International Journal of Scientific & Engineering Research Volume 3, Issue 7, June-2012 1
ISSN 2229-5518
Effects of Salinity on Growth and Total Lipid Con- tent of the Biofuel Potential Microalga Ankistro- desmus falcatus (Corda) Ralfs
Jayanta Talukdar, Mohan Chandra Kalita and Bhabesh Chandra Goswami
Abstract— Growth responses and total lipid content of a native strain of the biofuel potential freshwater oleaginous microalgae A.falcatus was studied owing to its inherently high lipid content for potential utilization as renewable biomass feedstock of biofuels. Influences of salinity in increasing order from 40 mM to 320 mM of NaCl in BG11 medium on growth (µ), total lipid (TL) content and calorific value (CV) were studied in triplicate batch mode culture at light intensity 35 µmol/m2/s, temperature 25 ± 2 0C and 16:8 hrs light and dark diurnal cycles. Enhanced growth and total lipid contents were observed with increasing salinity up to 160 mM NaCl. The highest specific growth (µ=0.313 d-1) and least doubling time (T =2.21 days) with maximum increase in cell numbers (2.9 x 105 ml-1) were recorded in medium supplemented with 160 mM of NaCl compared to control medium (µ=0.209 d-1, T2=3.32 days and 1.52 x 105 ml-1 respectively). Improved total lipid (55.3%), carbohydrate (14.5%), and protein (4.8%) contents were also determined compared to control medium (lipid 38.3%, carbohydrate 12.6%, and protein 3.1%, respectively). W ith maximum energy value of 27.9 ± 0.15 kJg-1, a close correlation (R2 = 0.955) between lipid content and calorific value was observed. W ith the support from present research findings, the native strai n of freshwater oleaginous microalga A. falcatus could be a potent candidate for production of renewable biomass feedstock of biofuels. The present research findings will be supportive towards further culture optimization for increased biomass yield with c oncomitant lipid content and
improved of fatty acid profile in mass cultivation.
Index Terms— Ankistrodesmus falcatus, Biofuel, Biomass, Calorific value, Lipid, Microalgae, Oleaginous, Renewable, Salinity
—————————— ——————————
WING to the global concern of energy crisis and the ap- proaching depletion of relatively low-cost petroleum- sourced primary energy, in addition to its staggering effects on the global economy, coupled with the consequences of burning fossil fuels towards environment, the quest for car- bon neutral renewable sources alternative energy and devel- opment of energy efficient technology has become the center stage of current energy research and policies. The use of bio- mass as a renewable source of alternative energy has been greatly motivated from the standpoint of both global energy
crisis and environmental issues.
Energy from renewable biomass sources has gained global
attention with the potential to substitute for fossil fuels in all
energy markets – in the production of heat, electricity, and
fuels for transport. Concerted efforts are now underway glo-
bally to harness alternative energy from renewable biomass
sources generated via photosynthesis to move towards more
sustainable bio-based economies where biomass-derived
products substitute for petrochemical-derived products [1].
Development of first and second generations of biofuels,
which are based on different categories of biomass feedstock
and their downstream processing and typically obtained from
————————————————
![]() Mr Jayanta Talukdar is currently pursuing doctoral research in the Depar t- ment of Biotechnology, Gauhati University, Guwahati – 14, Assam, INDIA, Ph: 0091-9954395143. E-mail: jayantabiotech.gu@gmail.com
Mr Jayanta Talukdar is currently pursuing doctoral research in the Depar t- ment of Biotechnology, Gauhati University, Guwahati – 14, Assam, INDIA, Ph: 0091-9954395143. E-mail: jayantabiotech.gu@gmail.com
![]() Dr Mohan Chandra Kalita, Professor, Department of Biotechnology, Gauhati
Dr Mohan Chandra Kalita, Professor, Department of Biotechnology, Gauhati
University, Guwahati –14, Assam, INDIA. E-mail: mckalita@sify.com
![]() Dr Bhabesh Chandra Goswami, Professor, Department of Chemistry, Gauh a- ti University, Guwahati – 14, Assam, INDIA. E-mail: bcg2005@sify.com
Dr Bhabesh Chandra Goswami, Professor, Department of Chemistry, Gauh a- ti University, Guwahati – 14, Assam, INDIA. E-mail: bcg2005@sify.com
starch, vegetable oil and cellulosic crops, require cultivated land and water supplies that can compete with land use for food production [2 - 4]. Extensive cropping of plants for bio- fuel production, which will certainly take place with the in- creasing fuel demand, raises a food vs fuel dilemma, and also natural resource demand problems [5 - 8]. With such limita- tions of first and second generation biofuels, microalgae based third and further generation of biofuels have gained great in- terest [9 - 14].
Microalgae are considered to represent the most promising among existing renewable biomass sources to generate biofu- els. They are also likely to have much lower adverse effect on the environment and on the world’s food supply than conven- tional biofuel-producing crops. The unique diversity of micro- algae and the spectrum of species available for amenability for biofuel production as compared to other advanced biomass feedstock have placed microalgae on the priority list. Various species may be selected to optimize the production of different biofuels. Microalgae biomass can be utilized to produce varie- ties of fuels, such as liquid fuels and gas, gas-or oil-based bio- fuels, bioethanol or methanol, biohydrogen, and biodiesel [2], [3], [5], [10 - 25]. Moreover, microalgae offer a diverse spec- trum of valuable products and pollution solutions, such as food, nutritional compounds, omega-3 fatty acids, animal feed, organic fertilizers, biodegradable plastics, recombinant proteins, pigments, medicines, pharmaceuticals, and vaccines [26].
