Table 2: Heat treatment schedule for crystallization of bioactive glasses
International Journal of Scientific & Engineering Research, Volume 3, Issue 2, February-2012 1
ISSN 2229-5518
Elastic Properties of substituted 45S5 Bioactive
Glasses and Glass - Ceramics
Ankesh Kumar Srivastava, Ram Pyare and S. P. Singh
Abstract — CuO, Fe2O3, MnO2 and ZnO substituted 45S5 bioactive - glasses were prepared. Glass - derived Bioactive Glass - ceramics were obtained through controlled crystallization of bioactive glasses. The formed crystalline phases in bioactive glass - ceramics were identified using X - ray diffraction (XRD) analysis. Density and ultrasonic wave velocities of bioactive glasses and glass - ceramics were measured and used to study elastic properties: Young’s modulus, shear modulus, bulk modulus and Poisson’s ratio, of bioactive glasses and glass - ceramics.
The results indicate that the addition of CuO, Fe2O3, MnO2 and ZnO contents in 45S5 bioactive glass enhances its density and elastic properties. The bioactive glass - ceramics exhibits higher density and elastic properties when compared with their corresponding bioactive glasses.
Poisson’s ratio
—————————— ——————————
The most widely researched bioactive material is 45S5 bioactive glass [Composition wt. % 45 SiO2 - 24.5 Na2O -
24.5 CaO - 6 P2O5], where S denotes the network former SiO2 in 45% by weight followed by a specific Ca/P molar ratio 5 [1]. It was invented by Hench in 1969. The key compositional features that are responsible for the bioactivity of 45S5 bioactive glass are its low SiO2 (glass network former) content, high Na2O and CaO (glass network modifiers) content, and high CaO/P2O5 ratio [2].
An ideal bioactive material requires both good biochemical and biomechanical compatibility. A particular advantage of
45S5 bioactive glass is its ability to bond to both hard and
soft tissues [3]. However, a disadvantage of 45S5 bioactive glass is its brittleness. As a consequence, it has poor mechanical properties and is generally restricted to clinical non - load bearing situation [4].
The mechanical strength of a glass depends on its elastic
properties, shape, surface/internal defects and the character of the force to which it is subjected. These factors inevitably degrade the mechanical strength of a material to 1/10 or lower of its intrinsic (theoretical) strength [5]. Thus, one cannot obtain the intrinsic strength by using destructive mechanical test on a big specimen. The ultrasonic non - destructive testing has been found to be one of the best techniques to study the elastic properties of glasses.
The present work aims to measure the density and ultrasonic wave velocities (both longitudinal and shear) for CuO, Fe2O3, MnO2 and ZnO substituted 45S5 bioactive glasses and glass - ceramics. These values have been used to evaluate Young’s, bulk and shear modulus and Poisson’s ratio of bioactive glasses and glass - ceramics.
Fine grained quartz was used as the source of SiO2 while Na2O and CaO were introduced in the form of anhydrous sodium carbonate [Na2CO3] and anhydrous calcium carbonate [CaCO3] respectively, P2O5 was added in the form of ammonium dihydrogen orthophosphate [NH4H2PO4] and CuO, Fe2O3, MnO2 and ZnO was added as such for preparation of bioactive glasses. All the batch materials were of analytical grade chemicals and used without further purification. The compositions of bioactive glasses are given in Table 1. The weighed batches were melted in alumina crucibles for 3 hours in an electric furnace at the temperature 1400 ± 10 0C. The homogeneous melts were cast into preheated stainless steel moulds of the required dimensions. The prepared bioactive glass samples were directly transferred to a regulated muffle furnace at the temperature 500 0C for annealing. After 1 h, the muffle furnace was left to cool to room temperature at a rate of 30
0C/ h.
In order to obtain the bioactive glass - ceramics, the bioactive glass samples were heated in the muffle furnace in two step regime at the deduced temperatures and times as shown in Table 2. These temperatures were obtained from differential thermal analysis (DTA) of bioactive glasses. Each bioactive glass sample was heated slowly to the first nucleation temperature for the formation of sufficient nuclei sites and after holding for the definite time, was then further heated to reach the second chosen crystal growth temperature for performing the perfect crystal growth process and after a second hold for the specific time, the sample was left to cool inside the muffle furnace to room temperature at a rate of 20 0C/h.
