figures 4, 5, 6, and 8, anionic SDBS surfactant
decreases the IFT at CMC concentration in comparison
with C12TAB and therefore it is a more appropriate surfac-
Figure 4: Oil-Water IFT reduction for the solution of 0.5 wt% SDBS, 0.5 to 1.5 wt% of Na2CO3, and 30 oC at different NaOH concentrations
T=50 C, Surfactant (SDBS) = 0.5 %wt

0.5 %wt Na2CO3  1 %wt Na2CO3
1 %wt Na2CO3
10
1 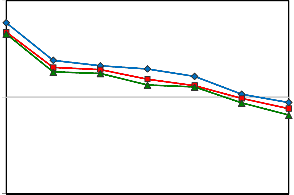
0.1
0 0.2 0.4 0.6
NaOH concentration (mol/lit)
Figure 5: Oil-Water IFT reduction for the solution of 0.5 wt% SDBS, 0.5 to 1.5 wt% of Na2CO3, and 50 oC at different NaOH concentrations
Corresponding Author: E-mail addresses: elyas.golabi@iauo.ac.ir (Elyas Golabi)
1. Department of Petroleum Engineering, Omidiyeh Branch, Islamic Azad University, Omidiyeh, Iran.
2. Department of Chemical Engineering, University of Isfahan, Isfahan, Iran.
3. EOR Research Center, School of Chemical and Petroleum Engineering, Shiraz University, Shiraz, Iran.
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Experimental Study of Anionic and cationic surfactants effects on reduce of IFT and wet-tability alteration in carbonate rock 6
ISSN 2229-5518
T=70 C, Surfactant (SDBS) = 0.5 W t%
0.5 %wt Na2CO3  1 %wt Na2CO3
1 %wt Na2CO3
10
1
0.1
0 0.2 0.4 0.6
NaOH concentration (mol/lit)
Figure 6: Oil-Water IFT reduction for the solution of 0.5 wt% SDBS, 0.5 to 1.5
wt% of Na2CO3, and 70 oC at different NaOH concentrations


T=30 C T=50 C
Surfactant (SDBS)= 0.5 %wt, Na2CO3= 1.5 %wt
10
Surfactant (C12 TAB)


T=30 C T=50 C
1
0.1
0 0.2 0.4 0.6
NaOH concentration (mol/lit)
1
0.1 0.2 0.3 0.4 0.5
C12 TAB (wt%)
Figure 7: Oil-Water IFT reduction for the solution of 0.5 wt% SDBS, 1.5 wt% of
Na2CO3, and 30, 50 and 70 oC at different NaOH concentrations
Figure 8: Oil-Water IFT reduction for the solution of 0.05 wt% C12TAB,
Corresponding Author: E-mail addresses: elyas.golabi@iauo.ac.ir (Elyas Golabi)
1. Department of Petroleum Engineering, Omidiyeh Branch, Islamic Azad University, Omidiyeh, Iran.
2. Department of Chemical Engineering, University of Isfahan, Isfahan, Iran.
3. EOR Research Center, School of Chemical and Petroleum Engineering, Shiraz University, Shiraz, Iran.
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Experimental Study of Anionic and cationic surfactants effects on reduce of IFT and wet-tability alteration in carbonate rock 7
ISSN 2229-5518
3.1. Wettability Alteration Measurements Using Contact Angle
Method
The contact angle measurement was applied to find the tendency of the specified reservoir rock to the oil and water solution. The oil and limestone core were used in this part of study were obtained from Aghajari reservoir.
First part measured the contact angle of reservoir rock in the presence of brine. According to droplet shape and the contact angle between oil droplet and the reservoir rock saturated with oil, the rock demonstrated as oil-wet (58 degree).
Table 5 shows the variation of the reservoir rock wettability
in the presence of brine-surfactant solution containing 0.5 wt% surfactant SDBS, 1.5 wt% Na2CO3 and 0.6 mol/lit
NaOH at 25, 30, 50 and 70 oC. According to table 5, SDBS solution was able to make a remarkable wettability altera- tion of reservoir rock to water-wet. Therefore an increase in temperature had positive effect on the degree of wettability so the higher temperature was seen the most effectiveness on the wettability alteration.