High lipid content with favorable fatty acid compositions, faster growth rate, ease of harvest, and dominance in nature with the ability to adapt in the prevalent climatic conditions are some of the desirable criteria for choosing the right species for biofuel production [21]. Isolation of native strains from
IJSER © 2012
International Journal of Scientific & Engineering Research Volume 3, Issue 7, June-2012 2
ISSN 2229-5518
local water bodies can provide the best information regarding the selection of appropriate strain(s) in terms of biomass and lipid productivity, dominance in the native algal flora, and hence the likelihood of being the most suitable selection as a biomass feedstock [27 - 29]. In addition to the species selection, nutritional requirement for culture optimization is another essential factor that significantly affects the growth rate and yield of products [30]. Few microalgae species can double its biomass in less than 24 hours under optimum culture condi- tions [10]. Such high yield and high density biomass is ideal for intensive cultivation and can provide an excellent biomass source of biofuels. Oil levels 20 to 50% by dry weight biomass are quite common, and some can exceed 80% [31]. Significant increases in lipid contents have been reported in microalgae under various stress conditions during cultivation such as ni- trogen deprivation [30], [32], high light intensity [33], high salinity [34], [35], phosphate limitation [36], or co-immobilized in alginate beads with bacterium Azospirilium brasilense [37], [38]. Stress conditions also have negative influence on growth, resulting low overall biomass production. Therefore, it is more appropriate to apply stress conditions in the later stage of growth after initial biomass production [39].
Besides production of biodiesel from microalgae oil, Illman et al. [18] reported the use of dried powder of the microalgae Chlorella as fuel together with a mixture of 85% cellulose powder and 15% diesel to run a diesel engine. A high calorific value of the microalga is essential to be act as a fuel, which chiefly depends on the lipid content besides carbohydrate and protein. An increasing calorific value is directly related to an increase in lipid content [18].
The present study was focused on improvisations of growth, lipid content and calorific value of a native strain of the freshwater microalgae A.falcatus towards potential utiliza- tion as renewable biomass feedstock of biofuels. The principal objectives were to investigate the influences of medium consti- tuents and salinity on growth, total lipid yield and calorific value of the A.falctus biomass.
TABLE 1
COMPONENTS OF CULTURE MEDIA
![]()

Constituents (g/L) BBM BG11
NaNO3 1.0 1.5
CaCl2 2H2O 0.025 0.036
MgSO47H2O 0.075 0.075
K2HPO4 0.075 0.4
NaCl 0.025 Nil
EDTA Nil 0.001
Citric acid Nil 0.006
Na2CO3 Nil 0.2
FeCl3 6H2O 0.005 Nil
NaMoO4 4.2 x 10-5 2.1 x 10-5
ZnSO47H2O 4.4 x 10-4 2.2 x 10-4
MnCl24H2O 3.6 x 10-3 1.8 x 10-3
CuSO45H2O 1.6 x 10-5 0.8 x 10-5
H3BO4 5.7 x 10-3 2.8 x 10-3
Native strain of freshwater microalga A. falcatus was isolated from water samples collected from the wetland Deepor Beel (a Ramsar site), latitude 26º 13´ N and longitude 91º 66´ E of Gu- wahati, Assam, India. Isolation of the microalga and its mono- culture was raised in BG11 [40] medium after several dilutions and streaking into agar plate (1% w/v agar in BG11 medium) according to Kawai et al. [41]. Purity of the culture was pe- riodically checked under optical microscope (LOBAMED, Model: ATC 2000) as well as streaking into agar plates. Sub- culture was done after every third week of inoculation.
Morplogical characteristics were studied under the optical microscope. Microphotographs were taken with the help of a digital camera (SONY Model: DSC-W100; 8.1 MP). The micro- alga was initially identified by comparing the morphological data with standard literature.
Constituents of culture media used in this investigation are given in Table 1. Mono-culture of the isolated strain was raised and maintained aseptically in autoclaved BG11 medium under laboratory conditions having: irradiation 35 to 40
µmoles/m2/s; temperature 25 ± 2 º C, and photoperiod of 16:8 hrs light and dark diurnal cycles. The pH of the medium was adjusted to 7.5 with either 1N HCl or 1N KOH prior to autoc- laving.
A batch culture in triplicate was conducted using 100 ml of BG11 and Bold basal (BB) [42] media in Erlenmeyer flasks to compare the growth responses of the studied microalga under stated laboratory conditions. The pH of the media was ad- justed to pH 7.5 as stated earlier. Growth study was conducted for a period of 12 days of culture duration.