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 2, February-2012 2
ISSN 2229-5518
Table 1: Composition of bioactive glasses
Code for glasses | Composition (wt %) | |||||
CuO substituted 45S5 bioactive glasses | ||||||
SiO2 | Na2O | CaO | P2O5 | CuO | ||
C1 | 44.00 | 24.50 | 24.50 | 6.00 | 1.00 | |
C2 | 43.00 | 24.50 | 24.50 | 6.00 | 2.00 | |
C3 | 42.00 | 24.50 | 24.50 | 6.00 | 3.00 | |
C4 | 41.00 | 24.50 | 24.50 | 6.00 | 4.00 | |
Fe2O3 substituted 45S5 bioactive glasses | ||||||
SiO2 | Na2O | CaO | P2O5 | Fe2O3 | ||
F1 | 44.00 | 24.50 | 24.50 | 6.00 | 1.00 | |
F2 | 43.00 | 24.50 | 24.50 | 6.00 | 2.00 | |
F3 | 42.00 | 24.50 | 24.50 | 6.00 | 3.00 | |
F4 | 41.00 | 24.50 | 24.50 | 6.00 | 4.00 | |
MnO2 substituted 45S5 bioactive glasses | ||||||
SiO2 | Na2O | CaO | P2O5 | MnO2 | ||
M1 | 44.00 | 24.50 | 24.50 | 6.00 | 1.00 | |
M2 | 43.00 | 24.50 | 24.50 | 6.00 | 2.00 | |
M3 | 42.00 | 24.50 | 24.50 | 6.00 | 3.00 | |
M4 | 41.00 | 24.50 | 24.50 | 6.00 | 4.00 | |
ZnO substituted 45S5 bioactive glasses | ||||||
SiO2 | Na2O | CaO | P2O5 | ZnO | ||
Z1 | 44.00 | 24.50 | 24.50 | 6.00 | 1.00 | |
Z2 | 43.00 | 24.50 | 24.50 | 6.00 | 2.00 | |
Z3 | 42.00 | 24.50 | 24.50 | 6.00 | 3.00 | |
Z4 | 41.00 | 24.50 | 24.50 | 6.00 | 4.00 | |
Base 45S5 bioactive glass | ||||||
SiO2 | Na2O | CaO | P2O5 | |||
45S5 | 44.00 | 24.50 | 24.50 | 6.00 |
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 2, February-2012 3
ISSN 2229-5518
![]()
Table 2: Heat treatment schedule for crystallization of bioactive glasses
Sample | Nucleation | Growth | ||||
Temperature (0C) | Time (hours) | Temperature (0C) | Time (hours) | |||
CuO substituted 45S5 bioactive glasses | ||||||
C1 | 518 | 6 | 675 | 3 | ||
C2 | 510 | 6 | 651 | 3 | ||
C3 | 504 | 6 | 631 | 3 | ||
C4 | 499 | 6 | 616 | 3 | ||
Fe2O3 substituted 45S5 bioactive glasses | ||||||
F1 | 527 | 6 | 682 | 3 | ||
F2 | 523 | 6 | 665 | 3 | ||
F3 | 519 | 6 | 650 | 3 | ||
F4 | 516 | 6 | 640 | 3 | ||
MnO2 substituted 45S5 bioactive glasses | ||||||
M1 | 525 | 6 | 678 | 3 | ||
M2 | 520 | 6 | 657 | 3 | ||
M3 | 517 | 6 | 640 | 3 | ||
M4 | 514 | 6 | 627 | 3 | ||
ZnO substituted 45S5 bioactive glasses | ||||||
Z1 | 528 | 6 | 686 | 3 | ||
Z2 | 524 | 6 | 671 | 3 | ||
Z3 | 521 | 6 | 661 | 3 | ||
Z4 | 518 | 6 | 651 | 3 | ||
Base 45S5 bioactive glass | ||||||
45S5 | 533 | 6 | 717 | 3 |
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 2, February-2012 4
ISSN 2229-5518
Identification of the crystalline phases after heat - treatment of bioactive glass samples was carried out by X - ray diffraction (XRD) analysis. The bioactive glass - ceramics were examined using a X - ray diffractometer, adopting Ni filter and Cu target with voltage of 40 KV and a current of
25 mA. The XRD patterns were recorded in a 2θ range of 10
- 700. The JCPDS - International Center for Diffraction Data
Cards was used as a reference data for the interpretation of
XRD patterns in the present work.
