International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 641
ISSN 2229-5518
Development of Improved Minimal Medi- um for Acetoin Metabolism by B. subtilis
Strains
Pratibha Sharma
Abstract— Acetoin is an economical important compound, widely used as a flavoring agent. It is naturally produced by Bacillus subtilis (B. Subtilis). Besides, advantage using B.Subtilis is that, it can be grown on a variety of carbon sources. This property contributes in studying carbon regulation in the organism. Therefore, characterization of its growth, in lab conditions, on complete medium and minimal medium needs to be done. A comparative analysis of existing minimal media is performed to identify an improved minimal medium for acetoin accumulation to characterize its growth. The study identifies the importance of iron, magnesium and manganese for acetoin accumulation.
Keeping objective in mind an improved minimal medium ( composition of improved medium TSM is: 0.5 % glucose, 40 mg/L tryptophane,
0.02 g MgSO
4,
0.44 g K 2 HPO 4 , 2 g Na-glutamate, 0.004 g FeCl 3 .6H 2 O, 0.004 g, 0.05 M Tris Buffer) was developed. This minimal
medium shown a 10 % enhancement in acetoin accumulation in comparison to existing minimal medium. The comparable biomass
synthesis with complex medium (LB) was observed in TSM provided with glucose, glycerol and xylose as a sole carbon source. Furthermore, capability of 168Δldh and 168ΔacuA was compared with B. subtilis 168. Therefore, TSM can be used as a minimal medium for various studies requiring change of carbon, nitrogen and phosphorus source.
Index Terms— Acetoin, Bacillus subtilis, Complex medium, Minimal medium, Micronutriants, Nitrogen source, Sole carbon source.
—————————— ——————————
1 INTRODUCTION
HE B. subtilis is a rod shaped gram positive; catalase posi- tive ubiquitous bacterium commonly recovered from wa- ter, soil, air and decomposing plant tissues (Alexander,
1977). It was found non-pathogenic to humans and plants, therefore, identified as a generally recognize as safe (GRAS) organism by the US Food and Drug Administration. There- fore, Bacillus products are used in the food industry. Bacillus subtilis has a high capacity for the production of the extracellu- lar enzymes (proteases, amylases, cellulases, chitinases, etc.) and there are examples where successful productions of intra- cellular enzymes have been observed. The presence of cellular quality control systems in case of B. subtilis that removes mis- folded and incomplete synthesized proteins; due to this rea- son, the protein produced is of high quality (Zweers et al.,
2008). Protein phosphorylation studies have also been carried
out with B. subtilis (Macek et al., 2007). Synthesis of pyruvate,
acetate, acetoin and 2,3 butanediol is observed on glycolytic
substrate by B. subtilis.
Acetoin is an important metabolite for maintaining the homeostasis of microorganisms as well as it is an interme- diate for synthesis of butanediol. Butanediol is also a second- ary product of acetoin metabolism. Previously, Magee and Kosaric (1987), reviewed the data of different authors, stressed that butanediol yield varied dramatically depending on the type of natural medium, its preparation, initial concentration of the carbon source, and other micronutrients. Therefore, if one wants to study metabolite patterns in cell, optimization of
————————————————
• Pratibha Sharma is currently pursuing PhD degree program in Depart- ment of Biosciences and Bioengineering in Inidian Institute of Technology Bombay, India. PH-02225764246, E-mail: pratibha.sharma@iitb.ac.in
medium condition is very important.
Acetoin production phenomena are growth related. Therefore, a medium that provide fast growth is likely to in- crease the no. of cell that contains acetoin synthesis enzyme. Thus, enhancement in acetoin accumulation may observe. A high nutrient medium like complex medium is likely to sup- port high density of growth but it may interfere with assay system. Additionally, studies on regulation or accumulation of metabolite are preferable to do in minimal medium as it pro- vides a choice of variation of carbon, nitrogen, phosphorus sources, and their concentrations. Therefore, it is important to optimize a medium that support fast growth of organism, ac- cumulation of acetoin and does not interfere with assay sys- tem.
Therefore, keeping this in mind we have studied acetoin metabolism on various minimal medium (Chemically defined medium, Basal minimal medium and Tris base medi- um) used for growth of B. subtilis 168. These medium differ in nitrogen, carbon, phosphorus sources and buffering condi- tions. We varied previous mentioned conditions to optimize a medium that support fast growth, acetoin accumulation and do not interfere with assay system. This medium was further tested for use of various carbon substrates and its use for cul- tivating different mutant strains of B. subtilis 168.