Salinity induced influences on growth responses of the micro- alga strain were studied in batch culture using 100 ml of BG11 medium in 250 ml Erlenmeyer flask, supplemented with NaCl in increasing order (40, 80, 160 and 320 mM). BG11 medium without addition of NaCl was used as control. Initial growth study was conducted in batch mode using. To get sufficient biomass for quantitative estimation of lipid and other bio- chemical test, cultures were raised to 500 ml in 1000 ml Erlen- meyer flask under similar growth conditions. Exponentially growing culture (20% v/v) was used as inoculums. Studies were conducted in triplicate under similar growth conditions, as stated earlier. Cells harvested after 21days of culture dura- tions were used compare total cellular lipid, carbohydrate and protein content in each of the treatments. Calorific values were also compared from the same biomass produced.
Growth was determined from the respective growth curves developed by plotting the observed cell numbers (cells/ml) against time of observations (days). Cell numbers were deter-
IJSER © 2012
International Journal of Scientific & Engineering Research Volume 3, Issue 7, June-2012 3
ISSN 2229-5518
mined from direct cell counts using a Neubour Haemocytome- ter. For each count average value from the data generated from ten replicates of each study. The specific growth rate (µ) and doubling time (T2) were calculated according to Levasseur et al. [43] using equations (1) and (2):
µ = ln (N2/N1) / (t2-t1) (1) T2 = 0.6931/µ (2) Where, N1 and N2 are the biomass at time (t1) and time (t2),
respectively.
Biomass was determined as dry cell weight (DCW) and measured gravimetrically. A known volume of culture was filtered through pre-weighed and pre-combusted GF/C filter paper. The filtered cell mass was oven dried at 80 °C for 6 hrs until constant weight. It was cooled down to room tempera- ture in desiccators and measured the dry weight of the sample using an analytical balance with a precision of 0.1 mg. Biomass was expressed in grams per liter (g/L).
Total cellular protein and carbohydrate contents were deter- mined from a known amount of freeze dried cell biomass har- vested at the end of the logarithmic phase of growth according to Lowry [44] and Hedge et al. [45].
Total lipid content was determined quantitatively from the biomass harvested at the end of experiment. Cultures were harvested by centrifugation at 2000 g for 2 minutes, rinsed with 1% NaCl solution, followed by rinsing with double- distilled water twice. The biomass was lyophilized using a freeze-dryer (ALPHA 1- 4, Germany) for 36 - 48 hrs and kept in desiccators until constant weight. Total lipid was extracted from 1 g of finely grinded lyophilized cell biomass with 2:1 mixture of chloroform and methanol according to Bligh and Dyer [46]. The residue was re-extracted (2 to 3 times) with chloroform. The filtrate was taken in a separatory funnel, and lipids were obtained in the bottom chloroform layer formed upon addition of 0.9% NaCl and collected in a grease-free cleaned pre-weighted glass vial. The solvent was removed using a rotary vacuum evaporator (JSGW, India) at 50 0C to near dryness and dried in desiccators under reduced pressure over anhydrous silica gel (ACS, Sigma-Aldrich, USA). The dried lipid extract was measured gravimetrically and ex- pressed as percent total lipid dry cell weight (% DCW).
Biomass energy value in terms of calorific value (CV) was de- termined using an automatic adiabatic bomb calorimeter (Model: RBC 106/09) according to DIN 51900 T3 (testing of solid and liquid fuels, determination of gross calorific value by the bomb calorimeter and calculation of net calorific value; method with adiabatic jacket) [18]. A crucible containing mois- ture-free (dry) algal biomass (1 g) was ignited in presence of oxygen (99.99% purity) at 3000 kPa. Calorific value was calcu calculated according to equation (3),
A.falcatus grown in BG11 and BB media revealed similar growth patterns, although growth rates were varied (Fig. 1A- B). After an initial lag phase of about 2 to 3 days, the alga grew exponentially from day 4 onwards. The exponential growth phase in BB medium lasted until day 8 with the maximum specific growth rate (µ) of 0.498 d-1 and the least doubling time (T2) of 1.39 days. The exponential growth phase in BG11 me- dium was observed in between days 4 and 6 with compara- tively slower growth rate (µ=0.385 d-1 and T2=1.8 days). In- crease in net cell numbers was recorded in culture grown in BB medium (4.18 x 105 mL-1) compared to that of BG11 (2.82 x
105 mL-1). Similarly, total cellular lipid, carbohydrate, and pro-
tein contents also differed between the cultures. Increased li-
pid (43.6%), carbohydrate (13.5%), and protein (5.1%) contents
by dry weight of biomass were recorded in the cultures grown
in BB medium compared to those of BG11 medium (lipid
38.5%, carbohydrate 12.5% and protein 3.1%). The results were
significant, considering the enhanced growth as well as im-
proved lipid content. The growth stimulating activity of BB
medium might be attributed to the presence of a low level of
NaCl (0.025 g/L) in its constituents. BG11 medium on the oth-
er hand does not contain any NaCl.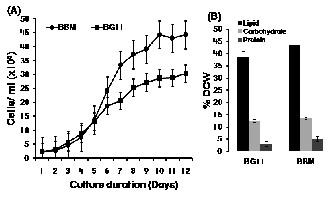
Fig. 1(A-B) Influences of culture media compositions on A.falcatus (A) Growth characteristics in BG11 and BB media; (B) Comparision of biochemical contents. Error bars represent standard error (n=3)
Possible stimulating influences of salinity on growth and lipid content of the tested A. falcatus strain were further con- firmed by the growth behavior of the stated microalga grown in BG11 medium supplemented with NaCl in increasing order (Fig. 2A-B). The strain was found to grow in all the five con- centrations of NaCl (40 to 320 mM). An improved growth was observed in salinity up to 160 mM. The best result was ob- tained in medium supplemented with 160 mM of NaCl fol- lowed by 80 mM and 40 mM of NaCl. Growth rate was, how- ever retarded in medium containing 320 mM of NaCl. Me- dium containing 160 mM of NaCl also showed a net increase in cell numbers (2.9 x 105 ml-1) compared to that of control medium (1.52 x105 ml-1) at the terminal day of the experiment (Fig. 2A). The highest specific growth rate (0.313 d-1) and least doubling time (2.21 days) were observed in medium supple-
IJSER © 2012
International Journal of Scientific & Engineering Research Volume 3, Issue 7, June-2012 4
ISSN 2229-5518
mented with 160 mM of NaCl compared to control medium (µ=0.209 d-1, T2=3.32 days). However, further increase in salt concentration at 320 mM retarded the growth of the studied microalga.
