International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 20
ISSN 2229-5518
Effects of Salvia officinalis L. (sag) leaves Extracts in Normal and Alloxan-Induced Diabetes in White Rats
Kareem T. Khashan1, Karar Abbas Al-khefaji2
1 Assistant professor of Biotechnology, Department of Biology, College of Science, University of Kufa, Kufa, Iraq
2 Department of Biology, College of Science, University of Kufa, Kufa, Iraq
ABSTRACT: This study was conducted at the laboratories of the faculty of Science / university of Kufa from October 2012 to April 2013. The study was undertaken to investigate the effectn of aqueous and ethanolic extracts of Sage (Salvia officinalis L) leaves at concentration (100) mg/kg in dosage on albino rats for 14 days, on blood glucose , serum cholesterol and triglycerides (TG) level in induced-diabetic rats by alloxan (150) mg/kg compared with the reference drug Glibenclamide, Also, an evaluation of the active commercially available Sage Oil were analyzed by TLC . Results showed significant reduction (P< 0.05) of fasting blood glucose level in alloxan-induced diabetic rats treatment with plant extracts and glibenclamide drug as compared with infected control group. And the sage leaves extracts gave a good results, even better than glibenclamid drug for lowering blood sugar. The results also, showed a slight increase in fasting blood glucose level in normal rats when treatment with plant extracts as compared with healthy control group and showed a significant increase (P< 0.05) in the level of cholesterol compared with the healthy control group , also shown significantly decreased (P< 0.05) in the level of TG when treatment of diabetes rats with alcoholic and aqueous extracts of the plant leaves compared with the healthy control group . Rf values of spots and UV spectra (with and without adding the specific agents ) and compared with the literature data it was determined that the isolated compounds were Anetole,Thuijone,Camphor,α-Humulene,α-Terpinol,Gernaiol and Limonene
Keywords: Salvia officinalis, Diabetes mellitus, Alloxan, Blood sugar, Fasting, Alcoholic, Anetole
INTRODUCTION
—————————— ——————————
medicinal (Dweck, 2000; Miura et al., 2002). S. officinalis
is the specie of the genus Salvia with the highest EO
Diabetes Mellitus is a metabolic disorder characterized by

hyperglycemia due to effects in insulin secretion, action or both. Chronic hyperglycemia in diabetes is associated with long term damages, dysfunction and eventually the failure of organs, especially the eyes, kidneys, nerves and cardiovascular system (Vinik & Vinik, 2003). Currently available therapy for diabetes include insulin and various oral anti-diabetic agents such as sulfonylureas, metformin and α-glucosidase inhibitors. Each of the above oral agents suffers from a number of serious adverse effects (Zhang & Moller, 2000; Moller, 2001). Thus, it appears useful to look for new methods in treatment of diabetes. Medical plants are world widely used and many of they were
production (Giannouli & Kintzios, 2000), additionally, many other active compounds that gives it it's medicinal and aromatic properties and makes it a rich source of bioactive compounds (Giannouli & Kintzios, 2000; Dweck, 2000; Barnes et al., 2002; Lima, 2006).Salvia genus is a rich source of biologically active water soluble components, namely phenolic acids and flavonoids,Caffeic acid , rosmarinic acidand 1,8-cineole, cis-thujone, trans- thujone, camphor and borneol as a major volatile components (Lima et al., 2005 , Giannouli & Kintzios,
2000) . Common sage, since ancient times, has been an ingredient in perfumes, a flavoring in a variety of food preparations, and a medicinal plant used in folk medicine
*Corresponding author: Kareem T khashan
Corresponding author e-mail: dr.kareem63@yahoo.com
Tel: +9647830105020
es, for
der to effect
for the treatment of a variety of ailments (Malamas & Marselo, 1992), where many studies mentioned that sage have many of biological activities, such as antioxidant,
(Pushparaj et al., 2000; Alarcon-Aguilar et al., 2002;
Hosseinzadeh et al., 2002; Kameswararao et al., 2003;
Singh et al., 2007). Salvia officinalis L. (common sage, garden sage or Dalmatian sage) is a medicinal and aromatic plant of the Lamiaceae (= Labiatae) family, native to Mediterranean countries, which today is cultivated all over the world (Lima, 2006). The botanical name of sage is a clear reference to the important curative properties of the plant: the genus name Salvia comes from the Latin salvāre meaning “to save” or “to heal” and officinalis means
antibacterial, hyperglycemic and anti-inflammatory activities (Cherevatyî et al., 1980; Baricevic et al., 2001; Alarcon-Aguilar et al., 2002; Lima, 2006). Also other studies, conducted on Sage extracts and their EO, have shown it's hypotensive properties, anti-spasmodic effect and central nervous system-depressant activities (Newall et al., 1996). Addition to therapeutic effects for metabolic and endocrine diseases (Istudor, 2001). S. officinalis L. is among the plants that are claimed to be beneficial to
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 21
ISSN 2229-5518
diabetic patients, and previous studies have suggested that some of its extracts have hypoglycemic effects in normal and diabetic animals (Alarcon-Aguilar et al., 2002; Eidi et al., 2005; Lima et al., 2006; Eidi & Eidi, 2009).Within a short period of time thin layer chromatography has become a most important technique for the identification, characterization and determination of chemical compounds as well as complex mixtures. In the authors' laboratory the technique has been used with considerable success for the analysis of essential oils and their constituents. The purpose of the current study was to examine the hypoglycemic effects of aqueous and ethanol extracts of Salvia officinalis leaves in normal and alloxan- induced diabetic rats.