2 Materials And Methods
2.1 Materials
List of strains used in this study with their source is provided in Table 1.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 642
ISSN 2229-5518
Chloramphenicol (5µg/ml) (Sigma Chemical Co., USA) was used for growth and selection of B. subtilis strains.

Other then acetoin (Sigma chemicals Co., USA), all the chemi- cals of analytical grade and media components used in this work were purchased from Sisco Research Laboratories, India.
Table 1
due to presence of some inhibitory component present in the complex medium.
To demonstrate the effect of complex medium on sensiti- tivity of assay, commercially produced acetoin was dissolve in water and LB as solvent and a standard colorimetric assay was performed. A straight line with (correlation equation of y =
Strains name
Bacillus subtilis 168
168Δacu
Genetic con-
stitution Source
trpC2 BGSC* Sharma &
0.99x ) was observed for acetoin concentration range between
0.1 to 1.2 g/L that suggest westerfeld assay is can be used to
measure acetoin using either of solution medium. However, a
five fold decrease in sensititivity for westerfeld assay (colori-
metric assay) was observed in LB as solution in comparison to water as solvent (Fig 1b). A low sensitivity of assay direct to-
A trpC2 acuA::cam
168Δldh trpC2 lctE::lctE- lacZ2 cat
Noronha,
2012
Cruz Ra-
mos 2000
wards the error in measurement especially in low concentra- tion of metabolites.
Therefore, quantification of acetoin production and other metabolites would likely be difficult in a complex medium.
List of strains and their source
* Bacillus genetic stock center
2.2 Methods
The B. subtilis strains were grown in LB medium (Luria Ber- tani, Miller) (Hi media Laboratories Pvt. Ltd., Mumbai, India)
at 37°C and 200 rpm agitation speed. CDD, BMM and TSS
medium were used as a minimal medium for growth (Har-
wood and cutting, 1990). Composition of these medium are as
follows
CDD: 1% Glucose, 2g K2 SO4, 10.8g K2 HPO4, 6g KH2 PO4, 1g
Na-Citrate, 0.02g MgSO4, 0.1% Na-gluatamate, 50 µM MnCl2 ,
20 mg/L Tryptophan
BMM: 1% Glucose, 0.75 g K2 HPO4, 0.75 mg KH2 PO4, 0.3 g (NH4 )2 SO4 ), 0.06 g MgSO4 .7H2 O, 0.003 g FeSO4 . 7H2 O, 0.0021 g MnSO4 .7H2 O, 20 mg/L Tryptophan
TSS: 0.05 M Tris, 40 µg each of FeCl3 6H2 0 and trisodium citrate dihydrate per ml, 2.5 mM K2 HPO4 , 0.02% MgSO4 7H2 0,
0.2% glutamine, 0.2% trisodium citrate dihydrate.
The growth of B. subtilis was monitored as a function of OD600 on spectrophotometer. Measurement of acetoin and glucose was performed on HPLC using a Bio-Rad Aminex HPX-87H.
5 mM Sulfuric acid was used as mobile phase and column
temperature was kept at 65°PC. A colorimetric assay, for sensi-
tivity of acetoin measurement, was performed (Westerfeld,
1945).
3 RESULTS AND DISCUSSIONS
3.1 Growth of B. subtilis 168 in complex medium
To demonstrate the deviation of standard acetoin assay on complex medium, B. subtilis 168 was grown on Luria broth (complex medium) and acetoin was measured with respect to time. A standard sigmoid curve with distinct lag, exponantial, and stationary phase was observed for growth by B. subtilis (Fig 1a). The maximum OD600 observed at 11 hours was 5.7. However, only a trace (0.5 mg/L) of acetoin synthesis was observed. This suggests low level of acetoin synthesis resulted
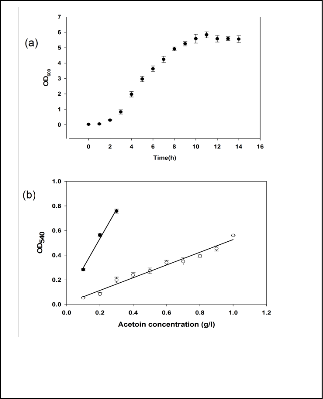
Hence, it becomes a primary requirement to optimize a medi- um that supports sufficient growth of B. subtilis 168 and max- imizes acetoin production.