However, slightly reduced CV was calculated in biomass grown under salinity level of 320 mM.
A linear trend of increasing CV with increasing lipid con- tent (R2 = 0.955) of A. falcatus was observed (Fig. 3). In all cases the lipid content increased when the strain was cultivated in medium containing NaCl. In both cases the CV was also found to increase.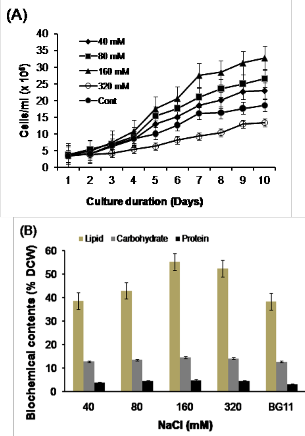
TABLE 2
GROW TH CHARACTERISTICS, LIPID CONTENT AND CALORIFIC

![]()
VALUE OF A. FALCATUS STRAIN UNDER SALT STRESS CONDITIONS
Salinity (mM) | Specific growth (µd-1) | T2 (days) | cells/ml (x105) | Lipid (%)a | CV (kJgm-1)b |
Control | 0.209 | 3.32 | 1.5 | 38.3 | 19.1 |
±0.20 | ±0.05 | ||||
40 | 0.263 | 2.64 | 1.93 | 38.6 | 19.7 |
±0.12 | ±0.11 | ||||
80 | 0.275 | 2.52 | 2.28 | 43.0 | 23.6 |
±0.15 | ±0.05 | ||||
160 | 0.313 | 2.21 | 2.90 | 55.3 | 27.9 |
±0.01 | ±0.15 | ||||
320 | 0.179 | 3.87 | 0.97 | 52.4 | 26.7 |
±0.22 | ±0.05 |
a values are mean of three independent values; b values are mean of five independent values.
‘±’ represents standard error of means
Fig. 2 (A-B). Influences of salinity on (A) growth and (B) bio- chemical contents of A.falcatus. Error bars represent standard error (n=3).
Total lipid, carbohydrate and protein contents were also found to increase in medium containing NaCl up to 160 mM (Table 2). The studied A. falcatus strain under a salinity stress revealed improved total lipid content (Fig. 2B). The highest lipid content (55.3% DCW) was recorded in the cultures con- taining 160 mM of NaCl, which was nearly ≈1.4 fold increase in total lipid content than that of cultures grown in control medium (38.3%). However, slightly reduced amount of total lipid content (52.5%) was recorded in culture grown at a salin- ity level of 320 mM. Similarly, carbohydrate and protein con- tent were also found to be increased with the increasing salini- ty. The highest carbohydrate (14.5% DCW) and protein (4.8% DCW) contents were recorded in the medium containing 160 mM of NaCl and the least (carbohydrate 12.6% and protein
3.1%) in control medium.
A.falcatus biomass grown in control medium revealed a low
CV (19.1 kJ/g), which was improved with increasing level of
salinity up to 160 mM (Table 2). The highest CV (27.9 kJ/g)
was determined in biomass under salinity of 160 mM of NaCl.
The studied native strain of microalga A.falcatus revealed high lipid content with the potentialities to be used as renewable biomass feedstock for biofuel production. Our results have demonstrated that the strain favors BB medium over BG11 for growth. In BB medium the highest cell numbers (4.43 x 106 ml-
1) were recorded after 12 days of cultivation with an initial cell numbers of 2.5 x105 ml-1 at the time of inoculation. Marginal increase in biochemical contents such as lipid (43.6%), carbo- hydrate (13.5%) and protein (5.1%) by dry weight of biomass were also recorded in the cultures grown in BB medium than those of BG11 medium (lipid 38.5%, carbohydrate 12.5% and protein 3.1%). The growth stimulating activity of BB medium might be attributed to the presence of a low level of NaCl (0.025 g/l) in its constituents. BG11 medium on the other hand does not contain any NaCl. The results were in parity with our earlier report on A.falcatus strain [47]. Similar results on en- hanced growth in BB medium were also reported in Haemato- coccus pluvialis [48], [49].