MATERILS AND METHODS
Collection and Classification of the plant: Sage was obtained from Mashhad, Iran and the plant samples were identified in botany laboratory, department of biology, Sciences faculty / university of Kufa. The leaves of plant were collected and dried, and then the dried plant samples were ground well into a fine powder and stored in darkish bags for later use. The treatment was conducted at laboratory conditions at the Plant research laboratory / Sciences faculty / university of Kufa. Preparation of aqueous and alcoholic extract : The alcoholic extraction process was conducted according to Hajzadeh and coworkers (2011) , (30g) of dried plant powder was packed in a filter paper type Watman (No.1) and extracted in a soxhlet apparatus using (450 ml) ethanol (90%) at (60 C°) for (13h). After extraction, the extract was filtered and concentrated by rotary evaporator, than dried by oven at (45 C°). The dry material was collected in closed bag and maintained at (4 C°) until use. For preparation of aqueous extract, (25g) of the dried plant powder were suspended in (500 ml) distilled water by rotation magnetic stirrer for an hour and a half, then it left for a period of (24 h). The mixture was filtered by filter paper type Watman (No.1) and centrifuged for (10 min) at (3000 r/m) to remove particulate substances. Then the extract was dried by oven in (45 C°), collected dry material and kept in (4 C°) until use (Harborn, 1984).
Thin layer chromatography analysis and reagents
Vanillin-sulfuric spray reagent was prepared by dissolving
1.5 gm vanillin in 2% ethanolic sulfuric solution( Ciesla et al 2001). Thin layer chromatography has been applied to the analysis of essential oils and their constituents from in vitro and in vivo of medicinal plant ( Ciesla et al 2001 ) . Rf-data are given for terpene compounds. Vanillin sulfuric acid (5% w./v.) is used as spray reagent and sensitivity limits are reported for the compounds examined. . The
plates silica gel TLC were then air-dried for twenty minutes and activated by heating in an oven at 100C for 15
minutes ,mobile phase solvent system composed of toluene
;acetate Ethyle solvent system in (7:93) v/v was used (Eukasz M. , 2010). Then placed into a specially designed chamber. The plates were developed to the distance of 90 mm. Then the plates were dried at room temperature for 15 min, prior to derivatization. Heating for 5 min at 105C the chromatoplate after spraying was found to bring about further characteristic color changes and increased moreover the sensitivity of the reagent, making it suitable for the detection of trace constituents . Plate images by the camera visualized under visible and UV light at 254 and
366 nm (Stafford et al ,2005 ) .Tentative identification of spots was achieved by comparison of values with those of authentic reference standards.
Animal Models: Albino rats were used in the experiment, Their weight ranged between (150-250 g), of either sex roughly the same age (4-6) months. These animals were subjected to identical laboratory conditions throughout the period of the research. The animals were divided into seven groups, each group (6) animals (3) males and (3) females: Group (1) dealing with natural food and water were considered as normal control .
Group (2) injected with alloxan drug and left without treatment, given distilled water only for 14 days and considered as diabetic control.
