International Journal of Scientific & Engineering Research, Volume 3, Issue 8, August 2012
Issn 2229-5518
M. Dhakshnamoorthy*, S. Vikram and R. Vasanthakumari
Abstract- The effect of iso-propylidene and aryl ether linked dianhydride and diamine monomers such as 4,4’-(4,4’-isopropylidine diphenoxy) bis (phthalic anhydride) (IDPA), 4,4’ oxydianiline (ODA) and 4,4’-(4,4’-isopropylidine diphenyl-1,1’-diyldioxy) dianiline (IDDA) on dielectric properties of two polyimides (PI) namely PI-1 (IDPA-ODA) and PI-2 (IDPA-IDDA) synthesized by a conventional two-stage method was studied. The poly amic acids(PAA) obtained in the first stage were thermally treated at 100 - 300 °C in the second stage to obtain the polyimide films by solution casting. The inherent viscosity of PAAs was around 1.0 dL/g and the PIs obtained, exhibited good solubility in polar aprotic solvents such as N-methyl pyrrolidone, N, N-Dimethyl acetamide, N, N-Dimethyl formamide, etc. due to flexible backbones. The PIs showed 10 % degradation temperature of 530 and 541°C in TGA analysis and wide angle X-ray diffraction profiles confirm that the PI films were amorphous in nature. The viscoelastic behavior and glass transition temperature (Tg) of PI films were determined by dynamic mechanical analyser and the values were 216.7 and 238.8 °C. The films showed high tensile strength, modulus and optical transparency upto 88 % and the dielectric constants were measured at various frequencie s and temperature levels and the polyimide film derived from IDPA and IDDA monomers showed dielectric constant value as low as 2.25 at 5 MHz, 100 °C.
Index Terms- Aryl ether linkage, dielectric constant, Polyimide film, Thermal property.
—————————— ——————————
In recent years, polyimides have become most
demanding for high performance polymers because of their
outstanding properties such as thermal stability, mechanical
and electrical. Among these polyimides, aromatic polyimides
are well known for their excellent mechanical and chemical
properties, like resistance to acids as well as their solubility in
polar aprotic solvents [1-5]. The modifications in this type of
polyimides have been made and used in aerospace, opto-
electronics, liquid crystal display (LED) and other needful
industries [6-10]. In-addition with these Polyimides have also
been used as sensors, membranes in fuel cell, gas separation,
ultrafiltration and nanofiltration applications due to their
flexibility, toughness and thermo-oxidative resistance [11-14,
24-26]. Most of the aromatic polyimides have high melting
temperature and poor solubility in most of the aromatic
solvents due to the rigid backbone and strong interaction
between the polymer chains. Many modifications have been
made to improve the flexibility and solubility with reservation
of other advantageous properties by introducing flexible
linkages, non-coplanar units or bulky substituents [15-23].
Most of the polyimides are light brown in colour which is due to inter and intramolecular charge transfer (CT) interaction between alternating electron-donating diamine and electron accepting dianhydride components. It has been shown that the polyimides with unsymmetric structure of
————————————————
Rahman University, Chennai,India, E-mail:vikram625001@gmail.com
Nanotechnology Centre, B S Abdur Rahman University, Chennai, India.
dianhydride or diamine components prevent the close packing of chains and also the CT interactions. It is also found that the introduction of bulky groups with unsymmetric structure into the polyimides rendered the polymer to have better solution processability, improved flexibility, less colour and lower dielectric constant [29 - 30].
In this study, we report the synthesis and characterization of two polyimides from symmetrical monomers with high thermal stability, good solubility, high transparency with lowest dielectric constant attainable so far. The effect of phthalic iso-propylidene group and ether linkage on thermal stability, dielectric property and optical property will be discussed. In addition to the bulky, packing-disruptive
–CH3 groups, the unsymmetrical structure of the bis(ether anhydride) or bis(ether amine) component will prevent the extended close packing of chains and also the CT interactions. Thus, the polymer would be expected to exhibit high transparency, flexibility, good solubility and low dielectric constant. By comparative studies of these polymers with their non tert butyl bulky group analogs, we confirmed in this paper that the introduction of both bulky group and the ether linkage into the polyimide structures rendered the polymer better solution processability, improved flexibility, transparency and lower dielectric constant values.
