International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 221
ISSN 2229-5518
Roy U.B. and Vijayalaxmi K.K.
Abstract:
The medicinal properties of Piper betle are well known since time immemorial, and traditionally the leaves are used to treat various diseases like halitosis, boils and abscesses, conjunctivitis, constipation, swelling of gums, cuts and injuries and burns. Lot of research on the anti-bacterial, anti- protozoan, anti-fungal, antioxidant, immunomodulatory, antimutagenic, antileishmanial and neurostimulatory properties of betel leaf and its essential oil has been carried out. However, not much data is available on the antitumor properties of betel leaves and their derivatives. In Our present investigation the cytotoxic activity of ethanolic extract of Piper betle leaves was evaluated using murine (Ehrlich Ascites Carcinoma and Melanoma B-16 cells) and human (HeLa, Raji) cancer cell lines by employing MTT assay and Trypan-blue dye exclusion method. It was observed that there was a concentration-dependent cell death in various cultured cell lines. Even though Piper betle displayed cytotoxicity towards both normal and tumor cell lines, the toxicity on tumor cells was far greater than that on normal cells indicating selective toxic effect of the plant extract on the tumor cells. This was evident from the finding that the IC50 values for tumor cells were comparatively very low than their normal counterparts, while the percentage inhibition of tumor cells was higher than that of normal cells. It was also indicative that Piper betle extract exhibited a dose dependent and time dependent cell killing. A significantly increased rate of cell death was observed with an increase in the concentration of the extract and the time of incubation with the extract, irrespective of the cell type. In both murine and human cell lines it was a general observation that the cells grown as suspension were comparatively more sensitive to the extract than the adherent cell types; the latter requiring a higher concentration of the extract to bring about the same rate of cell death.
Keywords: Piper betle, antioxidant, antitumor, cell viability, MTT assay.
Piper betle, commonly called betel leaf is the leaf of a vine belonging to the Piperaceae family, and has long been recognised as one of the medicinal plants that has tremendous health benefits. Betel leaf is mostly consumed as betel quid or paan, with or without tobacco. Betel leaves play a vital role in Indian tradition, customs and rituals. The medicinal properties of betel leaf are well known since time immemorial. According to traditional Ayurvedic medicine, chewing betel leaf is a remedy for bad breath. It acts as aphrodisiac and is known to kill or inhibit the growth of the deadly bacteria that cause typhoid, cholera, tuberculosis, etc. It is used as a stimulant and as an antiseptic. Traditionally the leaves are used to treat various diseases like halitosis, boils and abscesses, conjunctivitis, constipation, swelling of gums, cuts and injuries. The essential oil contained in the leaves is known to possess anti-bacterial, anti-protozoan and anti-fungal properties. Piper betle is extensively used in Chinese folk medicine. The leaves are used to prevent decay of teeth, used as carminative and the pills are given as anodyne and astringent [1]. In vitro experiments on human saliva using crude extracts of Piper betle was performed [2].
-------------------------------------------------------------------------
Roy U.B., Assistant Professor, Dept. of Zoology and Genetics, Govt. Science College, Bangalore, India. royub09@gmail.com.
Vijayalaxmi K.K., Professor, Dept. of Applied Zoology, Mangalore
University, Mangalagangothri, India.
It was found that Piper betle has bioenhancer activity on salivary peroxidise and plays an important role in oral hygiene [3]. It exhibited antibacterial and antifungal activity. The effect of Piper betle on the overall microbial activity [3] and the effectiveness of Piper oil in suppressing the oral pathogenic activity of fungi, S. faecalis and Candida albicans [4] were demonstrated. Hydroxychavicol, one of the major constituents of Piper betle showed antifungal activities against a broad spectrum of clinically significant human fungal sps., including Candida sp., Aspergillus and dermatophytes. It exhibited a concentration dependent killing [5].
Piper betle flower or leaf contains aromatic phenolic compounds which have been found to stimulate the release of catecholamines in vitro. Thus, betel chewing may affect parasympathetic, GABAnergic and sympathetic functions indicating its effects on central and autonomic nervous systems [6]. Piper betle extracts showed antiplatelet and antioxidative effects. The aqueous components of inflorescence Piper betle extracts are potential ROS scavengers and may prevent platelet aggregation possibly via scavenging of ROS or inhibition of TXB(2) production [7]. It was demonstrated that hydroxychavicol derived from Piper betle is a potent inhibitor of xanthine oxidase [8]. Investigations of the influence of varieties of betel leaf were carried out on the
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 222
ISSN 2229-5518
digestive enzymes of pancreas, intestinal mucosa and liver. It was found that betel leaves do not influence bile secretion and composition, but significantly stimulate the
pancreatic and intestinal lipase activity [9].