Furthermore, NaCl aided influence on growth was further
confirmed from our salinity experiment conducted with with
five levels of NaCl (0.0 mM, 40 mM, 80 mM, 160 mM and 320
mM). Enhanced growth rates were observed up to an opti-
mum level of NaCl concentration (≈160 mM). However, an
increase in salt concentration beyond 320 mM retarded the
growth. Similar findings on enhanced growth at low salinity
level were also reported in Chlorella vulgaris and Chlorococcum
humicola [50]. Significant increase in growth rate of C.vulgaris
was reported at low salinity level of 50-100 mM. Further in-
IJSER © 2012
International Journal of Scientific & Engineering Research Volume 3, Issue 7, June-2012 5
ISSN 2229-5518
crease in salinity beyond 200 mM retarded the growth in both C.vulgaris and C.humicola. Rao et al. (2007) reported increased biomass production in B.braunii at low salinity level of 17-34 mM. However, the growth rate was significantly reduced at higher concentration of NaCl. The findings have supported the facts that the adaptability of algae to saline condition vary according to their salt tolerance level as well as vary from spe- cies to species. Based on their tolerance extent the algae are grouped as halophilic (salt is required for optimum growth) and halotolerant (having response mechanism to survive in saline condition). In both cases, the algae produce some sec- ondary metabolites that protect them from salt injury and also balance as per the surrounding osmotic [51].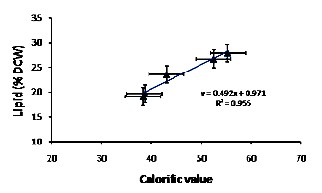
Fig. 3 Calorific value and total lipid content of A. falcatus biomass under salinity stress. A close corelation (R2=0.955) of linear increse in calorific value with increase in total lipid content of A.falcatus biomass. Error bars represent standard error (n=3)
Enhanced lipid and other biochemical contents (such as ke- to carotenoid astaxanthin) in response to NaCl induced stress condition were reported in a number of microalgae species [35], [48], [49], [52]. From our salinity experiment, the highest lipid content (55.3% DCW) was recorded in the cultures con- taining 160 mM of NaCl, which was nearly ≈1.4 fold increase in total lipid content than that of cultures grown in control BG11 medium (38.3%). On the other hand cultures grown in
320 mM concentration of NaCl yielded 52.5% slightly less than
that of 160 mM. This might be due to less amount of total bio-
mass produced at the end of the culture. Similarly, carbohy-
drate and protein content were also found to be increased with
the increasing salinity. The highest carbohydrate (14.5% DCW)
and protein (4.8% DCW) contents were recorded in 160 mM of
NaCl concentrations and the least (carbohydrate 12.6% and
protein 3.1%) in control medium. Similar observations were
also reported by Rao et al. [35] in B.braunii culture.
A. falcatus cell biomass grown in normal BG11 medium re-
vealed a much lower CV (19.1 kJ/g) (Table 2), which is in close agreement with Illman et al. [18]. The principal contribution to the CV of cell biomass is from their total cellular carbohydrate, protein, and lipid content [18], [53], [54]. Microalgae grown
under normal conditions were reported to have CVs between
18 and 21 kJ/g, which is much lower than the CV of diesel (42
kJ/g) [18]. However, improved lipid contents and thereby
improved CVs were also reported [18], [53], also evident from our results (Table 2) and can be seen in fig. 3. The tested A. falcatus strain grown in BG11 medium containing elevated level of NaCl yielded an increased level of total lipid content (Table 2) revealed increasing CVs. Similar results were also reported by Ilmann et al. [18] in C.vulgaris and C.emersonii.
It is envisaged that microalgae biomass would be used as renewable biofuel for electricity generation using static diesel engines [18], [54] for which large microalgae biomass produc- tion with high productivity and high lipid content must need to be maintained. The native A. falcatus strain achieved a better growth and thereby higher biomass yield in BG11 medium supplemented with 160 mM NaCl compared to control me- dium (0.0 mM NaCl). The lipid content reached up to 55.3% with an increasing CV (27.9 kJ/g), under the salt stressed con- dition. As seen from fig. 3, a linear increase of CVs with close correlation between increasing lipid content and thereby in- creasing CVs was observed. The resultant R2 = 0.955 value thus signify that intercellular lipid content together with car- bohydrate and protein contents largely influenced the resul- tant overall CV of the tested A. falcatus cell biomass. The re- sults were in accordance with previous reports [18], [53 - 55].
Salinity induced improved growth rate, total lipid content, and energy value in terms of calorific value were recorded in the studied native strain of oleaginous freshwater microalga A. falcatus biomass grown in BB medium that contain low level of salinity. Our test with BG11 medium supplemented with NaCl in increasing order further confirmed the salinity in- duced improved growth and lipid content leading to an over- all improvement in calorific value. An improved calorific val- ue of the biomass supports the possibility of utilizing A.falcatus biomass directly for energy generation. In view of our present findings, the native A.falcatus strain could be a potent renewable biomass feedstock of biofuels. Although we strongly feel that further culture optimization is needed pre- cede mass production, our present findings will be supportive for future studies.