Group (3) normal rats dosage alcoholic extract of the Sage leaves (100) mg/kg of body weight as single dose per day for 14 days.Group (4) normal rats dosage aqueous extract of the Sage leaves (100) mg/kg of body weight as single dose per day for 14 days (Eidi et al., 2005; Upendra et al.,
2011; Ahmadi & Elahe, 2012). Group (5) diabetic rats
dosage alcoholic extract of the Sage leaves (100) mg/kg of body weight as single dose per day for 14 days. Group (6) diabetic rats dosage aqueous extract of the Sage leaves (100) mg/kg of body weight as single dose per day for 14 days. Group (7) diabetic rats dosage Glibenclamide drug (0.6) mg/kg of body weight as single dose per day for 14 days (Pari & Umamaheswari, 2000; Eliza et al., 2009; Erejuwa et al., 2011). The dosage process was taken place by using a plastic tube connected with syringe. After the haling of material (Sag leaves extracts and Glibenclamide drug) to the inside of the tube, given to the animals by the inputting of the plastic tube orally and through the esophagus into the stomach to ensure the entry of the material and the animals take it have fully . Diabetes was induced in rats by alloxan, where animals were starved for
18 hours before being injected with alloxan (150 mg/kg) of
body weight (Arbeeny & Bergquist, 1991) with an intraperitoneal single dose (Hadcock et al., 1991), alloxan was prepared before injection directly by using colder
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 22
ISSN 2229-5518
Citrate Buffer. Three days later, it was measured blood sugar level in rats treated with alloxan, animals that showed blood glucose level over (200 mg/dl) considered to have diabetes (Teixeira et al., 2002). It has been measuring blood sugar at a rate of once every two days throughout the dosage period (14) days on the same way as above through the wound the vein of guilt to sure the animals does not return to the natural state. At the end of the experiment, which lasted 14 days, The animals were fasted for 12 hours, after which take (3-5 ml) blood from heart by heart puncture. The blood Put in test tubes and left for 30 minutes until clotting, then took to the centrifuge (3000 r/m) for 15 minutes to separate serum and save it in special tubes, after that measured the level of blood glucose by a spectrophotometer and using measuring blood glucose kit.
Statistical Analysis: Results were analyzed statistically using a complete randomized design (CRD), and tested significantly with least significant differences (L.S.D) at level (P< 0.05) to indicate the significant of results (Al- Rawy and Kalafallh, 2000).
RESULTS AND DISCUSSION
The presence of sage plant phytochemical compounds was also detected by thin layer chromatography. TLC is a standard technique, which separates the organic compounds from lower molecular weight according to their polarity (Li et al,2004 ; Lima,2006). the developing solvent was able to separate different chemicals having different retention factor (Rf value) present in plant extracts .
The results show in the figure (1C ) present ( 7) spots with Rf value(0.32,0.37,0.72, 0.74, 0.81, 0.98,1.00) in alcoholic sage leaves extracts and shown 4 spots in TLC plat from aqueous sage leaves extracts(0.81, 0.77,0.74 and 0.32 ) (Figure 1B) , the results were documented and used for the comparison of the obtained profiles with the fingerprint of the authenticated reference material represent of Anetole,Thuijone,Camphor,α-Humulene,α- Terpinol,Gernaiol and Limonene).Shimoni et al,2003:Ovrar et al,2010with alcoholic extracts and (Limonine,Camphor,Thuijone and and Anethole )with aqueous extracts( Kart-Georg et al,2003, Kosales et
al,2005: Citoan et al,2010: ovrar et al,2010.). It is expected that more active compounds can be detected by
TLC bioautography, if different solvent systems, from the
TLC profiles of essential oil separated ,it appeared that were consisted of more than one constituents. In contrast in the preparation , the result farther suggested that the sage leaves do not seen contain any alkaloids compounds in free form by using Acetone , Water , NH4OH ( 90,7,3
)v/v/v (Al-Rubaei,,1999) Figure 1 ( E). In almost all the
investigated samples the presence of band corresponding
to identical compounds was indicated with an arrow
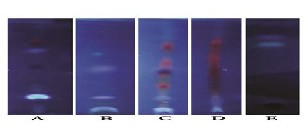
S.officinals plant.
Figure
1: Basic properties of chromatographically isolated compounds
from S. officinals leaves alcoholic and aqueous extracts
C. Rf value (0.32,0.37,0.72, 0.74, 0.81, 0.98,1.00) B. Rf value (0.81, 0.77,0.74 , 0.32 )
E.Rf value (no spots)
Estimation of the serum blood sugar level in normal and diabetic rats:
Results (Table 1) was indicated that the induced of diabetes in experimental animals led to a significant increase (P<
0.05) in the level of blood sugar (410.25 ± 6.38 mg/dl) compared with the healthy control group (89.25 ± 1.89 mg/dl), Results have shown presence significantly
decreased (P< 0.05) in the level of blood sugar when treatment of diabetes rats with alcoholic and aqueous extracts leaves plant as it was reaching (209 ± 5.87 mg/dl) and (209.5 ± 3.86 mg/dl), respectively, as well as they treatment with Glibenclamide drug (255 ± 9.33 mg/dl) compared with the infected control group. But alcoholic and aqueous extracts gives significantly decrease (P< 0.05) in the blood sugar level more than it by using Glibenclamide drug.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 23
ISSN 2229-5518
Also, the results, showed occurring a significant increase (P< 0.05) in the level of blood sugar when treatment of healthy animals with alcoholic and aqueous extracts (95.75
± 2.78 mg/dl) and (92 ± 2.48 mg/dl), respectively, compared with the healthy control group.