Co-administration of Piper betle with ethanol resulted in significant reduction of lipid levels and lipid peroxidation markers such as thiobarbituric acid ractive substances and hydroperoxides , and there was increase in the levels SOD, Catalase, Glutathione peroxidise, GSH, Vitamins C and E, indicating the neuroprotective effect of Piper betle against alcolol [10]. The leaf extracts had a chemopreventive role in protecting against carbon tetrachloride-induced liver fibrosis in rats [11]. The extracts significantly inhibited the elevated aspartate aminotransferase and alanine aninotransferase brought about by carbon tetrachloride intoxication. It also attenuated total GST and enhanced SOD and catalase activities.
Piper betle inhibited the production of allergic mediators by bone marrow- derived mast cells and lung epithelial cells. The extracts significantly decreased histamine and GM-CSF produced by an Ig E- mediated hypersensitive reaction, and inhibited eotaxin and IL-8 secretion in TNF- alpha and IL-4 induced allergic reactions [12]. Crude methanolic extracts of Piper betle showed a mixed type-1 and type-2 cytokine responses, thus suggesting a remarkable immunomodulatory property of this plant. The extracts were found to potentiate significant enhancement of both humoral as well as cell-mediated immune responses in mice [13].
The nonmutagenicity of betel leaf and its antimutagenic action against environmental mutagens were demonstrated [14]. It was found that the water and acetone extracts of betel leaf were nonmutagenic to S.typhimurium strains with and without S9 mix. Both the extracts suppressed the mutagenicity of betel quid mutagens in a dose dependent manner. They reduced the mutagenicity of benzo(a)pyrene and dimethylbenzanthracene . Ethanolic extract of Piper betle showed antileishmanial activity via programmed cell death as evidenced by morphological changes, loss of mitochondrial membrane potential and cell cycle arrest at the sub-G0/G1 phase [15].
Even though lot of research has been done on various biological properties and medicinal uses of Piper betle, our knowledge of Piper betle and its derivatives in cancer treatment is very limited. A potent anticancer agent is the one which is cytotoxic and mitostatic, with minimum side effects on the normal cells and tissues of the body. Our present investigation aimed at screening for cytotoxic activity of ethanolic extract of Piper betle leaves against murine and human cancer cell lines.
Two murine and two human cancer cell lines were used. Murine cell lines chosen were Ehrlich Ascites Carcinoma (EAC) and Melanoma B-16 cells, and the human cell lines were HeLa and Raji cells. Mouse peritoneal normal lymphocytes and macrophages (MPNLM) and peripheral blood mononuclear cells (PBMC) served as the normal counterparts for murine and human cell lines respectively. EAC cells were obtained by aspirating the cells from the abdomen of a tumor mouse on the 10th day after tumor transplantation, maintained in the animal house of the Department. HeLa, Raji and melanoma cells were procured from National Centre for Cell Sciences, Pune, India. HeLa and melanoma cells were maintained and cultured in DMEM and for other cell types RPMI-
1640 was used, both procured from Himedia Laboratories
Pvt. Ltd., Mumbai, India.
Matured leaves of high quality were collected from healthy wines grown under natural conditions without exposure to pesticides and chemical fertilizers. The leaves collected were thoroughly washed with distilled water and blot dried in the laboratory. Ethanolic extract of the leaves was prepared using a Soxhlet extractor. During each extraction around 300 grams of the finely chopped leaves were extracted with 1000 ml of distilled alcohol in the extractor for about 8-9 cycles. The extract obtained was concentrated in a rotary vacuum evaporator (Laborata 4003, Heidolph Rotovac, Germany) at 40°C. The thick concentrate obtained after the evaporation was further dried in a water bath at 45°C. A greenish-black paste thus obtained was weighed and stored in a deep freezer (-20°C) for future use. For in vitro studies the extract was dissolved in dimethyl sulphoxide (DMSO) solution. Serial dilutions were made to get different concentrations of the extract ranging from
5μg/ml to 300 μg/ml in 0.1% DMSO.
