International Journal of Scientific & Engineering Research, Volume 4, Issue ŗŖ, ber 2013 ISSN 2229-5518
Evaluation of Physio-chemical and Thermal properties of Soy Protein Concentrate and Different Binary Mixtures Based Graft Copolymers
Jaspreet Kaur, Balbir Singh Kaith, Rajeev Jindal*
Abstract— Present investigation deals with the modification of biopolymer to improve physio-chemical and thermal properties. In air synthesis of graft copolymers of soy protein concentrate (SPC) with vinyl monomer mixtures of ethylmethacrylate (EMA) with methylmethacrylate (MMA), methylacrylate (MA) and ethylacrylate (EA) was carried-out using ascorbic acid/ potassium persulphate (AAc/ KPS) redox initiator system. Graft copolymers formed were characterized using techniques like FTIR, SEM and XRD. Initial optimization of different reaction parameters was carried out for graft copolymerization of principal monomer EMA onto soy protein concentrate to get maximum graft yield (134.12%). The maximum graft yields with binary monomer mixtures; EMA+MMA, EMA+EA and EMA+MA were 120.54%, 142.38% and 293.58%, respectively. The graft copolymers were studied for thermal stability and were found to show high thermal stability. Modified protein was found to show improved chemical resistance toward acid and base. Moreover, grafted protein also showed enhanced moisture retardance.
Index Terms— Soy protein concentrate, graft copolymerization, binary monomer mixture, moisture resistance, thermal stability.
—————————— ——————————
IJSER
573
I
INTRODUCTION
ncreased consumption rate of petroleum based polymers resulted in the problem of waste management and envi- ronmental hazards. Polymers from natural resources have offered scientists a possible solution to the problem associated with traditional non-biodegradable polymers. Biopolymers from natural resources such as starch, cellulose and proteins have been regarded as an alternative material due to their re- newability, abundance and biodegradable nature [1], [2], [3]. Various vegetable proteins like corn, silk, wheat and soy have been investigated for various applications [4], [5], [6]. Due to abundance and relatively low cost soy is attracting much at- tention. Soy protein obtained from plant Glycine max contains about 20% of oil and 50% of proteins [7]. Among the commer- cial available varieties of soy i.e. soy flour, soy protein concen- trate and soy protein isolate, soy protein concentrate (SPC) contains about 65% proteins and 18% carbohydrates. SPC can be obtained from defatted soy flour by removal of soluble car- bohydrates [8]. Soy proteins have been used by scientists for developing adhesives, plastics, composites and elastomers [9],[10], [11] but the problem of low mechanical strength and high moisture absorbance required the modification of soy protein. Soy proteins have been modified using alkali, urea, guanidine hydrochloride and sodium dodecylsulphate [12],
————————————————
Jaspreet Kaur Bhatia is assistant Professor at Lyallpur Kahalsa College, Jal- andhar, Punjab, India PH: 0181-2465104 E. Mail: jaspreetbhatia81@gmail.com
Balbir is Kaith is Professor at Department of Chemistry, National Institute of Technology, Jalandhar, Punja, India, PH: 9780684883 E. Mail: bskaith@yahoo.co.in
Rajeev Jindal is Associate Professor at Department of Chemistry, National Institute of Technology, Jalandhar, Punja, India, PH: 09779914264 E.Mail: r_jindal@nitj.ac.in
[13], [14]. Cross linking [15], acylation [16], blending with oth- er polymers [17] and enzymatic modifications [18] are the oth- er methods to modify the soy proteins.
Graft copolymerization is one of the techniques employed for the incorporation of desired properties into the backbone [19], [20], [21]. Graft copolymerization of different vinyl monomers onto natural polymers like starch [22], [23], chitosan [24], [25], casein [26], silk [27] and wool [28] has been reported. However, only a very few authors have studied soy protein isolate graft copolymers [29], [30], whereas no report was found about the graft copolymerization onto soy protein concentrate. Graft copolymerization of binary monomer mixtures is of special importance as it results in the incorporation of properties of both monomers [31], [32], [33]. Grafting of binary monomer mixture inducts the tailor-made properties for specific applications.
In the present investigation the graft copolymerization of
binary vinyl monomer mixtures was carried-out onto SPC in aqueous medium using ascorbic acid (AAc) and potassium persulphate (KPS) as an initiator system. Graft copolymers formed were evaluated for their thermal behaviour. The behaviour of graft copolymers towards acid, base and moisture were studied to find out the effect of modification of soy protein concentrate on its physio-chemical properties.
EXPERIMENTAL
Materials
Ethylmethacrylate (EMA), methylmethacrylate (MMA),
IJSER © 2013
http://www.ijser.org
ethylacrylate (EA) and methylacrylate (MA) used were ob- tained from e-Merck chemicals. Ascorbic acid (AAc) and po- tassium persulphate (KPS) were procured from S.D. Fine chemicals ltd.
Synthesis of graft copolymers
Soy protein concentrate (SPC) was obtained from defatted soy flour after removal of sugar and other minor constituents us- ing aqueous alcohol process [34]. SPC (0.5 g) was immersed in known amount of distilled water in reaction flask. A definite ratio of AAc - KPS was added to the reaction flask followed by drop by drop addition of monomer. The reaction was carried- out for specific time interval at a definite temperature. Homopolymer formed was removed on soxhlet extraction with acetone for 24 hours. Graft copolymer obtained was dried at 40 oC to constant weight. % graft yield (Pg), was cal- culated as [35] :
Percentage graft yield (Pg)= [ (W2 –W1)/W1] x 100
where W1 = initial wt. of sample and W2 = wt. of sample (after
ated SO4-* free radical ions [Eq. 1] which in the aqueous me- dium gave rise to OH* free radicals [Eq. 2]. Ascorbic acid re- leased an electron to OH* and get converted to ascorbic acid free radical [III, Eq. 3]. Persulphate ions further generated SO4-* in the presence of ascorbic acid free radicals [Eq. 4]. Thus, ascorbic acid free radicals and SO4-* free radical ions act as the primary free radicals to initiate the graft copolymeriza- tion by generating the active sites on the monomer and the backbone [Eqs. 5-7]. Further propagation resulted in the grow- ing active c hains [Eq. 8] and graft copolymers are formed by reactions between active backbone and growing monomer chains [Eqs 9-11]. The reaction between two live chains resulted in the termination of growing chains [Eq. 11 or 12]. Ascorbic acid free radicals also play important role in termination by inter- acting with the active chains [Eqs. 13 and14].
Initiation
removal of homopolymer).
S O 2- -
SO -* HSO -
(1)
AH2 +