These results agreed with other studies conducted to investigate the effect of sage on hyperglycemia by other researchers using a different extracts and experimental methodology (Alarcon-Aguilar et al., 2002; Eidi et al.,
reducing effect of the serum cholesterol level, may be due
to the decomposition of saponin to sapognin in the gastrointestinal tract, which activates the secretion of bile acids by the liver. It was noted that saponin have formed with cholesterol insoluble complexes in the cavity of
TABLE 1
TABLE 1: EFFECT OF SAGE LEAVES EXTRACTS ON BLOOD SUGAR LEVEL IN NORMAL AND DIABETIC RATS.
Blood sugar level
2005; Eidi & Eidi, 2009).
Groups
mean ± S.E.M.
Estimation of the serum blood sugar level in normal and diabetic rats:
As The results (Table 2) was indicated to the presence of a
significantly increase (P< 0.05) in the total cholesterol level in diabetic control animals (112.2 ± 2.21 mg/dl) compared with the healthy control group (85.125 ± 1.89 mg/dl), these results agreed with what the many
Normal control 89.25 ± 1.89
Normal + Alcoholic extract 95.75 ± 2.78
Normal + Aqueous extract 92 ± 2.48
Diabetic control 410.25 ± 6.38
researchers have been reached (Bopanna et al., 1997; Al- A'miri, 2003), This cholesterol rise may be to increase the
Diabetic + Alcoholic extract
209 ± 5.87
cholesterol absorption by intestine due to increase activity of Acyl-Co-A: Cholesterol Acyl transferase that stimulated in the insulin absence, and the absence of insulin leads to
decrease of ApoE mRNA level thereby increasing the total cholesterol level (Maechler et al., 1993). In another explanation, the high level of cholesterol as a result of diabetes gets as a result of oxidation and the glycation process that occur on (LDL-C) or their receptors, where the large quantities of the cholesterol carried on (LDL-C) (Durlach et al., 1996). The treatment of diabetes rats with alcoholic and aqueous extracts of sage leaves, it has led to obtain significant decrease (P< 0.05) in the total cholesterol level (88.125 ± 2.29 mg/dl) and (87.375 ± 1.14 mg/dl), respectively, as well as they treatment with Glibenclamide drug (94.15 ± 2.78 mg/dl) compared with the infected control group, but the decrease in the total cholesterol level as a result of treatment with a Glibenclamide drug less than it when treatment with alcohol and aqueous extracts of plant leaves. The results showed, also, a significant decrease (P< 0.05) in total cholesterol level of healthy animals groups that treatment with alcoholic and aqueous sage leaves extracts as it was (81.6 ± 1.06 mg/dl) and (81 ±
1.06 mg/dl), respectively, when compared with the healthy control group. This agree with the results of Alayan (2006), Khattab and his group (2012) and Behradmanesh and his group (2013), which have shown the activity of sage plant in reducing the serum cholesterol level.The reason of this, perhaps due to the containment of sage leaves on high percentage of active components lectin and saponin, that responsible for hypolipidemic effects (Alayan, 2006). Lectin is proved to have a significant effect in lowering both serum and hepatic cholesterol (Okazaki et al., 2005), Sauvaire and his group (1996) explained that the saponin
Diabetic + Aqueous extract 209.5 ± 3.86
Diabetic + Drug 255 ± 9.33
LSD (P< 0.05) 2.63
gastrointestinal tract, and these complexes inhibit the cholesterol absorption from the intestine, leading to excrete it with waste and thus lower its level in the blood. Also, the saponin have ability to sticking with bile acids and neutral fat in the intestine and inhibited its absorption, and then reduced its level in the blood and stimulate the liver to convert cholesterol into bile acids (Spiller, 1996). This influence effective of S. officinalis on the level of total cholesterol may be due to its contain sterols (Newall et al.,
1996; Capasso et al., 2003), which showed many of studies to be effective in reducing the level of total cholesterol in the blood (Moreau et al., 2002). As for the level of triglycerides, the results of table (2) showed there is a significant increase (P< 0.05) in triglycerides level of the diabetic control group as it was (104 ± 2.20 mg/dl) compared with the healthy control group (63.575 ± 2.78 mg/dl), and this agreed with study of Pari & Latha (2002) and Al-A'merri (2003). This rise returns to the metabolic disorders that associated of diabetes mellitus, where the body depends on the analysis of fats in adipose tissue to fill its needs of energy because inability to use of the high glucose that found in the blood, thus the result be the high level of serum TG (Howard, 1999). And the lack of insulin leads to inhibition the activity of the enzyme Lipoprotein Lipase (LPL), which causes the reduction of the process of triglycerides removing in chilomicrone and VLDL result to be converted into fatty acids and glycerol, and therefore
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 24
ISSN 2229-5518
rising its level in the blood (Bishop, 2000). When the diabetes rats was treatment with alcoholic and aqueous extracts of sage leaves has led to obtain significant decrease (P< 0.05) in the triglycerides level where its level reached (65.7 ± 1.67 mg/dl) and (63.25 ± 1.76 mg/dl), respectively, as gets this decrease when the diabetes rats had treatment with drug (70 ± 1.83 mg/dl) compared with the infected control group, but the aqueous and alcoholic extracts gave a significant decrease (P< 0.05) in the triglycerides level larger than the level of decrease by using the glibenclamide drug. The triglycerides level of healthy animals groups that treated with alcoholic and aqueous extracts of plant leaves has decrease significantly (P<
0.05), as was (59.5 ± 1.43 mg/dl) and (60.375 ± 1.52 mg/dl), respectively, compared with the healthy control group.