Both RPMI- 1640 and DMEM were prepared from powdered media (Himedia laboratories, Mumbai, India) with L- glutamine, without sodium bicarbonate (NaHCO3 ) [16]. The powder provided in the vial was dissolved in 1000 ml of autoclaved double distilled water (DDW). To 900ml of DDW, contents of 1 unit vial were added at room temperature with constant stirring. To this, 1500 mg of NaHCO3 ((Himedia laboratories, Mumbai, India) was added and stirred well. The pH of the solution was adjusted to a range of 7.1- 7.4 using 1N
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 223
ISSN 2229-5518
HCl or 1N NaOH. Final volume was made upto 1000ml with DDW and sterilized by filtering through autoclaved cellulose acetate membrane filter with a pore size of 0.2
μm and diameter of 47 mm (Sartorius AG, Germany) in a filter unit, using vacuum pump. To the filtered media 2.5 ml of antibiotic- antimycotic solution (Himedia Laboratories, Mumbai, India) was added and stored in a refrigerator at 2-8°C until further use. At the time of use media was supplemented with 10% Foetal Bovine Serum (FBS) procured from Himedia Laboratories, Mumbai, India.
Cultures grown in media supplemented with 10% Foetal Bovine Serum were viewed using an inverted phase contrast microscope to observe growth and morphology of the cells. When the pH of the medium turned acidic, indicating overgrowth of cells, centrifugation was done for suspension cultures (EAC, Raji). Cells were centrifuged at 1500 rpm for 5 minutes and the media was removed. Fresh RPMI-1640 media was added and the cells were mixed thoroughly to obtain single cell suspension. Cells were then counted using a haemocytometer and re-seeded at a concentration of
1x106 cells/ml into fresh culture flasks containing RPMI-
1640 media supplemented with 10% FBS. The flasks were tilted gently to ensure uniform distribution of cells, which were then cultured in a CO2 incubator in a humidified atmosphere with 5% CO2 at 370 C. In case of adherent cell types (HeLa, Melanoma B-16), after the cells attained confluence or when the media turned acidic, the media was removed by aspiration and the cells were washed with sterilised phosphate buffered saline. The cells were then trypsinised with 0.25% trypsin for 2-3 min and subsequently washed with DMEM media without FBS. The cells were mixed with fresh media and finally reseeded at a concentration of 1x106 cells/ml into fresh culture flasks containing DMEM media supplemented with 10% FBS. The flasks were tilted gently to ensure uniform distribution of cells which were then cultured in a CO2 incubator in a humidified atmosphere with 5% CO2 at 370 C [16].
This was done by trypan-blue dye exclusion method [16]. The viable cells exclude the dye as they are non permeable to the dye and remain clear or white, whereas the non-viable cells take up the stain as they are permeable, and thus appear blue when visualized under the microscope. 0.4 % solution of the dye (Himedia, Mumbai, India) prepared in distilled water and filtered through cellulose acetate filter with 0.2 μm pore size and
47 mm diameter (Sartorius AG, Germany) was used.
100μl of cell suspension containing 1x105 cells was added to each of the wells containing 300μl of respective media
with supplements in a 24 well plate. Different concentrations of the extract in 100μl were added to get a final volume of 500μl; 0.1% DMSO served as control. The
cells were cultured in a CO2 incubator in a humidified atmosphere with 5% CO2 at 370 C. All the treatments were prepared in quadruplicates. Cell viability was determined at 24, 48 and 72 hr. time intervals for each test group. 50μl of cell suspension was mixed with an equal volume of the dye and loaded onto a haemocytometer. The slides were left undisturbed for 1- 2 min. The total number of cells (stained+unstained) and the number of stained (dead) cells was counted. Percentage viability was calculated using the formula;
No. of Dead Cells
Percentage = --------------------------- X 100
Viability Total no. of Dead
And Live Cells
IC50 values for each treatment was determined by plotting percentage viability against concentration of the extract.