Archimedes principle was employed to obtain the density of bioactive glass and glass - ceramic samples using distilled water as buoyant. All the weight measurements have been made using a digital balance having an accuracy of ± 0.0001 g. Density of sample was obtained employing the relation [6] given below [6]:![]()
where is the weight of sample in air, is the weight of sample in buoyant and is the density of buoyant.
The ultrasonic wave velocities propagated in the polished bioactive glass and glass - ceramic specimens were obtained by measuring the elapsed time between the initiation and the receipt of the pulse appearing on the screen of an ultrasonic flaw detector via a standard electronic circuit. The size of the specimen was 20 mm x 10 mm x 3 mm according to ASTM Standard: E494 - 10. Two quartz transducers in this machine, which are X - cut transducer and Y - cut transducer, with has a resonant frequency of 5
MHz and acts as transmitter - receiver at the same time, were required to generate longitudinal and transverse ultrasonic waves respectively Burnt honey was used as bonding material between samples and transducers. The ultrasonic wave velocity can be calculated using the following equation [7]:![]()
where is the sample thickness and is the time interval. The elastic strain in an amorphous solid (such as glass) generated by a minor stress, can be described by two independent elastic constants, and [8]. For pure longitudinal ultrasonic waves, and for pure transverse ultrasonic waves, , where and , respectively, are the longitudinal and transverse ultrasonic wave velocities. Elastic properties: Young’s modulus , shear modulus , bulk modulus and Poisson’s ratio
, can be obtained by density and longitudinal
and shear ultrasonic wave velocities by using the following standard equations [9]:![]()
![]()
![]()
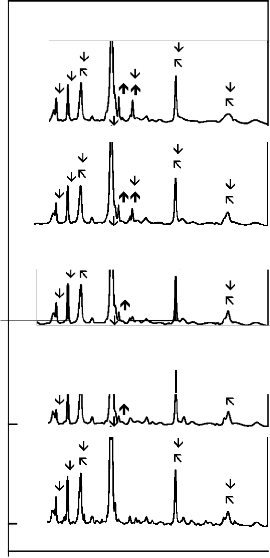
The X - ray diffraction (XRD) patterns for CuO substituted
45S5 bioactive glass - ceramics are shown in Figure 1. The XRD patterns of all the bioactive glass - ceramics show the presence of crystalline phase of sodium calcium silicate [Na2Ca2 Si3O9 (card number: PDF # 01 - 1078 & PDF # 02 -
0961), Na2CaSi3O8 (card number: PDF # 12 - 0671)]. Each CuO substituted bioactive glass - ceramics (C1, C2, C3 and C4) also, show the presence of crystalline phase of calcium silicate [CaSiO3 (card number: PDF # 42 - 0550)].
Experimental values of density of bioactive glasses and glass ceramics are given in Table 3. It has been observed that the increase of CuO, Fe2O3, MnO2 and ZnO contents in the base bioactive glass (45S5) causes an increase in its density. It also has been observed that the density of bioactive glass - ceramics are higher than their respective bioactive glasses.