Fig 1 (a) Growth curve of B. subtilis 168 in complex medium (L.B.) (b) Comparison of standard curve of acetoin in LB or water
as a solvent
To achieve this objective, a comparative study was per- formed for biomass production, glucose and acetoin metabo- lism by B. subtilis 168 in chemically defined medium (CDM), Basal minimal medium (BMM) and Tris base medium (TSS). These media differ in their composition of micronutrients and buffering solution.
3.2 Growth of B. subtilis 168 in minimal medium
To assess the suitability of minimal media (CDD, BMM
and TSS) for acetoin accumulation; B. subtilis was grown in
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 643
ISSN 2229-5518
medium providing 5 g/L of glucose as sole carbon source and biomass, glucose and acetoin metabolism was monitored at 3 hrs of growth interval.
a) Chemically defined medium (CDD)
The maximum OD600 ~ 1.8 was observed by B. subtilis 168 in chemically defined medium. However, the growth of or-
ganism was very slow making it difficult to distinguish be- tween the different stages of the growth of organism. A maxi- mum of 1 mg/L of acetoin synthesis and no residual glucose was observed in late stationary phase. The results indicates that major glucose was utilized towards biomass synthesis
Fig 2 Effect of using (a) CDD, (b) BMM and (c) on growth (OD600 ), glucose utilization and acetoin accumulation
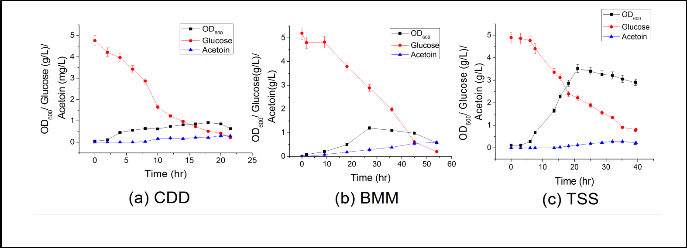
and byproduct formation or this medium is devoid of some micronutrients required for acetoin synthesis (Fig 2a).
b) Basal minimal medium (BMM)
B. subtilis 168 was grown in basal minimal medium (BMM ) by providing 5 g/l of glucose as a sole carbon source (Fig 2b). Likewise, chemically defined medium a slow growth and a maximum of OD600 ~1.2 was observed. However, in contrast, a
0.8 g/L of acetoin synthesis was observed. That suggest, a low acetoin synthesis by B. subtilis in CDD was a result of unavail- ability of some micronutrient required for acetoin synthesis.
c) Tris base medium
Contrary to CDD and BMM; a maximum OD600 ~ 3.2 was ob- served by B. subtilis in TSS medium (Fig 2c). As, an equal amount of glucose was provided in these medium; variation of biomass is due to the component of medium. Additionally, a 0.5 g/L of acetoin accumulation was observed at 30 hr of growth.
To check the interference of the medium component with the assay system a standard curve of acetoin was plotted by taking CDM, BMM and TSS as a solvent. Result shows compa- rable sensitivity with the water as a solvent (Data not shown).
3.3 Comparative analysis of BMM, CDD and TSS
Comparison of the components of BMM, CDD and TSS suggest that they differ in buffering medium, phasphorus source, nitorgent source and availability of iron and manga- nese. However, presence of tryptophane (B. subtilis 168 is an auxotroph for tryptophane) and magnisium source was com- mon. For comparative analysis, glucose (5 g/L) was kept con-

stant (Table 2).
TSS - Fe TSS BMM – Fe BMM
Fig 3a Fig3b
TSS TSS + Mn Mg 0.02 0.06 0.1 0.2
Fig 3c Fig 3d
Fig 3 Systematic characterization of medium components affecting acetoin accumulation by B. subtilis (a) Synthesis of acetoin in presence and ab- sence of iron in TSS (b) Synthesis of acetoin in presence and absence of iron in BMM (c) Effect of addition of MnSO 4 on acetoin synthesis in TSS (d) Synthesis of acetoin on using various concentration of MgSO 4 in TSS medium.