2 8 AH + 4 + 4
Characterization
( I) (II)
-* H O OH* HSO -
IR spectra were recorded with Perkin Elmer Fourier transform

SO4 + 2
+ 4 (2)
infrared (FTIR) spectrophotometer using KBr pellets. X-Ray

AH- + OH*
AH*
+ OH-
(3)
diffraction studies were performed on XPERT-PRO X-Ray
( II )
( III )
diffractrometer at 40 kV Iand 35JmA. The Ssamples were ER
scanned from 50 to 500 at 2θ scale using Cu Kα X-Ray radia-

2-
AH* + S2O8
4
A + SO -* +
4
HSO -
(4)
4
tions of 1.5418 A0. Scanning Electron Microscopic studies of SPC and its graft copolymers were carried-out on electron mi- croscope machine LEO 435 VP. TGA, DTA and DTG studies
(III)
Propagation
(IV)
[X* = AH* OR SO -* ]
were carried-out in the temperature range of 50O –700 OC at a
SPC +
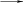

X* SPC* + X H
(5)
heating rate of 10 OC/minute on TG/DTA 6300, SII EXSTAR 6000.


M + X* X M*
(6)
Acid and base resistant studies
SPC

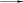
+ X M* SPC M*
+ X H
(7)
Acid resistance of the grafted vis-à-vis ungrafted sample was
SPC M* +
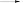


nM SPC (M)n M*
(8)
studied by putting a known weight of sample (0.1 g) in 25 ml
SPC*


+ nM SPC M(n-1) M*
(9)
1N HCl and the weight of each sample was noted at the inter-
X M* nM X M M*
(10)
val of every 6 hours till a constant weight was obtained. Simi- larly, base resistance was studied with 1N NaOH. % weight




+
Termination
(n)
loss was calculated as [36] :


SPC M

M* X M




M* SPC M X
% Wt. loss = [(Wi -Wf) / Wi] x 100
where, Wi = initial wt. of sample; Wf = final wt. of sample.
(n)
+ (n)
(2n+2)
Graft co-polymer
(11)
Moisture resistant studies








SPC M(n-1) M* + SPC M(n-1) M* SPC M(n-1) M2 M(n-1) SPC
(12)
Moisture absorbance studies were carried out as per ASTM D5229 standard. Percentage moisture absorbance was found


X M(n) M* +





*M M(n) X X M(2n+2) X
Hompolymer
(13)
by placing a known weight (Wi) of dry grafted and ungrafted samples in the appropriate environment having %RH= 80 for 24 hours. Final weights (Wf) of the samples were taken and %


SPC M(n)