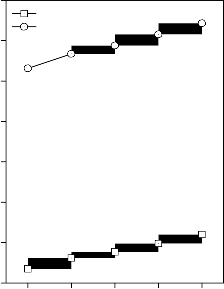
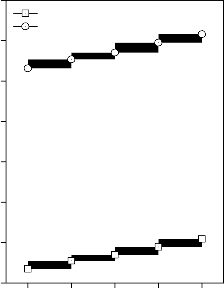
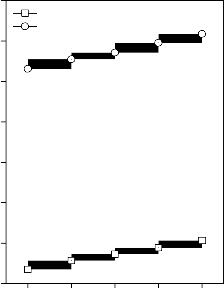
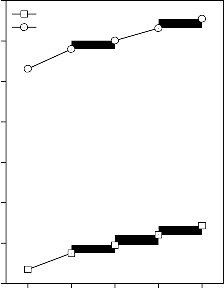
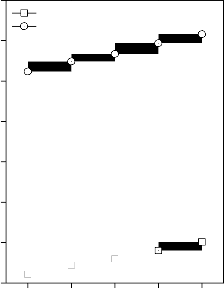
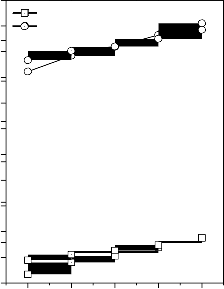
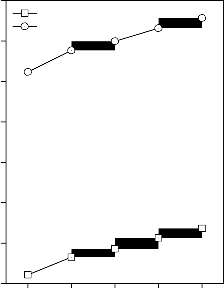
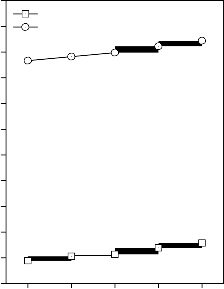
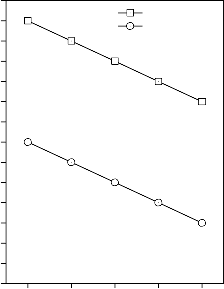
Values of longitudinal and shear ultrasonic wave velocities of investigated bioactive glasses and glass - ceramics are given in Table 3 while values of Young’s, shear and bulk modulus and Poisson’s ratio are listed in Table 4. Variation in Young’s, shear and bulk modulus and Poisson’s ratio of investigated bioactive glasses and glass - ceramics are shown in Figures 2, 3, 4 and 5 respectively. Both longitudinal and shear ultrasonic wave velocities and Young’s, shear and bulk modulus of base bioactive glass (45S5) increases with the incorporation of CuO, Fe2O3, MnO2 and ZnO contents in it.while a slight decrease in Poisson’s ratio was obtained. It also has been founded that the crystallization of bioactive glasses increases their both longitudinal and shear ultrasonic wave velocities, Young’s, shear and bulk modulus while decreases the Poisson’s ratio.
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 2, February-2012 5
ISSN 2229-5518
C4
C3
;::i "'
"'
rfl
'l)
:8
C2
"'
"'"' "'
Cl
45S5
10 15 20 25 30 35 40 45 50 55 60 65 70
28 (degree)
[..q Na Ca Sip
[ Na CaSip
[1ft CaSi0
2 2 9 2 8 3
Figure 1: XRD Patterns for CuO
ceramics
IJSER !b) 2012
substituted 4555 bioactive glass-
International Journal of Scientific & Engineering Research, Volume 3, Issue 2, February-2012 6
ISSN 2229-5518
Table 3: Density , longitudinal velocity and transverse velocity![]()
of bioactive glasses and glass ceramics
Sample | Glasses
| Glass - ceramics | ||||||
(g/cm3) (m/s) (m/s) | (g/cm3) | (m/s) | (m/s) | |||||
CuO substituted 45S5 bioactive glasses | CuO substituted 45S5 bioactive glasses - ceramics | |||||||
C1 | 2.732 5915 3370 | 2.943 | 6519 | 3744 | ||||
C2 | 2.742 5927 3381 | 2.954 | 6531 | 3757 | ||||
C3 | 2.759 5940 3394 | 2.972 | 6547 | 3772 | ||||
C4 | 2.772 5961 3411 | 2.986 | 6569 | 3789 | ||||
Fe2O3 substituted 45S5 bioactive glasses | Fe2O3 substituted 45S5 bioactive glasses - ceramics | |||||||
F1 | 2.723 5913 3368 | 2.925 | 6517 | 3743 | ||||
F2 | 2.731 5925 3380 | 2.932 | 6530 | 3755 | ||||
F3 | 2.746 5937 3393 | 2.946 | 6544 | 3770 | ||||
F4 | 2.757 5958 3409 | 2.956 | 6562 | 3784 | ||||
MnO2 substituted 45S5 bioactive glasses | MnO2 substituted 45S5 bioactive glasses - ceramics | |||||||
M1 | 2.738 5927 3382 | 2.937 | 6533 | 3761 | ||||
M2 | 2.749 5941 3397 | 2.947 | 6550 | 3775 | ||||
M3 | 2.767 5965 3415 | 2.964 | 6571 | 3793 | ||||
M4 | 2.781 5983 3431 | 2.977 | 6587 | 3807 | ||||
ZnO substituted 45S5 bioactive glasses | ZnO substituted 45S5 bioactive glasses - ceramics | |||||||
Z1 | 2.727 5912 3366 | 2.928 | 6515 | 3741 | ||||
Z2 | 2.736 5921 3379 | 2.936 | 6527 | 3752 | ||||
Z3 | 2.752 5933 3387 | 2.951 | 6541 | 3766 | ||||
Z4 | 2.764 5947 3400 | 2.962 | 6556 | 3780 | ||||