The authors wish to thank Prof. P. J. Handique, HoD, Depart- ment of Biotechnology, Gauhati Univesity, Assam, India and H. C. Das, Head, Centre of Excellence for Energy Studies, Oil India Limited, ‘INTEGRA’, Guwahati, Assam, India. This work was supported in part by a grant from DRL-Tezpur (DRDO), Assam, India under Project No.DIH-116(DRL-D-1) and Govt. of Assam (No.PMA (H) 160/2010/83).
[1] Borowitzka, M.A., and Moheimani, N.R.: Sustainable biofuels from algae.
Mitig. Adapt. Strateg. Glob. Change, (2011). doi:10.1007/S11027-010-9271-9
[2] Hill, J., Nelson, E., Tilman, D., Polasky, S., and Tiffany, D.: Environmental, economic, and energetic costs and benefits of biodiesel and bioethanol fuels. PNAS, 30, 11206-11210 (2006). doi:10.1073 pnas.0604600103
IJSER © 2012
International Journal of Scientific & Engineering Research Volume 3, Issue 7, June-2012 6
ISSN 2229-5518
[3] Brennan, L., and Owende, P.: Biofuels from microalgae – A review of technol- ogies for production, processing, and extractions of biofuels and co-products. Renew. Sust. Energ. Rev., 14, 557-577 (2010). doi:10.1016/j.rser.2009.10.009
[4] Piccolo, T.: Aquatic biofuels. GlobeFish-FIIU, (2008). http://www.globefish.org/files/Aquaticbiofuels_638.pdf
[5] Walkar, D.A.: Biofuels – for better or worse? Ann. Appl. Biol., 156, 319–327 (2010). doi:10.1111/j.1744-7348.2010.00404.x
[6] Schenk, P., Thomas-Hall, S., Stephens, E., Marx, U., Mussgnug, J., Posten, C., Kruse, O., and Hankamer, B.: Second generation biofuels: high efficiency mi- croalgae for biodiesel production. BioEnergy Research, 1, 20-43 (2008). doi:10.1007/s12155-008-9008-8
[7] Searchinger, T., Heimlich, R., Houghton, R.A., Dong, F., Elobeid, A., Fabiosa, J., Tokgoz, S., Hayes, D., and Yu, T.H.: Use of US croplands for biofuels in- creases greenhouse gases through emissions from land use change. Science Express, 1-6 (2008). doi:10.1126/science.1151861
[8] Singh, A., Nigam, P.S., and Murphy, J.D.: Mechanism and challenges in commercialisation of algal biofuels. Bioresour Technol., 102, 26–34 (2011). doi:10.1016/j.biortech.2010.06.057
[9] Campbell, C.J.: The coming oil crisis. Multi-science Publishing Company and etroconsultants, S.A Essex, (1997). http://www.multi- science.co.uk/oilcrisis.htm
[10] Chisti, Y.: Biodiesel from microalgae. Biotechnol. Adv., 25, 294-306 (2007).
doi:10.1016/j.biotechadv.2007.02.001
[11] Li, Q., Du, W., and Liu, D.: Perspectives of microbial oils for biodiesel produc- tion. Appl. Microbiol. Biotechnol., 80, 749-756 (2008). doi:10.1007/s00253-008-
1625-9
[12] Rodolfi, L., Zittelli, G.C., Bassi, N., Padovani, G., Biondi, N., Bonni G, and Mario, R.T.: Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bio- eng., 102, 100–112 (2009). doi:10.1002/bit.22033
[13] Mata, T., Martins, A.A., and Caetano, N.S.: Microalgae for biodiesel produc- tion and other applications: A review. Renew. Sust. Energ. Rev., 14, 217-232 (2010). doi:10.1016/j.rser.2009.07.020
[14] Spolaore, P., Joannis-Cassan, C., Duran, E., and Isambert, A.: Commercial applications of microalgae. J. Biosci. Bioeng., 101, 87–96 (2006).doi:10.1263/jbb.101.87
[15] Miao, X., and Wu, Q.: High yield bio-oil production from fast pyrolysis by
metabolic controlling of Chlorella protothecoides. J. Biotechnol., 110, 85–93 (2004). doi:10.1016/j.jbiotec.2004.01.013
[16] Pirt, S.J., Lee, Y.K., Walach, M.R., Pirt, M.W., Balyuzi, H.H., and Bazin, M.J.: A tubular bioreactor for photosynthetic production of biomass from carbon dio- xide: design and performance. J. Chem. Technol. Biotechnol., 33B, 35-58 (1983). doi:10.1002/jctb.280330105
[17] Kosaric, N. and Velikonja, J.: Liquid and gaseous fuels from biotechnology:
challenges and opportunities. FEMS Microbiology Reviews, 16, 111-142 (1995). doi:10.1111/j.1574-6976.1995.tb00161.x
[18] Illman, A.M., Scragg, A.H., and Shales, S.W.: (2000) Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Mi- crob. Technol. 27, 631–635 (2000). PII: S0141-0229(00)00266-0
[19] Banerjee, A., Sharma, R., Chisti, Y., and Benerjee, U.C.: Botryococcus braunii:
A renewable source of hydrocarbons and other chemicals. Crit. Rev. Biotech- nol., 22, 245-279 (2002). doi:10.1080/07388550290789513
[20] Pienkos, P.T., and Darzins, A.: The promise and challenges of micro-algal derived biofuels. Biofuel Bioproducts Biorefin., 3, 431–440 (2009). doi:10.1002/bbb.159
[21] Gong, Y., and Jiang, M.: Biodiesel production with microalgae as feedstock:
from strains to biodiesel. Biotechnol. Lett., 33, 1269–1284 (2011).