M* +X* SPC M

(n+1) X
(14)
moisture absorbance was calculated as:
% Moisture absorbance = [(Wf -Wi) / Wi] x 100
where, Wi = initial wt. of sample; Wf = final wt. of sample..
RESULTS AND DISCUSSION
Mechanism
Potassium persulphate in the presence of ascorbic acid gener-
Where SPC = Soy protein concentrate; M = Monomer ; AH2 = Ascorbic acid
Effect of binary mixture concentration on percentage grafting
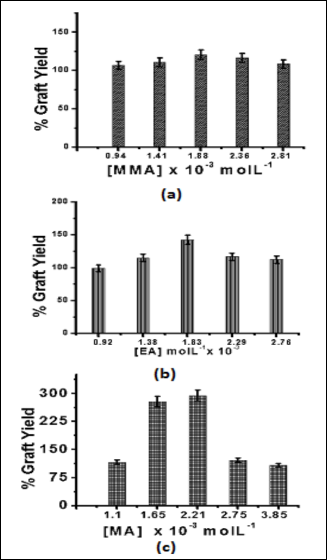
In case of graft copolymerization of EMA onto soy protein concentrate maximum graft percentage was found to be 134.12% and optimum conditions for maximum graft percent- age were: reaction time (minutes), 120; reaction temperature
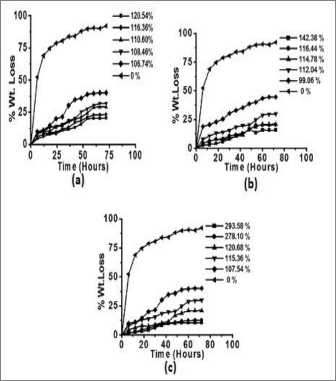
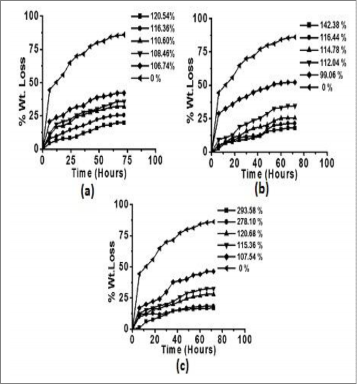
(OC), 40; solvent (ml), 100; AAc : KPS (molar ratio), 1:1.25; EMA concentration (mol L-1), 2.43x 10-3 and pH, 8.0 [37]. Graft- ing of different binary mixtures, EMA+MMA, EMA+EA and EMA+MA, using EMA as a principal monomer under pre- optimized reaction conditions showed graft percentage of 120.54%, 142.38% and 293.58%, respectively (Figs. 1-3).
polymer chains and as a result higher percentage of grafting is observed. Further, it is supported by the higher r1 value result- ing in higher copolymer formation and suppression of homopolymerization. In case of EMA+EA binary mixture though r1 value (0.91) is lower than r2 value (1.06) which ex- hibits the higher reactivity among the similar monomeric units giving rise to homopolymerization yet a higher graft yield was found. This can be explained on the basis that percentage graft yield also depends on rate of free radical transfer towards the backbone and monomeric moieties thereby resulting in more generation of free radical sites and hence higher graft yield [39]. Moreover, it also depends upon rate of propagation and termination and gave rise to higher graft yield. The low graft yield with EMA+MMA binary mixture was again due to ten- dency of MMA and EMA units to react with their own mono- mer radicals and resulted in more homopolymerization in place of copolymerization. Thus, gave rise to low graft yield. This is further supported by monomer reactivity ratios (r1=0.798, r2 =1.058) [40].
FTIR analysis
IJSER
FT-IR spectrum of SPC showed a broad peak at 3284.4 cm- 1 due to free –OH and –NH groups, peak at 1653.3 cm-1 due to C=O stretch of amide group (amide-I) and a peak at 1540.4 cm-1 due to N-H bending (amide-II) (Fig. 2a). SPC-g-
poly(EMA-co-MMA) showed peak at 1731.9 cm-1 due to ester carbonyl group of MMA along with peaks at 1242 cm-1 and
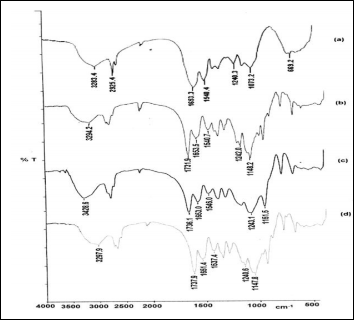
1148.2 cm-1 due to C-O stretchings (Fig. 2b). SPC-g-poly(EMA- co-EA) exhibited peaks at 1736.1 cm-1 due to C=O group of EA and at 1243.1cm-1 and at 1151.5cm-1 due to C-O stretch (Fig. 2c). In case of SPC-g-poly(EMA-co-MA) peaks due to C=O stretch of MA was observed at 1737.9 cm-1 and due to C-O stretch were obtained at 1240.6 cm-1 and 1147.8cm-1 (Fig. 2d). The presence of additional peaks in the IR spectra of graft gave evidence of grafting on protein backbone.
Fig. 1 Effect of concentration of (a) MMA (b) EA (c) MA in binary mixture on Pg; [EMA] = 2.39 x 10-3 Mol L-1
It is evident that in case of binary mixtures consisting of EMA with MA and EA higher percentage grafting has been found as compared to EMA alone (134.12%). The higher percentage of grafting in case of these binary monomer mixtures can be explained by the fact that addition of electron acceptor mono- mers increased the reactivity of EMA towards graft copoly- merization. The reactivity ratio values of these monomer mix- tures were calculated with the help of Price-Alfrey’s Q-e ap- proach [38]. The reactivity ratio values in case of binary mon- omer mixture EMA+MA were found to be r1 = 1.47, r2 = 0.65. Low r2 value shows that MA reacts with EMA in preference to their monomeric units thus producing more of growing co-
Fig. 2 FTIR spectra of (a) SPC (b) SPC-g-poly(EMA-co-MMA) (c) SPC-g- poly(EMA-co-MA) (d) SPC-g-poly(EMA-co-MA)
XRD Studies