Base 45S5 bioactive glass | Base 45S5 bioactive glass - ceramics | |||||||
45S5 | 2.707 5904 3353 | 2.912 | 6507 | 3728 |
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 2, February-2012 7
ISSN 2229-5518
Table 4: Young’s modulus , shear modulus , bulk modulus and Poisson’s ratio![]()
of bioactive glasses and glass ceramics
Sample | Glasses
| Glass - ceramics | ||||||||||
(GPa) (GPa) (GPa) | (GPa) | (GPa) | (GPa) | |||||||||
CuO substituted 45S5 bioactive glasses | CuO substituted 45S5 bioactive glasses - ceramics | |||||||||||
C1 | 78.10 31.02 54.22 0.259 | 103.37 | 41.25 | 70.06 | 0.253 | |||||||
C2 | 78.85 31.34 54.53 0.258 | 104.39 | 41.69 | 70.40 | 0.252 | |||||||
C3 | 79.89 31.78 54.96 0.257 | 105.78 | 42.28 | 71.00 | 0.251 | |||||||
C4 | 81.01 32.25 55.49 0.256 | 107.15 | 42.86 | 71.70 | 0.250 | |||||||
Fe2O3 substituted 45S5 bioactive glasses | Fe2O3 substituted 45S5 bioactive glasses - ceramics | |||||||||||
F1 | 77.75 30.88 54.02 0.259 | 102.67 | 40.97 | 69.59 | 0.253 | |||||||
F2 | 78.49 31.20 54.27 0.258 | 103.51 | 41.34 | 69.90 | 0.252 | |||||||
F3 | 79.46 31.61 54.64 0.257 | 104.75 | 41.87 | 70.32 | 0.251 | |||||||
F4 | 80.45 32.03 55.15 0.256 | 105.80 | 42.32 | 70.85 | 0.250 | |||||||
MnO2 substituted 45S5 bioactive glasses | MnO2 substituted 45S5 bioactive glasses - ceramics | |||||||||||
M1 | 78.77 31.31 54.43 0.258 | 104.01 | 41.54 | 69.96 | 0.252 | |||||||
M2 | 79.74 31.72 54.72 0.257 | 105.05 | 41.99 | 70.44 | 0.251 | |||||||
M3 | 81.03 32.26 55.43 0.256 | 106.60 | 42.64 | 71.11 | 0.250 | |||||||
M4 | 82.15 32.73 55.90 0.255 | 107.76 | 43.14 | 71.64 | 0.249 | |||||||
ZnO substituted 45S5 bioactive glasses | ZnO substituted 45S5 bioactive glasses - ceramics | |||||||||||
Z1 | 77.84 30.89 54.12 0.260 | 102.75 | 40.97 | 69.64 | 0.254 | |||||||
Z2 | 78.63 31.23 54.27 0.259 | 103.57 | 41.33 | 69.96 | 0.253 | |||||||
Z3 | 79.43 31.57 54.77 0.258 | 104.79 | 41.85 | 70.45 | 0.252 | |||||||
Z4 | 80.32 31.95 55.15 0.257 | 105.88 | 42.32 | 70.88 | 0.251 | |||||||
Base 45S5 bioactive glass | Base 45S5 bioactive glass - ceramics | |||||||||||
45S5 | 76.74 30.43 53.77 0.261 | 101.57 | 40.47 | 69.33 | 0.255 |
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 2, February-2012 8
ISSN 2229-5518
110
105
Glasses
Glass - ceramics
110
105
Glasses
Glass - ceramics
100
100
95 95
90 90
85 85
80 80
75
0 1 2 3 4
Weight % of CuO
75
0 1 2 3 4
Weight % of Fe O
(A)
2 3
(B)
110
105
Glasses
Glass - ceramics
110
105
Glasses
Glass - ceramics
100
100
95 95
90 90
85 85
80 80
75
0 1 2 3 4
Weight % of MnO
2
(C)
75
0 1 2 3 4
Weight % of ZnO (D)
Figure 2: Variation in Young’s modulus for (A) CuO, (B) Fe2O3, (C) MnO2 and (D) ZnO substituted 45S5 bioactive glasses and
glass - ceramics
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 2, February-2012 9
ISSN 2229-5518
474
72
42
70
Glasses
Glass - ceramics
44
Glasses
Glass - ceramics
42
4608 40
66
38 38
64
36 36
60
3548 34
56
32 32
54
3502
0 1 2 3 4
Weight % of CuO
30
0 1 2 3 4
Weight % of Fe O
(A)
2 3
(B)
44
Glasses
Glass - ceramics
42
44
Glass
Glass - ceramics
42
40 40
38 38
36 36
34 34
32 32
30
0 1 2 3 4