This is agrees with several studies that reported the impact
effective of the plant in reducing the triglycerides level
(Carla et al., 2009; Kianbakht et al., 2011; Khattab et al.,
2012), but its disagree with what Alayan (2006) was
reached, which reported lack the significant effect of sage aqueous extract on TG level in the blood. This lowering of triglycerides levels in treatment animals with S. officinalis extracts, due to the anti-oxidative role of the plant, It became clear his role effectively in preventing lipid peroxidation therefore prevent the lyses of lipid (Cuppett & Hall, 1998; Miura et al., 2002; Jaswir et al., 2005), as diabetes is one of the factors that cause oxidative stress (West, 2000).
Also, Carla and his group (2009) and Kianbakht and his
group (2011) reported that this effect may be due to the ability of S. officinalis to suppress the cholesterol biosynthesis, as a result of it contains active compounds have effected on the cholesterol metabolism by reducing its absorption or synthesized such as Thujone, which decrees the level of cholesterol and triglycerides.
The phytosterols in sage plant also have an impact affected
on the triglycerides level, where many of the studies confirmed the effective of these compounds in reducing the level of serum triglycerides (Plat & Mensink, 2009). In
addition, the improvement that made in the level of blood
glucose and increase insulin sensitivity as a result of treatment with sage leaves extracts and glibenclamide drug, lead to correct metabolic pathways and reduce the lyses of lipid in the tissues therefore decreases its level in the blood, this is referred to it by Swenson (1991). The results (Table 2) indicated also, that the aqueous extract of Sage leaves gave better effect in reducing the level of blood lipids than alcoholic extract and drug.
CONCLUSIONS
Our results showed significant reduction (P< 0.05) of fasting blood glucose level in alloxan-induced diabetic rats
treatment with plant extracts and glibenclamide drug as compared with infected control group. And the sage leaves extracts gave a good result, even better than glibenclamid
drug for lowering blood sugar. The results also, showed a slight increase in fasting blood glucose level in normal rats when treatment with plant extracts as compared with healthy control group and showed a significant increase (P< 0.05) in the level of cholesterol compared with the healthy control group , also shown significantly decreased (P< 0.05) in the level of TG when treatment of diabetes rats with alcoholic and aqueous extracts of the plant leaves compared with the healthy control group . Rf values of spots and UV spectra (with and without adding the specific agents ) and compared with the literature data it was determined that the isolated compounds were Anetole,Thuijone,Camphor,α-Humulene,α- Terpinol,Gernaiol and Limonene. Our results also indicated that improvements made in the level of blood glucose and increase insulin sensitivity as a result of treatment with sage leaves extracts and glibenclamide drug, lead to correct metabolic pathways and reduce the lyses of lipid in the tissues therefore decreases its level in the blood, also, the aqueous extract of Sage leaves gave better effect in reducing the level of blood lipids than alcoholic extract and drug.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 25
ISSN 2229-5518
REFERENCES
[1] Ahmadi R & Elahe A. The effects of salvia officinalis extract on serum level of creatine kinase and alkaline phosphatase in male rats. Razi Journal of Medical Sciences RJMS. 2012, 19(96): pp. 20-25.
[2] Al-A'miri AK. Effect of plant extracts on blood sugar
level in healthy and experimental diabetic male rats. M.Sc. Thesis. Faculty of Education, University of
TABLE 2
TABLE 2 : EFFECT OF SAGE LEAVES EXTRACTS ON
CHOLESTEROL AND TG LEVELS IN HEALTHY AND DIABETIC RATS
Tikrit. Iraq. 2003.