XRD pattern of soy protein showed the amorphous nature of soy protein. However, on grafting with vinyl monomers, crystallinity of the backbone sample was found to increase which is evident from increase in coherent length along with increase in d-spacing values and increase in Pg. The experi- mental data of XRD was computed for coherent lengths by using Scherrer equation [41]:
L = 0.9 λ / B cosθ
where λ is the wavelength of X-Ray radiations for Cu-Kα, equal to 1.5418 AO. θ is glancing angle in radians and B is the width of peak at half of the maximum intensity. Coherent lengths and d-spacings at different 2θ scale in case of SPC and grafted protein concentrate with different % graft yields are given in Table 1. Thus, with increase in Pg, anisotropy kept on increasing and SPC became more crystalline in nature on in- corporation of monomer chains with graft copolymerization process.
through covalent bonding.
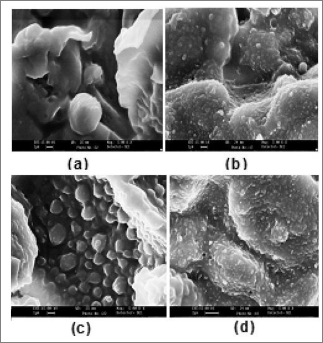
TABLE 1
. X-RAY DIFFRACTION STUDIES OF SPC AND GRAFTED SPC WITH
BINARY MONOMER MIXTURES
Sample | |
Pg | 2θ(o) | d- Spacing | Coherent length (L) |
Backbone |
-- | |
19.70 |
4.5067 |
14.15 |
| 120.54 116.36 110.60 | 17.30 17.48 18.13 | 5.1270 5.0745 4.8925 | 40.69 36.65 26.91 |
| | | | |
SPC-g-poly(EMA- | | | | |
co-MMA) | 108.46 | 18.13 | 4.8925 | 24.46 |
| 106.74 | 18.68 | 4.7507 | 23.75 |
| 142.38 116.44 114.78 | 17.30 18.57 18.66 | 5.1255 4.7772 4.7533 | 33.26 26.86 26.67 |
| | | | |
SPC-g-poly(EMA- | | | | |
co-EA) | 112.04 | 18.76 | 4.7294 | 23.02 |
| 99.06 | 19.08 | 4.6525 | 22.98 |
| 293.58 278.10 | 18.80 18.83 | 4.7212 4.6228 | 50.64 40.45 |
| | | | |
SPC-g-poly(EMA- | 120.68 | 18.99 | 4.6285 | 31.21 |
co-MA) | 115.36 | 19.68 | 4.5810 | 20.17 |
| 107.54 | 19.77 | 4.5103 | 17.38 |
IJSER
Fig. 3 Scanning Electron Micrograph of (a) SPC (b) SPC-g-poly(EMA-co-MMA)
(c) SPC-g-poly(EMA-co-EA) (d) SPC-g-poly(EMA-co-MA)
3.6 Thermal Studies
Thermogravimetric analysis of grafted and ungrafted soy pro- tein concentrate was carried-out as a function of % wt. loss vs temperature. Soy protein has a three dimensional structure involving sequence of amino acids. Covalent bonds present in soy proteins are either peptide bonds between amino acid res- idues or disulphide bonds. Proteins also have electrostatic- hydrophobic interactions and hydrogen bonding.
In case of SPC two phase decomposition was found in tem- perature range of 2180 - 501.40C with 62.7% wt. loss and 501.40- 561.70C with 21.3% wt. loss (Table 2). Initial decomposition corresponds to elimination of water and dissociation of qua- ternary structure of proteins. First phase of decomposition involved two stages, one in the temperature range of 2180- 358.90C (43.7% wt. loss) due to cleavage of peptide bonds of amino acid residues and second in the temperature range of 358.90-501.40C (19.0% wt. loss) corresponding to dissociation of S-S, O-O and O-N bonds. Second phase decomposition in- volved complete decomposition of proteins, resulting in the liberation of various gases like CO, CO2, and NH3 [42]. In case of grafted proteins with binary monomer mixtures, EMA+MMA, EMA+EA and EMA+MA, two phase thermal decomposition was found. SPC-g-poly(EMA-co-MMA) showed thermal decomposition in the temperature range of
Scanning Electron Microscopy (SEM)
A clear cut morphological differentiation has been observed in the scanning electron micrograph of SPC, SPC-g-poly(EMA- co-MMA), SPC-g-poly(EMA-co-EA) and SPC-g-poly(EMA-co- MA) (Figs. 3a-d). The heterogeneity of soy protein concentrate was found to increase on graft copolymerization. This exhibit- ed the incorporation of monomer chains onto SPC backbone
262.80 - 388.80C (76.7% wt. loss) and 388.80- 595.20C (12.2% wt.
loss). Thermal decomposition stages observed in case of SPC-
g-poly(EMA-co-EA) are in the temperature range of 241.50-
358.70C (79.1% wt. loss) and 358.70- 597.60C (14.7% wt. loss).
In case of SPC-g-poly(EMA-co-MA) thermal decomposition was observed at 241.90- 398.80C (72.9% wt. loss) and 398.90-
589.1OC (19.3% wt. loss).
TABLE 2