Weight % of MnO
2
(C)
Figure 3: Variation in
30
0 1 2 3 4
Weight % of ZnO (D)
shear modulus for (A) CuO, (B) Fe2O3, (C) MnO2 and (D) ZnO substituted 45S5 bioactive glasses and glass - ceramics
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 2, February-2012 10
ISSN 2229-5518
0.262
0.261
0.260
0.259
0.258
0.257
0.256
0.255
0.254
0.253
0.252
0.251
0.250
0.249
0.248
Glasses
Glass - ceramics
0.26724
0.261
72
0.260
0.259
0.2568
0.25676
0.256
64
0.255
0.254
0.25630
0.2528
0.251
56
0.250
0.254
0.24582
Glasses
Glass - ceramics
Glasses
Glass - ceramics
0 1 2 3 4
Weight % of CuO
0 1 2 3 4
Weight % of Fe O
(A)
2 3
(B)
74
Glasses
72 Glass - ceramics
74
Glasses
72 Glass - ceramics
70 70
68 68
66 66
64 64
62 62
60 60
58 58
56 56
54 54
52
0 1 2 3 4
Weight % of MnO
2
(C)
Figure 4: Variation in bulk modulus for (A)
52
0 1 2 3 4
Weight % of ZnO (D)
CuO, (B) Fe2O3, (C) MnO2 and (D) ZnO substituted 45S5 bioactive glasses and glass - ceramics
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 2, February-2012 11
ISSN 2229-5518
0.262
0.261
0.260
0.259
0.258
0.257
0.256
0.255
0.254
0.253
0.252
0.251
0.250
0.249
0.248
Glasses
Glass - ceramics

0 1 2 3 4
Weight % of MnO
2
(C)
Figure 5: Variation in
Poisson’s ratio for (A)
0.262
0.261
0.260
0.259
0.258
0.257
0.256
0.255
0.254
0.253
0.252
0.251
0.250
0.249
0.248
Glasses
Glass - ceramics
0 1 2 3 4
Weight % of ZnO (D)
CuO, (B) Fe2O3, (C) MnO2 and (D) ZnO substituted 45S5 bioactive glasses and glass - ceramics
The XRD patterns of all the bioactive glass - ceramics show the presence of crystalline phases. The reason for the ease of
IJSER © 2012
International Journal of Scientific & Engineering Research, Volume 3, Issue 2, February-2012 12
ISSN 2229-5518
crystallization of bioactive glasses can be correlated with
the presence of silicate and phosphate network, as well as the possible phase separation even in micro scale of the two phases on heat - treatment. It is well known that the addition of a few percentage of P2O5 to silicate glass compositions, promotes the volume nucleation and glass - ceramic formation [10]. There is some evidence for precipitation of phosphate crystals which subsequently act as heterogeneous nucleation sites for the subsequent crystallization of the major phases, although the detailed role of P2O5 remains to be discussed [11]. Previous studies [12], [13], [14] have shown that the heat - treatment of 45S5 bioactive glass at a nucleation temperature of 550 0C and followed by heating at a crystallization temperature of 680
0C produces a bioactive glass - ceramic containing the sodium calcium silicate [Na2Ca2Si3O9] as main crystallline phase. In all the bioactive glass - ceramics sodium calcium silicate [Na2Ca2 Si3O9 & Na2CaSi3O8] is present as main crystalline phase. Crystalline phase of calcium silicate [CaSiO3] is also observed in each CuO substituted bioactive glass - ceramics (C1, C2, C3 and C4). The studied bioactive glass - ceramics did not contain Cu, Fe, Mn and Zn as separate crystalline phases. This can be related to their relatively low content in bioactive glass composition.