[3] Alarcon-Aguilar FJ, Roman-Ramos R, Flores-Saenz JL
& Aguirre-Garcia F. Investigation on the hypoglycaemic effects of extracts of four Mexican medicinal plants in normal and alloxandiabetic mice. Phytother Res. 2002, 16: pp. 383–386.
[4] Alayan I. The effects of Salvia officinalis leaves on hyperlipidemia, glycemia, ulcer, inflammation and bactericidal activity. M.Sc. Thesis. School of Arts and Sciences, Lebanese American University. Lebanon.
2006.
[5] AL-Rawy K & Kalafallah A. Design and analysis of
agricultural experiments. Ministry of Higher Education and Scientific Research, Library Printing & Publishing
/ AL-Mousel University. 2nd edition, 2000. (In
Arabic).
[6] Al-Rubaei ,H.M .Effect of Datura Metel Mill in Musca Domestic , Ph.D thesis ,Collage of Scince, Baqbylone univirsety.1999 .
[7] Arbeeny C & Bergquist K. The effect of paravastation on serum cholesterol in hypercholesterolemic diabetic rabbits. Biochem. Biophys. Acta.1991, 1096 (3): pp.
238-244.
[8] Aziz BN. Some biochemical variables in cases of hunger, oxidative stress and experimental diabetes in rats. Effect of some medicinal plants and female sexual hormones. PhD thesis. Faculty of Veterinary Medicine, University of Mosul. Iraq. 1999
[9] Baricevic D & Bartol T. The
biological/pharmacological activity of the Salvia genus. In: S.E. Kintzios, (editor), SAGE - The Genus Salvia, Amsterdam, PA: Harwood Academic Publishers. 2000, pp. 143-184.
[10] Baricevic D, Sosa S & Della LR, Topical anti- inflammatory activity of Salvia officinalis L. leaves: the relevance of ursolic acid, J Ethnopharmacol. 2001,
75: pp. 125–132.
[11] Barnes J, Anderson LA & Phillipson JD. Herbal
Medicines, Pharmaceutical Press, London, 2002.
[12] Behradmanesh S, Fatemeh D and Mahmoud R.
Effect of Salvia officinalis on diabetic patients. Journal
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 26
ISSN 2229-5518
of Renal Injury Prevention J Ren Inj Prev. 2013. 2(2):
51-55.
[13] Bishop M, Duben–Engelkirk J and Fody E.
Clinical chemistry. 4th ed., Lippincott Williams and
Wilkins, Philadelphia, USA. 2000. 220–221.
[14] Bopanna K, Kannan J, Gadgil S, Balarman R and Rathod S. Antidiabetic and antihyperlipaemic effect of Nemm seed kernel powder on Alloxan diabetic Rabbits. J. Pharmacol. Indian. 1997. 29: 162-167.
[15] Capasso F, Gaginelia T, Grandolini G, & Izzo A.
Phytotherapy - A Quick Reference to Herbal Medicine, Naples, Italy. 2003, pp. 64-257.
[16] Carla MS, Alice AR, Marisa FA, Cristovao FL,
Manuel FF and Cristina PW. Sage tea drinking improves lipid profile and antioxidant defenses in Humans. Int. J. Mol. Sci. 2009. 10 (9): 3937-3950.
[17] Cherevatyî VS, Vashhenko TN & Shishkov GZ.
Comparative evaluation of the antibacterial action of different extracts from Salvia officinalis, Rastitel’nye Resursy. 1980, 16: pp. 137–139.
[18] Cuppett SL and Hall CA. Antioxidant activity of the Labiatae. In: Advances in Food and Nutrition Research, Academic Press, London. 1998. 245-271
[19] Duke JA. Handbook of Medicinal Herbs. Boca
Raton: CRC Press. 1985, pp. 420-421,565.
[20] Durlach V, Atlia N, Zahouani A, Leutenegger M
and Girard A. Atherosclerosis. 1996. 120: 155-165.
[21] Dweck AC. The folklore and cosmetic use of various Salvia species. In: Kintzios SE (Ed.), medicinal and aromatic plants—industrial profiles, Sage - The Genus Salvia, Harwood Academic Publishers, Amsterdam. 2000, 14: pp. 1-25.
[22] Eidi A & Eidi M. Antidiabetic effects of Sage (Salvia officinalis L.) leaves in normal and streptozotocin-induced diabetic rats. Diabetes & Metabolic Syndrome: Clinical research & Revies.
2009, 3: pp. 40-44.
[23] Eidi M, Eidi A & Zamanizadeh H. Effect of Salvia officinalis L. leaves on serum glucose and insulin in healthy and streptozotocin-induced diabetic rats. J Ethnopharmacology. 2005, 100(3): pp. 310-316.