THERMAL STUDIES OF SPC AND GRAFTED SPC WITH BINARY MONOMER MIXTURES
| |
1st stage | TGA |
2nd stage | | DTA
Exothermic peaks at differ- | DTG
Decomposition |
Sample | IDT (0C) | Decomposi- tion, 0C (% wt. loss) | Decomposi- tion, 0C (% wt. loss) | FDT (0C) | ent decom- position Tem- perature (µV) | Temperature, ˚C (Rate of wt. loss in mg/min) |
SPC |
218.0 |
218.0-501.0 (62.7%) | |
501-561.7 (21.3%) |
561.7 | 329.2(29.2µV), 500.4 (152.4µV) 503.8 (98.6 µV) | 63.2(0.0841), 320.5(0.439) 496.9 (0.884) |
SPC-g-poly(EMA-co- MMA) |
262.8 | 262.8-388.8 (76.7%) | | 388.8-595.2 (12.2%) |
595.2 | 371.50C (18.28µV) |
366.4 (0.718) |
SPC-g-poly(EMA-co-EA) |
241.5 | 241.5-358.7 (79.1%) | | 358.7- 597.6 (14.7%) |
597.6 |
397.7 (64.6µV) |
391.0 (2.258) |
SPC-g-poly(EMA-co-MA) |
241.9 | 241.9- 398.8 (40.4%) | | 398.9-589.1 (48.3%) |
589.1 |
389.8(92.3µV) |
389.3 (2.659) |
IJSER
The initial decomposition temperatures as well as final de- composition temperature of grafted proteins were found to be higher than that of SPC. Thus, the grafted soy protein was found thermally more stable than ungrafted PC. This in- crease in thermal stability was due to incorporation of polyvi- nyl chains onto SPC backbone through covalent bonding.
In case of DTA studies, SPC showed three exothermic peaks at 329.20C (29.2µV), 500.40C (152.4µV) and 503.80C (98.6 µV) cor-
responding to TGA decomposition stages of 2180-358.90C, 358.90-501.40C and 501.40-561.70C. Whereas, SPC-g-poly(EMA-
co-MMA), SPC-g-poly(EMA-co-EA) and SPC-g-poly(EMA-co- MA) showed their respective exothermic decompositions at 371.50C (18.28µV), 397.70C (64.6µV) and 389.80C (92.3µV), re-
spectively.
Thermal decomposition in case of DTG analysis of SPC,
showed exothermic peaks at 63.20C, 320.50C and 496.90C with
0.0841mg/min, 0.439 mg/min and 0.884 mg/min weight loss,
respectively. Whereas, in case of SPC-g-poly(EMA-co-MMA), SPC-g-poly(EMA-co-EA) and SPC-g-poly(EMA-co-MA) exo-
thermic peaks were found at 366.40C, 391.00C and 389.30C with 0.718mg/min, 2.258mg/min and 2.659mg/min weight loss, respectively (Table 2).
Acid and Base Resistant Studies
Acid and base resistance of grafted protein concentrate was found to higher as compared to ungrafted SPC. Moreover, acid and base resistance was found to increase as % grafting
increased (Figs. 4-5). This could be due to fact that poly(EMA), poly(MMA), poly(MA) and poly(EA) chains being highly hy- drophobic in nature, possess less chemical affinity for both acid and base. Thus, incorporation of hydrophobic monomer chains onto SPC backbone through graft copolymerization resulted in increased acid and base resistance. [43].
Fig. 4 Acid Resistance Studies of (a) SPC (b) SPC-g-poly(EMA-co-MMA) (c)
SPC-g-poly(EMA-co-MA) (d) SPC-g-poly(EMA-co-MA
TABLE 3
MOISTURE RESISTANCE STUDIES OF SPC AND GRAFTED SPC WITH
BINARY MONOMER MIXTURES
|
Sample | |
Pg |
% Weight Gain |
|
Backbone |
-- | | 62.225 |
| | 120.54 116.36 | 30.345 32.647 |
| | | |
| | 110.60 | 34.778 |
| SPC-g-poly(EMA-co-MMA) | 108.46 | 37.846 |
| | 106.74 | 39.042 |
| | 142.38 116.44 | 23.778 31.806 |
| | | |
| | 114.78 | 32.963 |
| SPC-g-poly(EMA-co-EA) | 112.04 | 36.775 |
| | 99.06 | 42.156 |
| | 293.58 278.10 | 20.014 21.965 |
| | | |
|
SPC-g-poly(EMA-co-MA) | 120.68 | 24.845 |
| | 115.36 | 33.856 |
| | 107.54 | 36.887 |
IJSER
mer has been found to be thermally more stable in comparison to backbone. These thermally and chemically stable products with high moisture retardance are important components for