. The increase of CuO, Fe2O3, MnO2 and ZnO contents in the base bioactive glass (45S5) leads to an increase its density because of replacement of a lighter element, Si (density =
2.33 g/cm3) with a heavier element, Cu (density = 8.96
g/cm3), Fe (density = 7.86 g/cm3), Mn (density = 7.21 g/cm3)
and Zn (density = 7.14 g/cm3). The density of bioactive glass
- ceramics are higher than their respective bioactive glasses due to densification.
In general, the increase in ultrasonic wave velocities (longitudinal and shear) and elastic modulus of glasses are related to the increase in connectivity of the glass network [15], [16]. An increase in longitudinal and shear ultrasonic wave velocities and Young’s, shear and bulk modulus of
45S5 bioactive glass due to increase of CuO, Fe2O3, MnO2 and ZnO contents in it, can be explained by the decrease in the inter - atomic spacing which means that the copper, iron, manganese and zinc ions with octahedral coordination are involved in the glass network as modifiers by occupying the interstitial positions which cause the increase in the average number of the network bonds per unit volume. Therefore, it can be suggested that CuO, Fe2O3, MnO2 and ZnO modification leads to an increase in the network connectivity of the studied bioactive glasses. A decrease in Poisson’s ratio of 45S5 bioactive glass with increasing of network modifiers is attributed to increase the
crosslink density (defined as the number of bridging bonds
per cation) of glass network [17].
It is generally accepted that the controlled heat treatment of
glasses above the glass transition temperature and crystallisation temperature leads the possible formation of crystalline phases along with the residual glassy phases [18]. The observed increase in ultrasonic wave velocities and elastic modulus, and decrease in Poisson’s ratio of bioactive glass - ceramics is mainly due to the densification during thermal treatments.
Addition of CuO, Fe2O3, MnO2 and ZnO contents in 45S5 bioactive glass increases its density and elastic modulus: Young’s, shear and bulk modulus while a decreases its Poisson’s ratio. Crystallization of bioactive glasses enhances their density and elastic properties.
[1] S.M. Best, A.E. Porter, E.S. Thian and J. Huang, “Bioceramics: Past, present and for the future”, Journal of the European Ceramic Society, vol. 28, pp. 1319 - 1327, 2008. [2] M.N. Rahaman, D.E. Day, B.S. Bal, Q. Fu, S.B. Jung, L.F. Bonewald and A.P. Tomsia, “Bioactive glass in tissue engineering”, Acta Biomaterialia, vol. 7, pp. 2355 - 2373,
2011.
*3+ L.L. Hench and O. Andersson, “An Introduction to Bioceramics”, editors, L.L. Hench and J. Wilson, World Scientific, Singapore, pp. - 41, 1993.
[4] V. Aina, G. Malavasi, A.F. Pla, L. Munaron and C.
Morterra, “Zinc - containing bioactive glasses: Surface reactivity and behaviour towards endothelial cells”, Acta Biomaterialia, vol. 5, pp. 1211 - 1222, 2009.
*5+ C.C. Lin, L.C. Huang and P. Shen, “Na2CaSi2O6 - P2O5 based bioactive glasses. Part 1: Elasticity and structure”, Journal of Non - Crystalline Solids, vol. 351, pp. 3195 - 3203,
2005.