doi:10.1007/s10529-011-0574-z
[22] Pokoo-Aikins, G., Nadim, A., EI-Halwagi, M.M., and Mahalec, V.: Design and analysis of biodiesel production from algae grown through carbon sequestra- tion. Clean. Tech. Environ. Policy, (2009). doi:10.1007/s10098-009-0215-6
[23] Hu, Q., Milton, S.M., Jarvis, E., Ghirardi, M., Posewitz, M., Seibert, M., and Darzins, A.: Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J., 54, 621–639 (2008). doi:10.1111/j.1365-
313X.2008.03492.x
[24] Melis, A., and Happe, T.: Hydrogen Production. Green Algae as a Source of Energy. Plant Physiol., 127, 740-748 (2001). www.plantphysiol.org/cgi/doi/10.1104/pp.010498
[25] Clarens, A.F., Resurreccion, E.P., White, M.A., and Colosi, L.M.: Environmen- tal Life Cycle Comparison of Algae to Other Bioenergy Feedstocks. Environ. Sci. Technol., 44, 1813-1819 (2010). doi: 10.1021/es902838n
[26] Pulz, O.: Valuable products from biotechnology of microalgae. Appl. Micro- biol. Biotechnol., 65, 635–648 (2004). doi:10.1007/s00253-004-1647-x
[27] Abou-Shanab, R.A.I., Hwang, J.H., Cho, Y., Min, B., Jeon, B.H.: Characteriza- tion of microalgal species isolated from fresh water bodies as a potential source for biodiesel production. Appl Energy, 88, 3300–6 (2011). http://dx.doi.org/10.1016/j.apenergy.2011.01.060
[28] Pérez, M.V.J., Castillo, P.S., Romera, O., Moreno, D.F., and Martínez, C.P.:
Growth and nutrient removal in free and immobilized planktonic green algae isolated from pig manure. Enzyme Microb. Technol., 34, 392–8 (2004). doi:10.1016/j.enzmictec.2003.07.010
[29] Odlare, M., Nehrenheim, E., Ribe, V., Thorin, E., Gavare, M., Grube, M.: Cul- tivation of algae with indigenous species – potentials for regional biofuel pro- duction. Appl. Energy, 88, 3280–5 (2011). http://dx.doi.org/10.1016/j.apenergy.2011.01.006
[30] Sanchez, S., Martinez, M.E., and Espinola, F.: Biomass production and bio-
chemical variability of the marine microalga Isochrysis galbana in relation to culture medium. Biochem. Eng. J., 6, 13–8 (2000). PII: S1369-703X(00)00071-1
[31] Day, J.G., Slocombe, S.P., and Stanley, M.S.: Overcoming biological constraints to enable the exploitation of microalgae for biofuels. Bioresour. Technol., (2011). doi:10.1016/j.biortech.2011.05.033
[32] Li, Y., Horsman, M., Wang, B., Wu, N., and Christopher, Q.L.: Effects of nitro- gen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol., 81, 629–636 (2008). doi:10.1007/s00253-008-1681-1
[33] Khotimchenko, S.V. and Yakovleva, I.M.: Lipid composition of the red alga Tichocarpus crinitus exposed to different levels of photon irradiance. Phyto- chemistry, 66, 73–79 (2005). doi:10.1016/j.phytochem.2004.10.024
[34] Araujo, G.S., Matos, L.J.B.L., Goncalves, L.R.B., Fernandes, F.A.N., and Farias, V.R.L.: Bioprospecting for oil producing microalgal strains: evaluation of oil and biomass production for ten microalgal strains. Bioresour. Technol., 102,
5248–5250 (2011). doi:10.1016/j.biortech.2011.01.089
[35] Rao, A.R., Dayananda, C., Sarada, R., Shamala, T.R., and Ravishankar, G.A.: Effect of salinity on growth of green alga Botryococcus braunii and its consti- tuents. Bioresour. Technol., 98, 560–564 (2007). doi:10.1016/j.biortech.2006.02.007
[36] Reitan, K.I., Rainuzzo, J.R., and Olsen, Y.: Effect of nutrient limitation on fatty
acid and lipid content of marine microalgae. J. Phycol. 30(6), 972–979 (1994). doi:10.1111/j.0022-3646.1994.00972.x
[37] Lebsky, V. K., Gonzalez-Bashan, L.E., and Bashan, Y.: Ultrastructure of coim- mobilization of the microalga Chlorella vulgaris with the plant growth- promoting bacterium Azospirillum brasilense and with its natural associative bacterium Phyllobacterium myrsinacearum in alginate beads. Can. J. Micro-
biol., 47, 1–8 (2001). doi:10.1139-cjm-47-1-1
IJSER © 2012
International Journal of Scientific & Engineering Research Volume 3, Issue 7, June-2012 7
ISSN 2229-5518
[38] de-Bashan, L.E., Bashan, Y., Moreno, M., Lebsky, V.K., and Bustillos, J.J.: In- creased pigment and lipid content, lipid variety, and cell and population size of the microalgae Chlorella spp. when co-immobilized in alginate beads with the microalgae-growth- promoting bacterium Azospirillum brasilense. Can. J. Microbiol., 48, 514–521 (2002). doi: 10.1139/W02-051
[39] Liu, Y., Ruan, R., and Kong, Q.: Mass culture of high oil content microalgae on wastewater and power plant flue gases. Chin. J. Bioprocess Eng., 3, 29–33 (2008). CNKI:SUN:SWJG.0.2008-03-004
[40] Nichols, H.W., and Bold, H.C.: Trichsarcina polyinorpha gene. et sp. nov. J.