Fig. 5 Base resistance studies of (a) SPC (b) SPC-g-poly(EMA-co-MMA) (c)
SPC-g-poly(EMA-co-MA) (d) SPC-g-poly(EMA-co-MA
Moisture Resistant Studies
It was found that grafting of binary monomer mixtures on soy protein concentrate backbone had made impact on the mois- ture absorbance behaviour of protein. SPC showed 62.2% wt. gain whereas SPC-g-poly(EMA-co-MA) showed 20% wt. gain in 24 hours. (Table 3). This was due to incorporation of hy- drophobic poly(EMA), poly(MMA), poly(MA) and poly(EA) chains onto sites vulnerable for moisture absorbance, thereby resulting in moisture retardancy. Moisture absorbance of soy protein concentrate decreased with increase in % grafting as with the increase in Pg more and more active sites, responsible for moisture absorbance, get incorporated with hydrophobic monomers [43].
4 CONCLUSION
The grafting of each monomer mixture of EMA+MMA, EMA+EA and EMA+MA onto soy protein concentrate in the presence of ascorbic acid/potassium persulphate as a redox initiator system has been found to influence the physico- chemical, thermal as well as morphological properties of pro- tein backbone. The incorporation of poly(EMA+MMA), poly(EMA+EA) and poly(EMA+MA) chains onto soy protein concentrate resulted in higher resistance towards the attack of acid and base. Moreover, lesser moisture absorbance has been observed in case of grafted protein. Further, the graft copoly-
various industrial applications.
REFERENCES
[1] J. F. Morsyleide, R. B. Chiou, E. S. Medeiros, D. F. Wood, L. H. C. Mattoso, W.
J. Orts and S. H. Imam, “Biodegradable Composites based on Starch/EVOH/glycerol Blends and Coconut Fibres”, J. Appl. Polym. Sci., vol. 111, no. 2, pp. 612–618, Jan. 2009.
[2] Z. Wang, O. Hu, X. Dai, H. Wu, Y. Wang and J. Shen, “Preparation and Char- acterization of Cellulose Fiber/Chitosan Composites”, Polym. Comp., vol. 30, no. 10, pp. 1517-1522, Oct. 2009.
[3] O. Orliac and F. Silvestre, “New Thermo-Molded Biodegradable Films Based on Sunflower Protein Isolate: Aging and Physical Properties”, Macromol. Sym., vol. 197, no. 1, pp. 193-206, July 2003.
[4] Y. Chen, S. Liu and G. Wang, “Synthesis and Physicochemical Properties of Graft Copolymer of Corn Starch and Acrylamide”, Polym. Comp., vol. 28, no. 1, pp. 47-56, Feb. 2007.
[5] S. O. Han, S. M. Lee, W. H. Park and D. Cho, “Mechanical and Thermal Prop- erties of Waste Silk Fiber-Reinforced Poly(Butylene Succinate) Biocomposites”, J. Appl. Polym. Sci., vol. 100, no. 6, pp. 4972–4980, Jun 2006.
[6] T. Takahashi, K. Hirayama, N. Teramoto and M Shibata, “Biocomposites composed of epoxidized soybean oil cured with terpene-based acid anhy- dride and cellulose fibres”, J. Appl. Polym. Sci., vol. 108, no. 3, pp. 1596–1602, May 2008.
[7] R. Kumar, V. Choudhary, S. Mishra, I. K. Verma and B. Mattiason, “Adhe- sives and Plastics Based on Soy Protein Products”, Ind. Crops Prod., vol. 16, no. 3, pp. 155-172, Nov. 2002.
[8] S. N. Swain, S. M. Biswal, P. K. Nanda and P. L. Nayak, “Biodegradable Soy-
Based Plastics: Oppurtunities and Challenges”, J. Polym. Env., vol. 12, no. 1, pp. 35-42, Jan. 2004.
[9] W. Y. Choi, C. M. Lee and H. J. Park, “Development of Biodegradable Hot- Melt Adhesive Based on Poly-e-Caprolactone and Soy Protein Isolate for Food Packaging System”, LWT-Food Sci. Tech., vol. 39, no. 6, pp. 591–597, Aug. 2006.
[10] C. M. Vaz, L. A. Graaf, R. L. Reisa and A. M. Cunha, “In Vitro Degradation