Distinctly, no acetoin synthesis was observed in CDD and major difference among all three medium was absence of iron in CDD. This suggests that iron is one of the most important cofactor required for the acetoin synthesis. To validate this B. subtilis was grown in TSS, BMM and TSS – Fe, BMM- Fe.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 644
ISSN 2229-5518

Fig 3 depicts westerfeld assay for acetoin measurement. A pink color suggests synthes. A trace of acetoin synthesis was detected in TSS-Fe in ccomparison to TSS (Fig 3a). Likewise, an absence of acetoin synthesis was observed for CDD media lacking iron (Fig 3b). Therefore, iron is likely an essential component for synthesis of acetoin.
Table 2
Comparison of composition of CDD, BMM and TSS media
variation of MgSO4 does not affect the acetoin synthesis. Therefore, a 90 % reduction in MgSO4 amount is possible from TSS medium.
As the maximum biomass production was observed on TSS medium, we have assumed that tris base medium and sodium glutamate are best as buffering medium and nitrogen source for the growth. Additionally, phosphorus source used was the di and mono potassium salt of sulphate.
By the systematic analysis of CDD, BMM and TSS, it is clearly observed that iron, magnesium and manganese are essential component for acetoin synthesis. However, 0.02 g MgSO4 is enough for acetoin synthesis.
Therefore, an improved tris base minimal medium with manganese (TSM) was developed that supports maximal biomass and acetoin synthesis. Composition of medium is as follows: 0.5 % glucose, 40 mg/L tryptophane, 0.02 g MgSO
4,
0.44 g K2 HPO4 , 2 g Na-glutamate, 0.004 g FeCl3 .6H2 O, 0.004 g,
0.05 M Tris Buffer.
3.4 Validation of TSM for growth and acetoin accumulation
Our prime objective was to improve minimal medium such that it support higher acetoin accumulation using various complex carbon sources as sole carbon sources by various modified B. subtilis strains. Therefore, validation of TSM for following properties is necessary:
Among CDD, BMM and TSS, availability of Mangen- ese sulphate was another variable. To study the involvement of manganese in acetoin synthesis, B. subtilis was grown in TSS medium supplemented with 21 mg of manganese sulphate, and colorimetric assay for acetoin measurement was observed (Westerfeld 1945). For control, measurement was performed in TSS medium (without addition of MnSO4 ). As shown in Fig
3c, a 10 % enhancement in acetoin synthesis was observed. That suggests manganese play some direct/indirect involve- ment in acetoin synthesis process and it should be added in the minimal medium for acetoin accumulation studies.
The variation in Concentration of mangenisium sul- phate was also observed between 0.02 to 0.2 g/L. To investi- gate, if concentration of mangnisium has any role on acetoin synthesis, B. subtilis was grown in TSS medium provided with
0.02, 0.06, 0.1 and 0.2 g/L of MgSO4 and synthesis of acetoin was monitored using westerfeld assay. As depicted in Fig 3d;
(a) Characterization of TSM using glucose as sole carbon source by B. subtilis
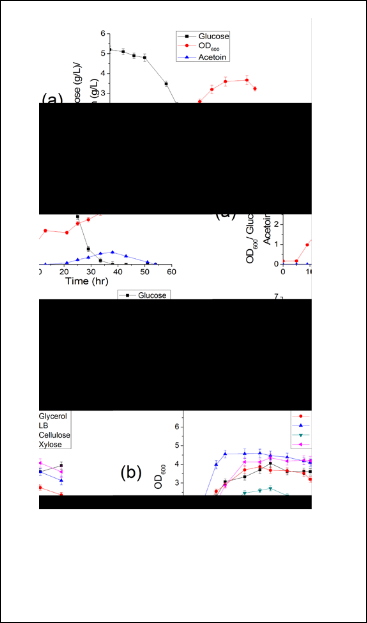
To achieve this, B. subtilis was grown in TSM medium con- taining 5 g/L of glucose as sole carbon source and meas- urement of biomass, glucose and acetoin synthesis was monitored during course of growth. As described in Fig 4a, a maximum OD600 ~ 3.5 was observed. which is compara- ble to TSS and a two fold higher then CDD and BMM me- dia. Besides, a 50 % enhanced accumulation was observd in TSM as compared to TSS. It is the maximal value among CDD, BMM and TSS. The observation suggests TSM is an improved medium for acetoin accumulation by B. subtilis.