[24] Eliza J, Daisy P, Ignacimuthu S & Duraipandiyan V. Normo-glycemic and hypolipidemic effect of costunolide isolated from Costus speciosus in streptozotocin-induced diabetic rats. Chem Biol Interact. 2009, 179(2-3): pp. 329-334.
[25] Erejuwa OO, Sulaiman SA, Wahab MS,
Sirajudeen KNS, Salleh MS & Gurtu S. Effect of Glibenclamide alone versus Glibenclamide and Honey on Oxidative Stress in Pancreas of Streptozotocin- Induced Diabetic Rats. International Journal of Applied Research in Natural Products. 2011, 4(2): pp.
1-10.
[26] Eukasz,M.C and Monika ,W.H) . Application of
Thin-Layer chromatography for the quality control and screening the free radical scavenging activity of selected pharmacuetical preparations containing Salvia officinals L.extract.Acta,Poloniae pharmaceutica Drug research.Vol.67 No.5(2010) pp48 in 485.
[27] Giannouli AL & Kintzios SE. Essential oils of
Salvia spp: examples of intraspecific and seasonal variation. In: S.E. Kintzios, (editor), SAGE - The Genus Salvia, Amsterdam, PA: Harwood Academic Publishers. 2000, pp. 69-80.
[28] Guarino MP, Afonso RA, Raimundo N, Raposo JF
& Macedo MP. Hepatic glutathione and nitric oxide
are critical for hepatic insulin-sensitizing substance action. Am J Physiol Gastrointest Liver Physiol. 2003,
284: pp. 588–594.
[29] Hadcock S, Richardson M, Winocour P & Hatton
M. Intimal alterations in rabbit aortas during the first 6 months of allaxon-induced diabetes. Canada. American Heart Association. Arteriosclerosis and Thrombosis.
1991, 11: pp. 517-529.
[30] Hajzadeh M, Rajaei Z, Ghamami A & Tamiz A.
The effect of salvia officinalis leaf extraction on blood
glucose in streptozotocin-induced Diabetic rats. School of medicine, Mashhad Uni. of med. Sci, Iran. Pharmacology–line. 2011, 1: pp. 213-220.
[31] Han YM, Oh H, Na M, Kim BS, Oh WK, Kim BY,
Jeong DG, Ryu SE, Sok DE & Ahn JS. PTP1B inhibitory effect of abietane diterpenes isolated from Salvia miltiorrhiza. Biol Pharm Bull. 2005, 28: pp.
1795–1797.
[32] Harborne JB. Phytochemical Methods - A Guide to Modern Techniques of Plant Analysis, 2nd ed. Chapman and Hall, London. 1984.
[33] Haugland RP, Johnson IB. Intracellular ion indicators in fluorescent and luminescent probes, (2nd edn). Academic Press.1999; p. 40.
[34] Hosseinzadeh H, Ramezani M & Danaei AR. Anti hyperglycemic effect of Securigera securidaca L. seed extract in mice. Phytother Res. 2002, 16: pp. 745-747.
[35] Hostettmann K, Wolfender JL. The search for biologically active secondary metabolites. Pesticid Sci
1997; 51: 471‐482.
[36] Istudor V. Farmacognozie. Fitochimie. Fitoterapie,
Editura Medicala, Bucuresti. 2001.
[37] Jaswir I, Che Man YB and Hassan TH.
Performance of phytochemical antioxidant systems in
refined-bleached-deodorized palm olein during frying. Asia Pacific Journal of Clinical Nutrition. 2005. 14:
402-413.
[38] Kameswararao B, Kesavulu MM & Apparao C.
Evaluation of antidiabetic effect of Momordica
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 27
ISSN 2229-5518
cymbalaria fruit in alloxan-diabetic rats. Fitoterapia.
2003, 74: pp. 7-13.
[39] Kianbakht S, Abasi B, Perham M and Hashem F.
Antihyperlipidemic effects of Salvia officinalis L. leaf extract in patients with hyperlipidemia: A randomized double-blind placebo-controlled clinical trial. Phytother. Res. 2011. 25(12): 1849-1953.
[40] Li WL, Zheng HC, Bukuru J & De Kimpe N.
Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol. 2004, 92: pp. 1–21.
[41] Lima CF, Andrade PB, Seabra RM, Fernandes- Ferreira M & Pereira-Wilson C. The drinking of a Salvia officinalis infusion improves liver antioxidant status in mice and rats. J Ethnopharmacol. 2005, 97: pp. 383–389.
[42] Lima, Christopher Fernando Macedo. Effects of
Salvia officinalis in the liver: Relevance of glutathione levels. PhD Thesis. School of Sciences, University of Minho. Portugal. 2006.