Behaviour of Biodegradable Soy Plastics: Effects of Crosslinking with Glyoxal and Thermal Treatment”, Polym. Deg. Stab., vol. 81, no. 1, pp. 65–74, Jan. 2003.
[11] V. Soldi, R. M. D. Soares and F. F. Scremin, “Thermal Stability of Biodegrada- ble Films Based on Soy Protein and Corn Starch”, Macromol. Symp., vol. 229, no. 1, pp. 258-265, Nov. 2005.
[12] U. Kalapathy, N. S. Hettiarachchy, D. Myer and K. C. Rhee, “Alkali-Modified
Soy Proteins: Effect of Salts and Disulfide Bond Cleavage on Adhesion and Viscosity”, J. Am. Oil Chem. Soc., vol. 73, no. 8, pp. 1063- 1066, Aug. 1996.
[13] W. N. Huang and X. Sun, “Adhesive Properties of Soy Protein Modified by Urea and Guanidine Hydrochloride”, J. Am. Oil Chem. Soc., vol. 77, no. 1, pp. 101-104, Jan. 2000.
[14] Z. K. Zhong and X. S. Sun, “Thermal and Mechanical Properties and Water
Absorption of Sodium Dodecyl Sulfate Modified Soy Protein (11 S)”, J Appl. Polym. Sci., vol. 81, no. 1, pp. 166-175, July 2001.
[15] Y. Wang, X. Mo, X. S. Sun, D. Wang, “Soy Protein Adhesion Enhanced by Gluteraldehyde Crosslink” J. Appl. Polym. Sci., vol. 104, no. 1, pp. 130-136, April, 2007.
[16] K. L. Frazen and J. E. Kinsella, “Functional Properties of Succinylated and
Acetylated Soy Protein”, J Agric. Food Chem., vol. 24, no. 4, pp. 788-795, July 1976.
[27] Y. Song, Y. Jin, D. Wei and J. Sun, “Graft Copolymerization of Methyl Meth- acrylate onto Silk Sericin Initiated by Ceric Ammonium Nitrate”, J Macromol. Sci. A Pure appl. Chem., vol. 43, no. 6, pp. 899–907, 2006.
[28] E. Said and H. E. Mosallamy, “Graft Copolymerization of Methyl Methacry-
late onto Wool Fibers Initiated by NaBO3/Fe(II) Redox System”, J Macromol. Sci. A Pure appl. Chem., vol. 39, no. 6, pp. 609-622, 2002.
[29] D. Xi, C. Yang, X. Liu, M. Chen, C. Sun and Y. Xu, “Graft Polymerization of
Styrene on Soy Protein Isolate” J. Appl. Polym. Sci., vol. 98, no. 3, pp. 1457–1461, Nov. 2005.
[30] C. Yang, X. Song, C. Sun, M. Chen, Y. Xu, X. Liu and Z. Ni, “Graft Copoly- merization of Soybean Protein Isolate and Methacrylic Acid”, J. Appl. Polym. Sci. ,vol. 102, no. 4, pp. 4023–4029, Nov. 2006.
[31] K. C. Gupta and K. Khandekar, “Acrylamide-Methylmethacrylate Graft Copolymerization onto Cellulose Using Ceric Ammonium Nitrate” J. Macromol. Sci. A Pure appl. Chem. vol. 40, no. 2, pp. 155–179, 2003.
[32] B. S. Kaith, A. Chauhan, A. S. Singha and D. Pathania, “Induction of Morpho- logical Changes in Hibiscus Sabdariffa Fiber on Graft Copolymerization With Binary Vinyl Monomer Mixtures”, Inter. J. Polym. Anal. Charac., vol. 14, no. 3, pp. 246–258, 2009.
[33] S. Rattan S, J. Maitra, B. N. Misra and I. Kaur, “Radiation Induced Graft Co- polymerization of Vinyl Monomers and Their Binary Mixture onto Rayon Fi- bre”, J. Appl. Polym. Sci., vol. 108, no. 5, pp. 3104–3113, June, 2008.
[34] D. Kaplan, “Biopolymers from renewable resources” Springer-verlag Berlin Hei-
delberg, New York, 1998.
[35] E. Princi, S. Vicini., E. Pedemonte, A. Mulas, E. Franceschi, G. Luciano and V.
IJSER
[17] Z. Zhong and S. X. Sun, “Properties of Soy Protein Isolate/Poly(Ethylene-Co-
Ethylacrylate-Co-Maleic Anhydride) Blends”, J. Appl. Polym. Sci., vol. 88, no. 2, pp. 407-431, April 2003.
[18] R. Kumar, V. Choudhary, S. Mishra and I. K. Verma, “Enzymatically Modi-
fied Soy Protein Part I. Thermal Behaviour”, J. Therm. Anal. Cal., vol. 75, no. 3, pp. 727-738, March, 2004.