4,4’-(4,4’-isopropylidene diphenoxy)bis (phthalic anhydride) (IDPA) (Sigma Aldrich m.p.184-187°C), 4,4’-Oxy dianiline (ODA) (Lotto chemicals), 4,4’-(4,4’-isopropylidene diphenyl-1,1’-diyl dioxy) dianiline (IDDA) (Sigma Aldrich m.p.127-130 °C) were used as received after drying them. The chemical structures of the monomers used are shown in Figure 1. N, N-Dimethyl acetamide (DMAc) solvent obtained
IJSER © 2012
International Journal of Scientific & Engineering Research, Volume 3, Issue 8, August 2012
Issn 2229-5518
from Merck chemicals was vacuum distilled and dried using molecular sieves.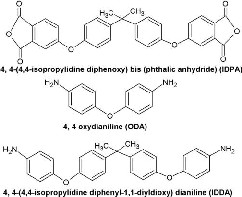
Fig 1. Chemical Structure of the monomers
The fourier transform infrared (FT-IR) spectra were recorded on a Perkin Elmer Fourier transform infrared spectrophotometer. The inherent viscosities of the poly (amic acid)s were measured with an Ubbelhode viscometer at 25°C at a concentration of 0.5 g/dl in DMAc at 25°C.
The crystallite particles size measurements were performed Wide-angle X-ray diffraction (WAXD) at room temperature (RT) (25 °C) on a Bruker D8 Focus X-ray diffractometer, using nickel filtered Cu Kα (λ=0.1541 nm, scintillator detector, operating at 40 kV and 30 mA). The step time was 1 second at 0.02°/ step between the 2θ angle of 5 to
50°.
The mechanical property using dynamic mechanical
analyses (DMA) were conducted for polyimide films using
Seiko Instrument DMS 6100. The frequency was set at 1 Hz
and the heating rate was 2 °C/min in air atmosphere. The
relaxation temperatures were determined from corresponding
peak top temperatures on the damping (tan δ) curves.
Thermogravimetric analyses (TGA) were determined
for polyimide films using Seiko Instrument TG/DTA6200,
EXSTAR 6000. The experiments were carried out on 5 - 7 mg
of samples in flowing of nitrogen gas (flow rate 140 ml/min)
and also in air atmosphere at a heating rate of 20 °C/min.
The optical transparency study by Ultraviolet-visible
(UV-Vis) spectra of the polymer films were recorded on a
CARY 5E UV-VIS-NIR spectrophotometer. Lloyd’s instrument
with an LF plus model 1 KN load cell was used to study the
tensile properties of the polyimide films. The specimens of 70
mm long, 5 mm wide, about 0.05 mm thick were cut and the
tensile properties were determined following the general
procedure in ASTM D882 using 4 – 5 specimens from each
film. The test specimen gauge length was 5 cm and the
crosshead speed for film testing was 0.5 cm/min.
The dielectric constants of the films were measured
on a HIOKI 3532 - 50 LCR HITESTER in the frequency range
from 50 Hz to 5 MHz. Using the gold electrodes were vacuum-
deposited on both surfaces of dried films. Experiments were performed at the temperature ranges from 40 °C upto 250 °C in a dry chamber.
The PAAs were prepared from dianhydride and
diamine via a two-step method. In first step, PAA-1 was
prepared by dissolving diamine ODA (0.155 g, 0.776 mmol) in
2 ml of CaH2-dried DMAc in a 50ml round bottom flask. After
the diamine monomer dissolved completely, an equimolar
amount (0.404 g - 0.776 mmol) of dianhydride IDPA was
added. PAA-2 was prepared similarly by the same procedure,
dissolving the diamine IDDA (0.317 g, 0.774 mmol) in 3 ml of
CaH2-dried DMAc and dianhydride IDPA (0.403 g, 0.774
mmol) was added. The mixture of dianhydride and diamine
were stirred at RT for 12 hrs under nitrogen atmosphere to
afford a viscous PAA precursor. The polymer solution was
poured into distilled water. The white precipitate of PAA was
collected by filtration, washed thoroughly with distilled water,
and dried at 60 °C for 6 hrs in vacuum oven to remove
moisture and solvent.