[43] Maechler P, Wollheim C, Bentzen C and Niesors E. Importance of exogenous cholesterol in diabetic rats: Effect of treatment with insulin or with an Acyl- CoA: Cholesterol acyl tranceferase inhibitor. Ann. Nutr. Metab. 1993. 37: 99-209.
[44] Malamas M and Marselo SM. The tradition of medicinal plants in Zagori, Epirus (Northwestern Greece), J. Ethnopharmacol. 1992, 37: pp. 197-203.
[45] Medical Economics Company. PDR for Medicinal
Herbs, 2nd ed. Montvale, N. J.: Medicinal Economics.
1998, pp. 655,656.
[46] Miura K, Kikuzaki H & Nakatani N. Antioxidant activity of chemical components from sage (Salvia officinalis L.) and thyme (Thymus vulgaris L.) measured by the oil stability index method. Journal of Agricultural and Food Chemistry. 2002, 50: pp. 1845-
1851.
[47] Moller DE. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001, 414: pp.
821-827.
[48] Moreau RA, Whitaker BD and Hicks KB.
Phytosterols, phytostanols and their conjugates in foods: structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res. 2002. 41: 457-
500.
[49] Newall C, Anderson LA & Phillipson JD. Herbal Medicine - A Guide for Health-Care Professionals, London. 1996, PP. 231-232,330-334.
[50] Okazaki Y, Katayama T and Hori K. Dietary lectin lowers serum cholesterol and raises fecal neutral sterols in cholesterol-fed rats. Journal of Nutritional Sciences and Vitaminology. 2005. 51(5): 343-348.
[51] Pari L & Umamaheswari J. Anti hyperglycemic activity of Musa sapientum flowers: effect on lipid
peroxidation in alloxan diabetic rats. Phytother Res.
2000, 14(2): pp. 136-138.
[52] Pari L and Latha M. Effect of Cassia Auriculata Flowers on Blood Sugar Levels, Serum and Tissue Lipids in Streptozotocin Diabetic Rats. Singapore Med. J. 2002. 43(12): 617-621.
[53] Plat J and Mensink RP. "Plant Stanol Esters Lower Serum Triacylglycerol Concentrations via a Reduced Hepatic VLDL-1 Production". Lipids. 2009. 44(12):
1149–1153.
[54] Pushparaj P, Tan CH & Tan BKH. Effects of
Averrhoa bilimbi leaf extract on blood glucose and lipids in streptozotocin-diabetic rats. J Ethnopharmacol. 2000, 72: pp. 69–76.
[55] Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y
& Sato Y. Gosha-jinki-gan (a herbal complex) corrects abnormal insulin signaling. Evid Based Complement Alternat Med. 2004, 1: pp. 269–276.
[56] Sauvaire Y, Bassiac Y, Leconte O, Petit P and Rebes G. Steroid saponins from fenugreek and some of their biological properties. Adv. Exp. Med. Biol. 1996.
405: 37-46.[59] Spiller RC. Cholesterol, fiber and bile acid. Lancet. 1996. 347: 415-416.
[57] Saxena A & Vikram NK. Role of selected Indian plants in management of type 2 diabetes: a review. J Altern Complement Med. 2004, 10: pp. 369–378.
[58] Singh SK, Kesari AN, Gupta RK, Jaiswal D & Watal G. Assesment of antidiabetic potential of Cynodon dactylon extract in streptozotocin diabetic rats. J Ethnopharmacol. 2007, 114: pp. 174-179.
[59] Stafford GI, Jager AK, van Staden J. Effect of storage on the chemical composition and biological activity of several popular South African medicinal
plants. J Ethnopharmacol 2005; 97:107‐115.
[60] Swenson TL. The role of cholesteryl estertransfer
protein in lipoprotein metabolism. Diabetes Metab. Rev. 1991. 7: 139–153.
[61] Teixeira C, Rava C, Silva P, Melchior R, Argenta
R, Anselmi F, Almeida C & Fuchs F. Absence of antihyperglycemic effect of Jambolan in experimental and clinical models. J. Ethano Pharmacol. 2002, 71: pp. 343 – 347.
[62] Upendra B, Sweta T, Praveen S, Shravan B & Mahendra M. Diuretic activity of extract of Salvia officinalis L. Asian Journal of Pharmacy & Life Science. 2011, 1 (1): pp. 24-28.
[63] Vinik AI & Vinik E. Prevention of the complications of diabetes. Am J Manag Care. 2003, 9: pp. 63-80.
[64] West IC. Radicals and oxidative stress in diabetes.
Diabetic Medicine. 2000. 17: 171-180
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 28
ISSN 2229-5518
[65] Zhang BB & Moller DE. New approaches in the
treatment of type 2 diabetes. Curr Opinion Chem Biol.
2000, 4: pp. 461-467.
IJSER © 2015 http://www.ijser.org