[19] J. L. Eguiburu, M. J. F. Berridi and J. S. Roman, “Graft Copolymers for Bio- medical Applications Prepared by Free Radical Polymerization of Poly(L- Lactide) Macromonomers with Vinyl and Acrylic Monomers”, Polymers, vol. 37, no. 16, pp. 3615-3622, Aug. 1996.
[20] I. H. Mondal, Y. Uraki, M. Ubukata and K. Itoyama, “Graft Polymerization of Vinyl Monomers onto Cotton Fibres Pretreated with Amines: Mechanical Property and Moisture Sorption”, Cellulose, vol. 15, no. 4, pp. 581–592, Aug. 2008.
[21] S. Kalia, B. S. Kaith, S. Sharma and B. Bhardwaj, “Mechanical Properties of Flax-g-Poly(Methylacrylate) Reinforced Phenolic Composites” Fibres Polym., vol. 9, no. 4, pp. 416-422, Aug. 2008.
[22] V. D. Athawale and V. Lele, “Graft Copolymerization onto Starch. II. Grafting of Acrylic Acid and Preparation of It’s Hydrogels”, Carbohyd. Polym., vol. 35, no. 1-2, pp. 21-27, Jan-Feb 1998.
[23] W. M. Choi, I. D. Jung, S. K. Kwon, C. S. Ha and W. J. Cho, “Synthesis and
Photobiodegradable Properties of Graft Copolymers of Vinyl Ketones and Starch”, Polym. Deg. Stab., vol. 61, no. 1, pp. 15-20, 1998.
[24] G. Huacai, P. Wan and L. Dengke, “Graft Copolymerization of Chitosan with Acrylic Acid Under Microwave Irradiation and Its Water Absorbency”, Carbohyd. Polym., vol. 66, no. 3, pp. 372-378, Nov. 2006.
[25] M. Y. Arıca, M. Yilmaz and G. Bayramoglu, “Chitosan-Grafted Poly(Hydroxyethyl Methacrylateco- Glycidyl Methacrylate) Mem branes for Reversible Enzyme Immobilization”, J. Appl. Polym. Sci., vol. 103, no. 5, pp. 3084–3093, March 2007.
[26] Y. Liu, J. Li, L. Yang and Z. Shi, “Graft Copolymerization of Methyl Methacry- late onto Casein Initiated by Potassium Dilluratocuprate(III)”, J. Macromol. Sci. A Pure appl. Chem., vol. 41, no. 3, pp. 305-316, March, 2004.
Trefiletti, “Thermal Analysis and Characterisation of Cellulose Grafted With
Acrylic Monomers”, Thermochem. Acta, vol. 425, no. pp. 173-179, 2005.
[36] B. S. Kaith, A. S. Singha and S. K. Gupta, “Graft Copolymerization of Flax Fibres with Binary Vinyl Monomer Mixtures and Evaluation of Swelling, Moisture Absorbance and Thermal Behaviour of the Grafted Fibres” J. Polym. Mater., vol. 20, no. 195–199, 2003.
[37] B. S. Kaith, R. Jindal and J. K. Bhatia, “Morphological and Thermal Evaluation of Soy Protein Concentrate on Graft Copolymerization with Ethylmethacrylate” J. Appl. Polym. Sci., vol. 120, no. 4, pp. 2183–2190, May 2011.
[38] N. Kawabata, T. Fueno, T. Tsuruta and J. Furukuwa, “A Consideration of the Q-e Scheme. IV. A Theoretical Approach to Price-Alfrey’s Polarity Term”, J. Chem. Sci. Japan, vol. 36, no.9, pp. 1168-1172, 1963.
[39] J. Brandrup and E. H. Immergut, “Polymer Handbook”, Ed. 2. Wiley Interscience, New York, 1975.
[40] G. Ham, “High Polymer”, Copolymerization, Wiley, New York, 1964.
[41] A. Sarkar, H. Mallik and N. Gupta, “Anisotropic Electrical Conduction in Gum Arabica-A Biopolymer”, Mater. Sci. Eng. C, vol. 20, no. 1-2, pp. 215-218, May 2002.
[42] S. N. Swain, K. K. Rao and P. L. Nayak, “Biodegradable Polymers; Part II.
Thermal Degradation of Biodegradable Plastics Cross-Linked from Formal- dehyde-Soy Protein Concentrate”, J. Therm. Anal. Cal., vol. 79, no. 1, pp. 33-38 Jan. 2005.

[43] B. S. Kaith, R. Jindal and J. K. Bhatia, “ Evaluation of Thermal Behavior of Microwave Induced Graft Copolymerization Ethylmethacrylate onto Soy Protein Concentrate” J. Macromol. Sci. Pure Appl. Chem., vol. 48, no. 4, pp. 299- 308, March 2011.