Phycol. 1, 34–38 (1969).
[41] Kawai, H., Motomura, T., and Okuda, K.: (2005) In: Algal culturing tech- niques, Anderson, R., A., (Eds.), Elsevier Academic Press, Burlington, MA, USA (2005), 133-144.
[42] Boussiba, S., and Vonshak, A.: Astaxanthin accumulation in the green algae
Haematococcus pluvialis. Plant Cell Physiol., 32 (7), 1077–1082 (1991). pcp.oxfordjournals.org/content/32/7/1077.abstract
[43] Levasseur, M.P., Thomson, A., and Harrison, P.J.: Physiological acclimation of marine phytoplankton to different nitrogen sources, J. Phycol., 29, 587–
595(1993). doi:10.1111/j.0022-3646.1993.00587.x
[44] Lowry, O.H., Rosebrough, N.J., Farr, A.L., and Randall, R.J.: Protein mea- surement with the folin phenol reagent, J. Biol. Chem., 193, 265-275 (1951). http://www.jbc.org/content/193/1/265.full.pdf+html
[45] Hedge, J.E., and Hofreiter, B.T.: In: Carbohydrate chemistry, 17th Edition.
Whistler, R., L., and Be Miller, J., N. (Eds.), Academic Press New York (1962). [46] Bligh, E.G., and Dyer, W.J.: A rapid method of total lipid extraction and purifi-
cation. Can. J. Biochem. Physiol., 37, 911-917 (1959). doi:10.1139/o59-099
[47] Kalita, N., Baruah, G., Dev Goswami, R.C., Talukdar, J., and Kalita, M.C.: Ankistrodesmus falcatus: A promising candidate for lipid production, its bio- chemical analysis and strategies to enhance lipid productivity. J. Microbiol. Biotech. Res., 1 (4), 148-157 (2011). http://scholarsresearchlibrary.com/JMB- vol1-iss4/JMB-2011-1-4-148-157.pdf
[48] Dominguez-Bocanegra, A.R., Legarreta, I.G., Jeronimo, F.M., and Campoco- sio, A.T.: Influence of environmental and nutritional factors in the production of astaxanthin from Haematococcus pluvialis. Bioresour. Technol., 92, 209-214 (2004). doi:10.1016/j.biortech.2003.04.001
[49] Imamoglu, E., Dalay, M.C., and Sukan, F.V.: Influences of different stress media and high light intensities on accumulation of astaxanthin in the green alga Haematococcus pluvialis. New Biotechnol., 26, 199–204 (2009). doi:10.1016/j.nbt.2009.08.007
[50] Abdel-Rahman, M.H.M., Ali, R.M., and Said, H.A.: Alleviation of NaClin- duced effects on Chlorella vulgaris and Chlorococcum hunmicola by ribof- lavin application. Int. J. Agric. Biol., 7(1), 58-62 (2005). http://www.fspublishers.org/ijab/past- issues/IJABVOL_7_NO_1/11.pdf
[51] Hart, B.T., Bailey, P., Edwards, R., Hortlek, K., James, K., McMohan,
A., Meredith, C., and Swading, K.: A review of the salt sensitivity of the Australian fresh water biota. Hydrobiologia, 210, 105–144 (1991). doi:10.1007/BF00034684
[52] Fodorpataki, L., Bartha, C.: Salt stress tolerance of a freshwater green alga under different photon flux densities. Sludia Universities Babes- Bolyai, Biologia, XLIX, 2, 85-93 (2004). adat- bank.transindex.ro/vendeg/htmlk/pdf5635.pdf
[53] Scragg, A.H., Illman, A.M., Carde, A., Shales, S.W.: Growth of micro- algae with increased calorific values in tubular bioreactor. Biomass and Bioenergy, 23, 67-73 (2002). PII:S0961-9534(02)00028-4
[54] Scragg, A.H., Morrison, J., Shales, S.W.: The use of fuel containing
Chlorella vulgaris in a diesel engine. Enzyme and Microbial Technol.,
33, 884-889 (2003). doi:10.1016/j.enzmictec.2003.01.001
[55] Bhola, V., Desikan, R., Santosh, S.K., Subburamu, K., Sanniyasi, E., and Bux, F.: Effects of parameters affecting biomass yield and ther- mal behaviour of Chlorella vulgaris. J. Biosci. Bioeng., 111 (3), 377-382 (2011). doi:10.1016/j.jbiosc.2010.11.006
IJSER © 2012