MTT assay was done as per the modified method of Mosmann [17]. MTT 3-(4, 5-dimethyl-thiazolyl)-2, 5- diphenyl-2H-tetrazolium bromide) is a yellow water soluble salt which is biologically active. MTT enters the proliferating cells and the mitochondrial enzymes of the cells reduce MTT into a purple coloured formazan. The formazan precipitate was extracted with DMSO and the absorbance was measured at 550 nm in a double beam UV- visible spectrophotometer (UV-1601, Shimadzu, Japan). MTT (Himedia, Mumbai, India) was prepared by dissolving 5 mg powder in 1ml phosphate buffered saline under hood. It was then filtered through cellulose acetate filter with 0.2 μm pore size and 47 mm diameter (Sartorius AG, Germany) and stored at 80C. 100μl of cell suspension containing 1x105 cells was added to each of the wells containing 300μl of respective media with supplements in a 24 well plate. Different concentrations of the extract in 100μl were added to get a final volume of
500μl; 0.1% DMSO served as control. The cells were
cultured in a CO2 incubator in a humidified atmosphere with 5% CO2 at 370C. All the treatments were prepared in quadruplicates and cytotoxicity was determined at 24, 48 and 72 hr. time intervals for each test group. The cells were treated with 200μl of MTT prepared in PBS and incubated for 4 hr. at 370C avoiding exposure to light. After 4 hr. MTT was removed and the formazan formed was extracted with 2ml of DMSO and the absorbance was read at 550 nm. The growth inhibition rate was calculated by the following formula [18];
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 224
ISSN 2229-5518
OD control well – OD treated well
Inhibition = --------------------------------------X 100
Rate OD control well
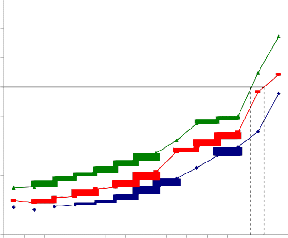
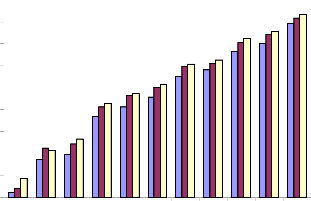
It was observed that there was a concentration- dependent cell death in various cultured cell lines. Although the normal cells were affected by the plant extracts, the IC50 values observed were very high, whereas the IC50 values for tumor cells were comparatively very low indicating the selective cytotoxicity of Piper betle extract. In case of MPNLM, the IC50 value was found to be 390.2μg/ml and 350.7μg/ml respectively, and at 24 hr. even at a concentration as high as 300μg/ml there was only 30% cell death (Fig.1a). The EAC cells showed lower IC50 values when compared to that of Melanoma B-16 cells indicating a greater sensitivity of the cells towards the extract. At a concentration of 50μg/ml there was 100% cell death at
72hr and the IC50 values for EAC cells were found to be
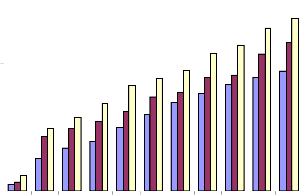
45.9, 24.2 and 9.4μg/ml at 24, 48 and 72 hr. respectively (Fig.2a). Induction of cell death required slightly higher concentrations of the plant extract for Melanoma cells when compared to EAC cells. The IC50 values were found to be 59.3, 42.7 and 18.2μg/ml at 24, 48 and 72 hr. respectively, from which it can be learnt that, for melanoma cells, doses double than that required for EAC cells were required for causing cell death (Fig.3a). The normal human lymphocytes were slightly less sensitive to Piper betle extracts when compared to murine counterpart. Even though cell death occurred in a dose- depended manner, the IC50 values were very high. Only at a concentration as high as 390μg/ml and for an incubation period of 72 hr, it was possible to obtain IC50 value. When the cells were incubated for 24 and 48 hr., the IC50 values could not be obtained even at 400μg/ml concentration (Fig.4a). HeLa cells showed comparatively higher IC50 values compared to Raji cells. The concentration which killed 50% of the cell population was
78.6, 37.3 and 16.9μg/ml for 24, 48 and 72 hr. incubation
respectively (Fig.5a). Raji cells were found to be the most sensitive of all cell types. The IC50 values were found to be very low indicating greater cell deaths at lower
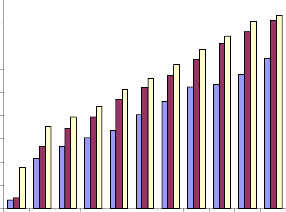
concentrations of the plant extract. At 72 hr. of incubation it was as low as 7.2μg/ml; at 48 hr., 28.7, and at 24hr. it was 45.7μg/ml (Fig.6a).
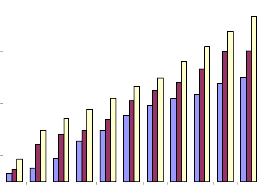
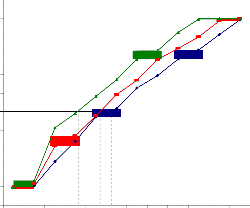
The results of MTT assay were in coordination with that of dye exclusion test with reference to MPNLM. At lower doses very less inhibition was observed and at the maximum dose, ie, 300μg/ml percentage inhibitions were found to be 40.25, 50.19 and 63.53 at 24, 48 and 72 hr. respectively (Fig.1b). For EAC cells it was found that both at 48 and 72 hr. there was around 50% inhibition at a concentration of 75μg/ml. and at a concentration of
300μg/ml, the percentage inhibition was found to be 79,
81.62 and 83 at 24, 48 and 72 hr. respectively. Not much
difference was observed with reference to the responses
of cells at 48 and 72hr (Fig.2b). Piper betle, had a lesser toxic effect on melanoma cells when compared to EAC cells; however, the results of MTT assay indicated that a
50% inhibition could be achieved at concentrations of 200,
150 and 100μg/ml at 24, 48 and 72 hr. time intervals
respectively, indicating a dose and time response (Fig.