(b) Characterization of growth of B. subtilis using various complex carbon sources
To elucidate the effectiveness of TSM with carbon sources other than glucose (Cellulose, glycerol and xylose) as sole carbon source, B. subtilis 168 was grown in TSM provided with 0.5 % of glucose, glycerol, xylose and cellulose. Result obtained was compared with growing B. subtilis in complex medium (LB). A 50 % lower biomass was detected in medi- um containing cellulose as sole carbon source in compari- son of LB. This could be due to the low cellulose activities by B. subtilis. However, a maximum OD600 ~ 5 was ob- served, this was comparable to medium medium contain- ing glucose, glycerol and xylose as sole carbon source (Fig
4b). Therefore, TSM may be used for studies using previ-
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 645
ISSN 2229-5518
ously mentioned carbon sources.
(c) Acetoin accumulation by various modified strains of B. subtilis using glucose as sole carbon source
To explicate that TSM is a suitable medium to grow genet- ically modified strains of B. subtilis; 168Δldh (lacking lac- tate dehydrogenase activity) and 168ΔacuA (lacking acetate utilization enzyme activity) were grown in TSM provided with 5 g/L of glucose as sole carbon source and acetoin synthesis was measured (Fig 4c).
4 CONCLUSION
Our results show that cofactors, iron and manganese, have an important role in acetoin accumulation. However, concentration of these metals is required in very small amount.
A minimal medium (TSM) for growth and acetoin accumulation of various mutant strains of B. subtilis 168 was developed. Composition of minimal medium is as follows: 0.5
% glucose, 40 mg/L tryptophane, 0.02 g MgSO
4,
0.44 g
K2 HPO4 , 2 g Na-glutamate, 0.004 g FeCl3 .6H2 O, 0.004 g, 0.05
M Tris Buffer. This medium was found supportive to the
growth of B. subtilis 168 provided with glucose, glycerol and
xylose as sole carbon source. Besides this, TSM can be used for
the study of various genetically modified strains of B. subtilis.
ACKNOWLEDGMENT
This work was supported in part by a grant from Council for Scientific and Industrial research, India (F.No.10-2(5)/2006(ii)- E.U.II.
Fig 4 Validation of TSM for (a) biomass and acetoin synthesis using glucose as sole carbon source (b) Growth of B. subtilis in TSM using glucose, glycerol, cellulose and xylose as sole carbon source (c) Comparison of acetoin accumulation by 168, 168Δldh and 168ΔacuA strains.
The strains 168 and 168Δldh have shown a comparative acetoin accumulation (0.65 g/L). However, a 20 % increase in acetoin accumulation was observed for 168ΔacuA due to less utilization of acetoin in absence of acetoin catabolite genes (Sharma & Noronha, 2012) (Fig 4c). Therefore, TSM can be used to cultivate various modified strains of B. subtilis.
REFERENCES
[1] B. I. Macek, J. V. Mijakovic, F. Olsen, C. Gnad, P. R. Kumar, Jensen and M. Mann. “The Serine/Threonine/Tyrosine Phosphoproteome of the Model Bacterium Bacillus subtilis.” Mol cell proteomics. vol. 6.4, pp. 694-707, 2007.
[2] C. R. Harwood, & S. M. Cutting, “Molecular biological methods for
Bacillus” New York: Wiley. vol. 707, 1990.
[3] H. Cruz Ramos, T. Hoffmann , M. Marino, H. Nedjari, E. Presecan-Siedel,
O. Dreesen, P. Glaser, D. Jahn, “Fermentative metabolism of Bacillus sub- tilis: physiology and regulation of gene expression”. J. bacterial, vol. 182. pp. 3072–3080, 2000.
[4] J. C. Zweers, I. Barak, D Becher, A. J. Driessen, M. Hecker, V. P.
Kontinen, M. J. Saller, L. Vavrova, J. M. van Dijl, “Towards the de- velopment of Bacillus subtilis as a cell factory for membrane proteins and protein complexes”. Microb Cell Fact. vol. 7:10, 2008.
[5] M. Alexander, “Introduction to Soil Microbiology”. John Wiley and
Sons, Inc., New York. 1977.
[6] R. J. Magee, N. Kosaric,“The microbial production of 2,3-butanediol.”
Adv Appl Microbiol. vol. 32, pp. 89–161, 1987.
[7] S. Pratibha, N. Santosh, “Systematic study of acuABC operon in Bacillus subtilis 168”. IPCBEE. vol 3, pp. 17–21, 2012.
[8] S. S. Westerfeld, “A colorimetric determination of blood acetoin.” J. Biol.
Chem. vol. 169, pp 495-501.
IJSER © 2013 http://www.ijser.org