Table 6 demonstrates the reservoir wettability in the pres- ence of brin-surfactant solution composed of 0.4 wt% (CMC concentration) surfactant C12TAB and brine, at 25,
30, 50 and 70 oC. The appearance of droplet and the contact
angle between the droplet and the rock indicates that
C12TAB has remarkably altered the reservoir rock wettabili- ty. Increasing solution temperature also led to more altera-
tion towards to water-wet condition.
According to the contact angle between the oil drop and
the rock (Table 5 and 6), surfactant C12TAB has altered the reservoir rock wettability more than SDBS at the same
temperature.
Table 5: Variation of the contact angle between reservoir rock and droplet of surfactant SDBS
Temperature (oC) | 25 | | 30 | | 50 | | 70 |
Contact Angle at 0.5 wt% | 98 | | 102 | | 109 | | 113 |
Table 6: Variation of the contact angle between reservoir rock and droplet of surfactant C12TAB
Temperature (oC) | 25 | | 30 | | 50 | | 70 |
Contact Angle at 0.4 wt% | 105 | | 108 | | 115 | | 119 |
4. Conclusion
1- At lower concentrations, more IFT reductions were ob- tained by anionic surfactant SDBS compared with C12TAB.
2- Surfactant C12TAB alters the reservoir rock wettability
more than SDBS surfactant at the same temperatures.
3- Increasing of temperature is effective parameter at re-
duction of IFT and wettability alteration toward water wet.
4- In use of SDBS surfactant, presence of alkaline cause
more reduction of IFT.
5- C12TAB at CMC concentration showed minimum IFT
value and after this concentration the variation of IFT was
constant.
5. References
[1] Almalik, M. S., Attia, A. M., 1997. Effects of alkaline flooding on the recovery of Safaniya crude oil of Saudi Arabia, J. Pet. Sci. Eng. 17, 3–4, 367-376
[2] Austad, T., Standnes, D. C., 2003. Spontaneous imbibi- tion of water into oil-wet carbonates, J. Pet. Sci Eng., 39(3-
4), 363-376.
Babadagli, T., 2003. Evaluation of EOR methods for heavy-
oil recovery in naturally fractured reservoirs, J. Pet. Sci Eng
37, 25-37.
[3] Babu, D.R., Hornof, V., Neale, G.H., 1986. Evaluation of
aqueous chemical systems for heavy oil recovery processes,
Fuel, 65, 4–7.
[4] Chen, H.L., Lucas, L.R.,. Nogaret, L.A.D, Yang, H.D.,
Kenyon, D.E., 2000. Laboratory monitoring of surfactant
imbibition using computerized tomography, SPE 59006
Presented at the SPE International Petroleum Conference
and Exhibition in Mexico, Villa Hermosa, Mexico.
[5] Dosher, T.M., Wise, F.A., 1976. Enhanced crude oil re-
covery potential-an estimate. Paper SPE 5800, J. Pet. Tech-
nol. 575-585.
[6] Golabi, E., Seyedeyn Azad, F., Ayatollahi, S. Sh., Hos-
seini, S. N., Akhlaghi, N., 2012. Experimental Study of Wet-
tability Alteration of Limestone Rock from Oil-Wet to Wa-
ter-Wet using Various Surfactants, SPE 157801.
[7] Graue, A., Aspenes, E., Bogno, T. , Moe, R. W., Ramsdal,
J., 2002. Alteration of wettability and wettability hetero-
geneity, J. Pet. Sci Eng 33, 3-17.
[8] Hatiboglu, C. U., Babadagli, T. 2007. Oil recovery by
counter-current spontaneous imbibition: effect of matrix
shape factor, gravity, IFT, oil viscosity, wettability, and
rock type, J. Pet. Sci Eng 59, 106-122.
[9] Hirasaki, G.J., Zhang, D.L., 2004. Surface chemistry of
oil rrecovery from factured, oil-wet, carbonate formations,
SPE J. 9 (2), 151–162.
[10] Li, J., Wong, W., GU, Y., 2004. Dynamic interfacial ten-
sion phenomena and wettability alteration of crude oil-
rock–alkaline-surfactant solution systems, SPE 90207, SPE
Technical Conference, Houston.