3b). For PBMC the results of MTT assay were in
proportion with that of dye exclusion test. There was 50%
inhibition at a concentration of 300μg/ml at 24 hr.; and
250μg/ml at 48 and 72 hr. (Fig.4b). For HeLA cells the
values were almost coinciding with that of melanoma cells. There was around 80% inhibition at 72 hr. and only
56.5% inhibition at 24 hr. when incubated with 300μg/ml of the extract. Lower doses showed lesser inhibition rate (Fig.5b). The extract had a significant level of toxicity on Raji cells also. 50% inhibition was observed at a concentration of 50μg/ml and 80% inhibition at 250μg/ml when incubated for 72 hr. At lower concentrations and lesser incubation periods the percentage inhibition was found to be low (Fig.6b).
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 225
ISSN 2229-5518

![]()
80 24 hr
48 hr
70 72 hr
60
50
40
30
20
10
0
Treatment
Fig.1a. IC50 value of ethanolic extract of Piper betle for MPNLM.
![]()
70 24 hr
48 hr
60 72 hr
50
40
30
20
10
0
Treatment
Fig.1b. Inhibition of proliferation of MPNLM by
alcoholic extract of Piper betle (MTT assay).
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 226
ISSN 2229-5518

110
100
90
80
70
60
50
40
30
20
10
0
Treatment
Fig.2a. IC50 value of ethanolic extract of Piper betle for
mouse EAC cells.
![]()
90 24 hr
48 hr
80
72 hr
70
60
50
40
30
20
10
0
Treatment
Fig.2b. Inhibition of proliferation of EAC cells of mouse by
alcoholic extract of Piper betle (MTTassay).
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 227
ISSN 2229-5518
110
100
90
80
70
60
50
40
30
20
10
0
Treatment
Fig.3a. IC50 value of ethanolic extract of Piper betle for
mouse melanoma cells.
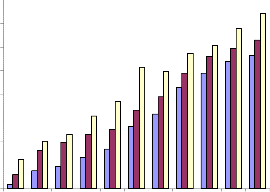
![]()
80 24 hr
48 hr
70 72 hr
60
50
40
30
20
10
0
Treatment
Fig.3b. Inhibition of proliferation of mouse melanoma cells by
alcoholic extract of Piper betle (MTT assay).
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 228
ISSN 2229-5518
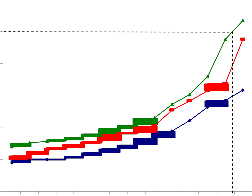
60
50
40
30
20
10
0
Treatment
Fig.4a. IC50 value of ethanolic extract of Piper betle for PBMC.
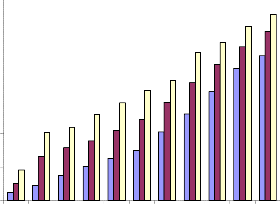
![]()
60 24 hr
48 hr
72 hr
50
40
30
20
10
0
Treatment
Fig.4b. Inhibition of proliferation of PBMC
by alcoholic extract of Piper betle (MTT assay).
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 229
![]()
ISSN 2229-5518
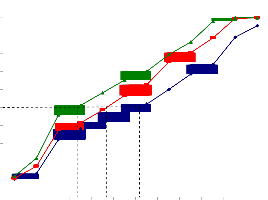
110
100
90
80
70
60
50
40
30
20
10
0
24 hr
48 hr
72 hr
Treatment
Fig.5a. IC50 value of ethanolic extract of Piper betle for
HeLa cells.
![]()
90 24 hr
48 hr
80
72 hr
70
60
50
40
30
20
10
0
Treatment
Fig.5b. Inhibition of proliferation of HeLa cells by
alcoholic extract of Piper betle (MTT assay).
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 230
![]()
ISSN 2229-5518
110
100
90
80
70
60
50
40
30
20
10
0
24 hr
48 hr
72 hr
Treatment
Fig.6a. IC50 value of ethanolic extract of Piper betle for
Raji cells.
![]()
90 24 hr
48 hr
80
72 hr
70
60
50
40
30
20
10
0
Treatment
Fig.6b. Inhibition of proliferation of Raji cells by
alcoholic extract of Piper betle (MTT assay).