[6] V. Rajendran, A.N. Begum, M.A. Azooz and F.H. ElBatal, “Microstructural dependence on relevant physical - mechanical properties on SiO2 - Na2O - CaO - P2O5 biological glasses”, Biomaterials, vol. 23, pp. 4263 - 4275,
2002.
[7] K.A. Matori, M.H.M. Zaid, H.A.A. Sidek, M.K. Halimah, Z.A. Wahab and M.G.M. Sabri, “Influence of ZnO on the ultrasonic velocity and elastic moduli of soda lime silicate glasses”, International Journal of the Physical Sciences, vol.
5(14), pp. 2212 - 2216, 2010.
*8+ B. Eraiah, M.G. Smitha and R.V. Anavekar, “Elastic properties of lead - phosphate glasses doped with samarium trioxide”, Journal of Physics and Chemistry of Solids, vol. 71, pp. 153 - 155, 2010.
[9 ] M.H.M. Zaid, K.A. Matori, L.C. Wah, H.A.A. Sidek,
M.K. Halimah, Z.A. Wahab and B.Z. Azmi, “Elastic moduli
IJSER © 2012 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 3, Issue 2, February-2012 13
ISSN 2229-5518
prediction and correlation in soda lime silicate glasses
containing ZnO”, International Journal of the Physical
Sciences, vol. 6(6), pp. 1404 - 1410, 2011.
[10] F.H. ElBatal and A. ElKheshen, “Preparation and characterization of some substituted bioglasses and their ceramic derivatives from the system SiO2 - Na2O - CaO - P2O5 and effect of gamma irradiation”, Materials Chemistry and Physics, vol. 110, pp. 352 - 362, 2008.
*11+ P.F. James, “Glass ceramics: new compositions and uses”, Journal of Non - Crystalline Solids, vol. 181, pp. 1 -
15, 1995.
[12] L.L. Hench, R.J. Splinter, T.K. Greenlee and W.C. Allen, “Bonding mechanisms at the interface of ceramic prosthetic materials”, Journal of Biomedical Materials Research, vol. 2 (part 1), pp. 117 - 141, 1971.
*13+ L.L. Hench and H.A. Paschall, “Direct chemical bonding of bioactive glass - ceramic materials and bone”, Journal of Biomedical Materials Research: Symp., vol. 4, pp.
25 - 42, 1973.
[14] V.R. Mastelaro, E.D. Zanotto, N. Lequeux and R. Cortes, “Relationship between short - range order and ease of nucleation in Na2Ca2Si3O9, CaSiO3 and PbSiO3 glasses”, Journal of Non - Crystalline Solids, vol. 262, pp. 191 - 199,
2000.
[15] M.S. Gaafar, F.H. ElBatal, M. ElGazery and S.A. Mansour, “Effect of Doping by Different Transition Metals on the Acoustical Properties of Alkali Borate Glasses”, Acta Physica Polonica A, vol. 115, pp. 671 - 678, 2009.
[16] A.N. Kannappan, S. Thirumaran and R. Palani, “Elastic
and Mechanical Properties of Glass Specimen by Ultrasonic Method”, ARPN Journal of Engineering and Applied Sciences, vol. 4(1), pp. 27 - 31, 2009.
*17+ A. Higazy and B. Bridge. “Elastic constants and structure of the vitreous system Co3O4 - P2O4”, Journal of Non - Crystalline Solids, vol. 72, pp. 81 - 108, 1985.
[18] A.V. Gayathri Devi, V. Rajendran and N. Rajendran, “Ultrasonic characterisation of calcium phosphate glasses and glass - ceramics with addition of TiO2”, International Journal of Engineering Science and Technology vol. 2(6), pp. 2483-2490, 2010.
————————————————
■ Ankesh Kumar Srivastava, Ram Pyare and S. P. Singh
Department of Ceramic Engineering, Institute of Technology
Banaras Hindu University, Varanasi - 221005: INDIA
■ Corresponding Author
Ankesh Kumar Srivastava
E - mail: ankesh.000@gmail.com
IJSER © 2012 http://www.ijser.org