[11] Liu, Q., Dong, M., Ma, S., 2006. Alkaline/surfactant
flood potential in western Canadian heavy oil reservoirs,
SPE 99791.
Corresponding Author: E-mail addresses: elyas.golabi@iauo.ac.ir (Elyas Golabi)
1. Department of Petroleum Engineering, Omidiyeh Branch, Islamic Azad University, Omidiyeh, Iran.
2. Department of Chemical Engineering, University of Isfahan, Isfahan, Iran.
3. EOR Research Center, School of Chemical and Petroleum Engineering, Shiraz University, Shiraz, Iran.
IJSER © 2012
http://www.ijser.org
The research paper published by IJSER journal is about Experimental Study of Anionic and cationic surfactants effects on reduce of IFT and wet-tability alteration in carbonate rock 8
ISSN 2229-5518
[12] Lorenz, P.B., Peru, D.A., 1989. Guidelines help select reservoir for NaHCO3 EOR, Oil & Gas J., 87, 37, 53-57.
[13] Martin, F.D., Oxley, J.C., Lim, H., 1985. Enhanced re- covery of a "J" sand crude oil with a combination of surfac- tant and alkaline chemicals, SPE 14293.
[14] Mohanty, K.K., 2003. Dilute surfactant methods for carbonate formations, Quarterly Progress report, DE-FC26-
02NT 15322, U.S. Department of Energy.
[15] Morrow, N. R., 1990. Wettability and its effect on oil
recovery, J. Pet. Technol., 1476-1484.
[16] Nedjhioui, M., Moulai-Mostefa, N., Morsli, A., Bens-
maili, A., 2005. Combined effects of poly-
mer/surfactant/oil/alkali on physical chemical properties, Desalination 185, 1-3, 543–550.
[17] Ramakrishnan, T. S., Wasan, D. T., 1983. A model for interfacial activity of acidic crude oil/caustic systems for alkaline flooding, SPEJ 23:4, 602-612.
[18] Somasundaran, P., Zhang, L. 2006. Adsorption of sur- factants on minerals for wettability control in improved oil recovery processes, J. Pet. Sci Eng 52, 198-212.
[19] Spinler, E.A., Zornes, D.R. Tobola, D.P., Moradi- Araghi, A., 2000. Enhancement of oil recovery using a low concentration of surfactant to improve spontaneous and forced imbibition in Chalk, SPE 59290.
[20] Standnes, D. C., Austad, T., 2003. Nontoxic low-cost amines as wettability alteration chemicals in carbonates, J. Pet. Sci Eng 39, 431-446.
[21] Tadros, T., 2005. Applied Surfactants: Principles and applications, Phase behavior of surfactant systems (Chapter
3), John Wiley & Sons, United Kingdom.
[22] Touhami, Y., Rana, D., Neale, G.H., Hornof, V., 2001.
Study of polymer-surfactant interactions via surface ten-
sion measurements, Colloid Polym. Sci. 279, 297–300
[23] Wardlaw, N. C., 1996. Factors effecting oil recovery
from carbonate reservoirs and predication of recovery, in
G. V. Chilingarian, S. J. Mazzullo, H. H. Rieke, eds., in car-
bonate reservoir characterization: A Geologic-Engineering
Analysis, Part II: Elsevier, New York, Development in Pe-
trolium Science 44, 867-903.
[24] Xie, X., Weiss, W.W., Tong, Z., Morrow, N.R., 2005.
Improved oil recovery from carbonate reservoirs by chemi-
cal stimulation, SPE 89424, 276-285.
[25] Yu, L. 2009. Spontaneous imbibition of seawater into
preferentially oil-wet chalk cores experiments and simula-
tion, J. Pet. Sci Eng 66, 171-179.
Corresponding Author: E-mail addresses: elyas.golabi@iauo.ac.ir (Elyas Golabi)
1. Department of Petroleum Engineering, Omidiyeh Branch, Islamic Azad University, Omidiyeh, Iran.
2. Department of Chemical Engineering, University of Isfahan, Isfahan, Iran.
3. EOR Research Center, School of Chemical and Petroleum Engineering, Shiraz University, Shiraz, Iran.
IJSER © 2012
http://www.ijser.org