The dried PAA powder was redissolved in DMAc solvent at 30% solid content (w/v) to get homogenized PAA solution. The solution was poured on a glass surface and heated sequentially at 100 °C for 1 h, 200 °C for 1h and 300 °C for 1h. After cooling to RT the polyimide film was soaked in water, a flexible PI film was self-stripped from the glass surface.
3 RESULTS AND DISCUSSION
The solubility of polyimides was tested in various
solvents. The polyimides IDPA-ODA and IDPA-IDDA were
soluble in polar aprotic solvents such as NMP, DMF, DMAc,
etc. at RT (Table I). The inherent viscosities (Table I) were
found at 0.872 and 1.091 for PAA1 and PAA2 respectively.
Even though the PAAs showed low in solution viscosity
values, they yielded quite flexible and strong PI film after
imidization.
Table I. Inherent viscosities and solubility behaviours of PIs.
S. No . | PI | N M P | DM -Ac | D M F | DM -SO | CH- Cl3 | TH F | ηinh (dL/g ) |
1. | IDPA ODA | ++ | ++ | ++ | ± | ± | ± | 0.872 |
2. | IDPA IDDA | ++ | ++ | ++ | ± | ± | ± | 1.091 |
++ = soluble; ± = partially soluble; - - = insoluble at room temperature
IJSER © 2012
International Journal of Scientific & Engineering Research, Volume 3, Issue 8, August 2012
Issn 2229-5518
The conversion of the PAA to the fully cyclized
polyimide was determined by FT-IR. Figure 2 and 3 show the
IR spectra of PAAs and polyimides respectively. The complete
conversion of o-carboxylic amide to the imide ring was
evidenced by the disappearance of the amic acid bands at 2500
- 3500 cm-1 together with the appearance of characteristic
molecular absorption vibrational bands at 1775 (asymmetrical
C=O stretch), 1713 (symmetrical C=O stretch), 1382 (C-N
stretch), 745 (C=O bending). (Figure 3)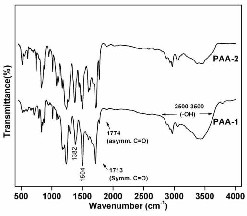
Fig 2. FT-IR spectra of poly(amic acid) powder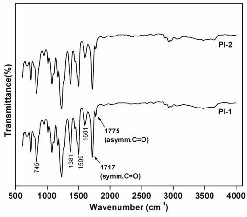
Fig 3. FT-IR spectra of polyimide films
Polyimide films were characterized by wide-angle
XRD studies and the diffraction patterns are shown in Figure
4. A broad diffraction peak intensity was observed around 2θ
= 10 - 25° for PI films which showed amorphous nature with
broad diffraction intensities peak due to flexible iso-
propylidene group present in the matrices.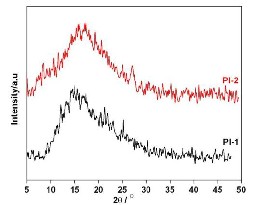
Fig 4. XRD
pattern of PAA and PI
The visco-elastic relaxation behavior of polyimide
films were measured by DMA. The specimen dimensions for
DMA measurements were about 40mm × 5mm × 0.03mm
(l×w×t). The storage modulus E’, loss modulus E’’ and a
measure of dissipation or damping tan δ (tan δ = E’/E’’) at 1
Hz oscillatory deformation were recorded in the temperature
range from 25 °C to 300 °C. The glass transition temperatures
were determined as the temperatures of the maxima of tan δ
(Figure 5 and 6) which showed 238.8 °C and 216.7 °C for PI
films 1 and 2 respectively. The Arrhenius plot of ln.