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 231
ISSN 2229-5518
Plant derived compounds have been playing crucial role as clinically useful anticancer agents [19]. In the past few years, large bodies of experimental studies have been performed to develop novel antitumor agents that are able to selectively inhibit important pathways that control cancer cell proliferation [20]. To evaluate the cytotoxic effect of ethanolic extract of Piper betle, cell viability and inhibition of cell proliferation was determined on four different cancer cell lines, two murine and two human. Murine cell lines chosen were Ehrlich Ascites Carcinoma (EAC) and Melanoma B-16 cells, and the human cell lines were HeLa and Raji cells. The results obtained were compared with that of the normal counterparts, MPNLM and PBMC (for murine and human cell lines respectively). Even though Piper betle displayed cytotoxicity towards both normal and tumor cell lines, the effect on tumor cells was far greater than that on normal cells. This indicates a selective toxic effect of the plant extract on the tumor cells. In both murine and human cell lines it was a general observation that the cells grown as suspension were comparatively more sensitive to the extract than the adherent cell types; the latter requiring a higher concentration of the extract to bring about the same rate of cell death. This was evident from the fact that the IC50 values for EAC and Raji cells were lower than that for Melanoma-B16 and HeLa cells. The normal cells showed much higher IC50 values indicating lesser toxic effect of the extract on them. In fact, it could be obtained only when the cells were incubated in very high concentrations of the extract for a period of 72hr and not earlier. It was also indicative that the cells showed a ‘dose and time’ response to Piper betle extract treatment with a dose dependent and time dependent cell killing. A significantly increased rate of cell death was observed with an increase in the concentration of the extract and the time of incubation with the extract, irrespective of the cell type.
The antitumor property of Piper betle may be due to the phytochemicals present in it, including polyphenols and alkaloids, most of which are potent free radical scavengers. Phenolic compounds such as epigallocatechin gallate, catechin, genistein and quercetin suppressed growth of breast cancer cells implying the importance of antioxidants towards the anti-proliferative effects of cells. Anti-cancer agents with antioxidant activities may exert their beneficial effects by balancing levels of ROS so as not to cause further proliferation of cancer cells while still allowing apoptosis to occur [21].
Promising new source of therapeutic agents has been discovered in plant secondary metabolites, irregularly occurring compounds that characterize certain plants or
plant groups [22]. Several phenolic compounds have been identified in the leaves of P. betle including β- sitosterol, dotriacontanoic acid, tritriacontane, stearic acid, hydroxychavicol, chevibetol and allylpyrocatechol, together with their glucosides [23], [24]. Identification of catechin, morin and quercetin through HPLC analyses was also done [21]. Studies of polyphenols also have produced compelling data for the antitumor activities of plant secondary metabolites in various types of cancers [25]. Several flavonoids have been shown to inhibit cancer development while exhibiting antioxidant activities in various animal models [26].
Hydroxychavicol, a component of P. betle leaf showed anti-proliferative effect towards oral carcinoma cell line [27]. Antioxidants may inhibit carcinogenesis through other non-antioxidant action such as by modulating signalling pathways involved in cellular functions such as proliferation, cell growth and differentiation, by influencing activities of cancer-related enzymes such as cyclooxygenase-2 and phase I or II metabolizing enzymes or by inducing cell cycle arrest [28].
Plant-derived extracts containing antioxidant principles showed cytotoxicity towards tumor cells and antitumor activity in experimental animals. Antitumor activity of these antioxidants is either through induction of apoptosis or by inhibition of neovascularization. The implication of free radicals in tumors is well documented. The free radical hypothesis supported the fact that the antioxidants effectively inhibit the tumor, and the observed properties may be attributed to the antioxidant and antitumor principles present in the plant extracts [29].
It was demonstrated that the aqueous extract of P. betle prevented formation of tumors when fed to rats in the initiation phase of induced-mammary carcinogenesis but could not inhibit tumor growth when fed to rats in the later stages of induced mammary carcinogenesis [30]. The anti-proliferative action of aqueous extract of Piper betle towards KB cells indicates its potential in treating oral cancer [31]. Moreover, the leaves of P. betle were found to have strong anti-tumor promoting activities in Raji cells [32].
Betel leaves are also reported to possess antioxidant activity besides antimutagenic and anticarcinogenic properties, particularly against the tobacco carcinogens, due to the presence of ingredients like hydroxychavicol and chlorogenic acid in it. The latter compound is also reported to kill the cancerous cells without affecting the normal cells unlike the common cancer drugs and relevant therapeutic means. Therefore, possibility of
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 232
ISSN 2229-5518
manufacturing a new blood cancer drug from it cannot be ruled out [33]. In conclusion we can say that Piper betle leaves have potent antitumor properties due to the
presence of a wide range of phytochemicals carrying out free radical scavenging activities as well as inducing selective toxicity against cancerous cells.