frequencies (Hz) corresponding to tan δ max against
temperature for PI-1 & PI-2 is shown in Figure 7 & 8. The
activation energies (ΔE) of polyimide films were found to be
781.43 and 981.01 kJ/mol for PI 1 and 2 respectively. The
higher value of ΔE for PI2 can be attributed to the presence of
bulky symmetrical isopropylidene groups in both the
monomers.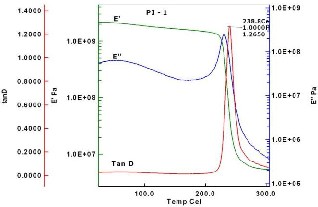
Fig 5. DMA curve of IDPA-ODA PI film 1
IJSER © 2012
International Journal of Scientific & Engineering Research, Volume 3, Issue 8, August 2012
Issn 2229-5518
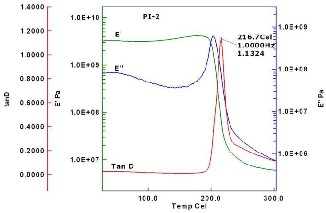
Fig 6. DMA curve of IDPA-IDDA PI film 2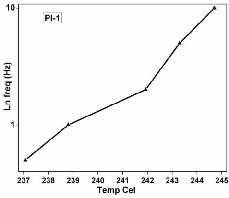
Fig 7. The curve of Temp. Vs ln freq. of PI-1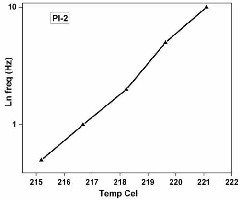
Fig 8. The curve of Temp. Vs ln freq. of PI-2
Typical TGA curves for polyimides are shown in
Figure 9 and 10 as determined at nitrogen (N2) gas and air
atmosphere. The PI-1 is found to be more thermally stable
compared to PI-2 and Thermal stability of polyimide (PI-1 &
PI-2) films at 10 wt% decomposition temperature values (Td)
was found to be 541.1 °C, 531.1 °C in N2 atm and 530.3 °C,
530.1 °C in air atm. Hence it is clearly evident for that in the
polyimides, the nature of decomposition and the groups
participating in it are same. Examination of the structures of
the polyimides illustrates that the common decomposable
group is the imide ring which is the target group for the
decomposition stage. As the nature of cleavage in the imide
ring is the same in all of them, the slight shift in their
decomposition temperature for PI-1 & PI-2 is attributed to the
influence of the electronic factors (electron releasing or
electron withdrawing) associated with phenoxide oxygen and
the iso-propylidene group of the dianhydride and diamine.
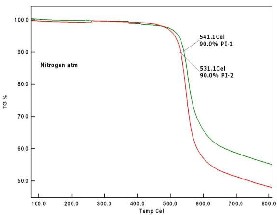
Fig 9. TGA curve of PIs in nitrogen atm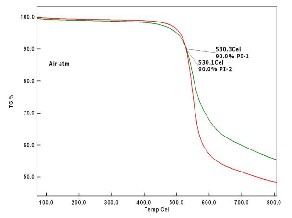
Fig 10. TGA curve of PIs in air atm
IJSER © 2012
International Journal of Scientific & Engineering Research, Volume 3, Issue 8, August 2012
Issn 2229-5518
The UV-Visible spectra of the polyimides with film
thickness of 70 - 90 μm are shown in Figure 11. Both the
polyimides show high transmission (85 - 88 %) in the
wavelength range of 800 - 1600 nm.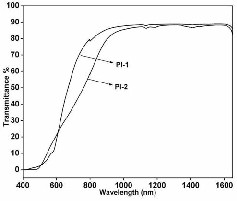
Fig 11. UV-Visible spectra of polyimide films
The iso-propylidene group and phthalic ether groups present in dianhydride and diamine moieties were effective in decreasing the charge transfer complex between polymer backbone chains through steric hindrance leads to increase in the intermolecular distance and thus decreased the interaction between the polyimide chains resulting in a good optical transparency.