The authors are thankful to BRNS-DAE, Govt. of India, for funding the research project which was sanctioned to Dr. K.K. Vijayalaxmi, Professor and Principal Investigator, Department of Applied Zoology, Mangalore University, Mangalagangothri, Mangalore, India.
[1] A. K. Nadkarni and K.M. Nadkarni, Indian Materia Madica, vol.2, Eastern Book Corporation, Mumbai, 2007.
[2] N. Kumar, and R. Tripathi, Putative role of Betel
(Piper betle L.) in oral hygiene, Plant peroxidise
Newsl., vol.15, pp.45-48, 2000.
[3] S. Bissa, D. Songara and A. Bohra, Traditions in
oral hygiene: chewing of betel (Piper betle L.)
leaves. Curr. Sci., vol. 92, pp. 26-28, 2007.
[4] S. Gupta, N. Kumar and S.M. Gupta, Antibacterial and antifungal activity in the extract and oil of Piper betle (Linn.) landrace Bangla Mahoba, Adv. Zool., vol. 31, pp. 16-20,
2009.
[5] I. Ali, In vitro antifungal activity of Hydroxychavicol isolated from Piper betle L., Ann. Clin. Microbiol. Antimicrob., vol. 9, pp. 7,
2010 .
[6] N.S. Chu, Effects of betel chewing on the central
and autonomic nervous systems, J. Biomed. Sci., vol. 8, pp. 229-236, 2001.
[7] D. Lei, Antioxidative and antiplatelet effects of aqueous inflorescence Piper betle extract, J. Agri. Food Chem., vol. 51, pp. 2083-2088, 2003 .
[8] K. Murata, Hydroxychavicol: a potent xanthine oxidase inhibitor obtained from the leaves of betel, Piper betle, J. Nat. Med., vol. 63, pp. 355-359,
2009.
[9] M.S. Prabhu, K. Patel, G. Saraswathi and K.
Srinivasan, Effect of orally administerd betel leaf (Piper betle Linn.) on the digestive enzymes of pancreas and intestinal mucosa and on bile production in rats, Indian J. Exp. Biol., vol. 33, pp.
752-756, 1995.
[10] R. Saravanan, N. Rajendra Prasad, and K.V.
Pugalendi, Effect of Piper betle leaf extract on
alcoholic toxicity in the rat brai, J. Med. Food, vol.
6(3), pp. 261-265, 2003.
[11] S.C. Young, C.J.Wang, J.J. Lin, P.L. Peng, J.L. Hsu and F.P. Chou, Protection effect of Piper betle leaf extract against carbon tetrachloride-induced
liver fibrosis in rats, Arch. Toxicol., vol. 81, pp.45-
55, 2007.
[12] M. Wirotesangthong, N. Inagaki, H. Tanaka, W.
Thankijcharoenpath, and H. Nagai, Inhibitory
effects of Piper betle on production of allergic
mediators by bone marrow-derived mast cells and lung epithelium cells, Int.Immunopharmacol, vol. 8, pp. 453-457, 2008.
[13] M. Singh, S. Shakya, V.K. Soni, A. Dangi, N.
Kumar and S.M. Bhattacharya, The n-hexane and
chloroform fractions of Piper betle L. trigger different arms of immune responses in BALB/c mice and exhibit antifilarial activity against human lymphatic filarid Brugia malayi., Int. Immunopharmacol, vol. 9, pp.716-728, 2009.
[14] M. Nagabhushan, A.J. Amonkar, A.V. D’Souza and S.V. Bhide, Nonmutagenicity of betel leaf and its antimutagenic action against environmental mutagens, Neoplasma, vol. 34, pp.
159-167, 1987.
[15] A. Sarkar, R. Sen, S. Ganguli, G. Mandal, and M.
Chatterjee, An Ethanolic extract of leaves of Piper
betle (paan) Linn. mediates its antileishmanial activity via apoptosis, Parasitol. Res., vol. 102, pp.
1249-1255. 2008.
[16] R. Ian Freshney, Culture of animal cells: A manual of basic technique, Johm Wiley and Sons, Inc., 5th Edn., pp. 161-162, 199-216, 2005.
[17] T. Mossmann, Rapid colorimetric assay for
cellular growth and survival: application to proliferation and cytotoxicity assays, J. Immunol. Methods, vol. 65, pp. 55-63, 1983.
[18] Z. Zheng, W. Zheng, Y. Huang, Z .Yang, J. Li, H.
Cai and W. Su, Detection of antitumor and
antimicrobial activities in marine organism associated actinomycetes isolated from the Taiwan Strait, China, FEMS Microbiology Lett., vol. 188, pp. 87-91, 2000.
[19] M. Soriful Islam, Most. Mauluda Akhtar, M.
Mostafizur Rahman, M. Atikur Rahman, Kanak Kanti Sarker and M. Firoz Alam, Antitumor and Phytotoxic Activities of Leaf Methanol Extract of Oldenlandia diffusa (Willd) Roxb., Global Journal of Pharmacology, vol. 3(2), pp: 99-106, 2009.
[20] F. Ciardiello, R. Caputo, R. Bianco, V. Damiano, G. Pomatico, A. Sabino De Placido, R. Bianco, and G. Tortora, Antitumor Effect and Potentiation of Cytotoxic Drugs Activity in Human Cancer Cells by ZD-1839 (Iressa), an Epidermal Growth Factor Receptor-selective Tyrosine Kinase Inhibitor, Clinical Cancer Research, Vol. 6, pp: 2053–2063, 2000.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 233
ISSN 2229-5518
[21] N. N. Abrahim, M. S. Kanthimathi and A. A.
Aziz, Piper betle shows antioxidant activities,
inhibits MCF-7 cell proliferation and increases
activities of catalase and superoxide dismutase,
BMC Complementary and Alternative Medicine,
12:220, 2012.
[22] A. C. Scheck, K. Perry, N. C. Hank and W.
D.Clark Anticancer activity of extracts derived
from the mature roots of Scutellaria baicalensis on human malignant brain tumor cells, BMC Complementary and Alternative Medicine , vol.
6(27), pp: 1-9, 2006.
[23] S. Bhattacharya, M. Subramanian, S.
Roychowdhury, A.K. Bauri, J.P. Kamat, S. Chattopadhyay, S.K. Bandyopadhyay, Radioprotective Property of the Ethanolic Extract of Piper betel Leaf, J Radiat Res, vol. 46, pp. 165-
171, 2005.
[24] V.S. Parmar, S.C. Jain, S. Gupta, S. Talwar, V.K.
Rajwanshi, R. Kumar, A. Azim, S. Malhotra, N.
Kumar, R. Jain, N.K. Sharma, O.D. Tyagi, S.L. Lawrie, W. Errington, O.W. Howarth, C.E. Olsen, S.K. Singh, J. Wengel, Polyphenols and alkaloids from piper species, Phytochemistry, vol.49, pp.1069-1078, 1998.
[25] C.S. Yang, J.M. Landau, M.T. Huang, H.L.
Newmark, Inhibition of carcinogenesis by
dietary polyphenolic compounds. Ann Rev Nutr.
vol.21, pp: 381-406, 2001.
[26] M. L.Chatterjee, S.K. Katiyar, R.R. Mohan, R.
Agarwal, A flavonoid antioxidant, silymarin, affords exceptionally high protection against tumor promotion in the SENCAR mouse skin tumerogenesis model, Cancer Res., vol.59, pp:
622-632, 1999.
[27] M.C. Chang, B.J. Uang, H.L. Wu, J.J. Lee, L.J.
Hahn, J.H. Jeng, Inducing the cell cycle arrest
and apoptosis of oral KB carcinoma cells by hydroxychavicol: roles of glutathione and reactive oxygen species, Br. J. Pharmacol., vol.
135(3), pp. 619-630, 2002.
[28] S. Wang, K.A. Meckling, M.F. Marcone, Y.
Kakuda, R. Tsao, Can phytochemical antioxidant rich foods act as anti-cancer agents? Food Res Int, vol.44, pp.2545-2554, 2011.
[29] D. viral, P. Shivanand, N.P. Jivani, Anticancer evaluation of Adiantum Venustum Don., J.Young Pharm., vol.3(1), pp. 48-54, 2011.
[30] A.R. Rao, A. Sinha, R.S. Selvan, Inhibitory action
of Piper betle on the initiation of 7,12-
dimethylbenz[a]anthracene-induced mammary carcinogenesis in rats, Cancer Lett., vol. 26, pp.207-214, 1985.
[31] R.A. Fathilah, R. Sujata, A.W. Norhanom, M.I.
Adenan, Antiproliferative activity of aqueous
extract of Piper betle L. and Psidium guajava L. on
KB and HeLa cell lines, Planta Med, vol.4, pp.987-
990, 2010.
[32] A. Murakami, A.M. Ali, K. Mat-Salleh, K.
Koshimizu, H. Ohigashi, Screening for the In Vitro Anti-tumor-promoting Activities of Edible Plants from Malaysia, Biosci Biotechnol Biochem, vol.64, pp.9-16, 2000.
[33] P. Guha, Betel Leaf: The Neglected Green Gold
of India, J. Hum. Ecol., vol.19 (2), pp. 87-93, 2006.
IJSER © 2013 http://www.ijser.org