The polyimide films were subjected to a tensile test,
and their tensile properties are summarized in Table 2. The
films had strength at break of 89.1 and 85.4 MPa for PI-1 & PI-
2 respectively showing a decrease in tensile strength was
observed, when ODA was replaced with IDDA. ODA
containing polymer (PI-1) had more rigidity and low
flexibility because there is only strengthened C-O-C bond and
no flexible iso propylidene group. Similar to tensile strength
which is suggested to be strongly dependent upon the
bridging groups, the percentage of elongation of the polymer
chain also depends on the properties of the bridge. As
expected, in PI-2 there is an increase elongation of iso
propylidene bridge than C-O-C bonding bridge.
Table II. Tensile strength of polyimide films
The plot dielectric constant vs. frequency at different
temperature ranging from 40 - 250 °C is shown in Figure 12
and 13 for PI-1 and PI-2 respectively. The dielectric constant
values measured at 5 MHz at different temperature showed in
Table III.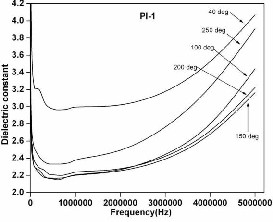
Fig 12. The curve of freq. Vs dielectric constant of PI film 1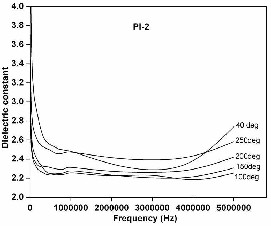
Fig 13. The curve of freq. Vs dielectric constant of PI film 2
It is observed that there is a decrease in dielectric constant with temperature from 40 to 200 °C at all the frequencies determined upto 5 MHz. Further it is interesting to find that PI-2 showed lower dielectric constant values ranging from 2.2 to 2.7 compared to PI-1 which showed in the range 3.1 to 4.0 at all the measured temperatures and frequencies. Chin-ping yang et al. prepared polyimides with keto groups in polymer chains which had dielectric constant of 3.47 at 1 MHz [14]. As shown in Table II, our polyimide film, PI 2 derived from IDPA and IDDA monomers shows dielectric constant as low as 2.25 at 5 MHz at 100°C. The low dielectric constant here is attributed to the fact that the introduction of the iso-propyli-
IJSER © 2012
International Journal of Scientific & Engineering Research, Volume 3, Issue 8, August 2012
Issn 2229-5518
-dene group and phthalic – ether group in both dianhydride and diamine leads to the formation of configuration of repeat units in the polymer backbone causing the decrease in the intermolecular force and packing ability of the resulting polymers. These results suggest that the PIs containing phthalic iso-propylidene groups loosen the polymer packing and increased the free volume, subsequently leading to reduced dielectric constant.
Table III. Dielectric constant of polyimide films
S. No. | PI film | Dielectric Constant (5 MHz) | ||||
S. No. | PI film | 40°C | 100°C | 150°C | 200°C | 250°C |
1. | PI-1 | 4.0709 | 3.4427 | 3.1649 | 3.2244 | 3.9088 |
2. | PI-2 | 2.7384 | 2.2546 | 2.3077 | 2.4215 | 2.5841 |
Two polyimides were prepared by the polycondensation reaction of IDPA with ODA and IDDA in DMAc solvent at RT and characterized for various properties. These aromatic polyimides showed good solubility in polar aprotic solvents, higher thermal stability, flexibility, toughness and optical properties. The XRD profiles of PI films showed that there was an absence of crystalline domains, revealing the amorphous nature of the films. PI-2 (IDPA-IDDA) showed lower dielectric constant values in the range of 2.2 – 2.7 compared to PI-1 (IDPA-ODA) at different frequencies up to 5
MHz. This low value is attributed to the presence of more free space between the polymer backbone chains created by the steric effect of bulky phthalic iso-propylidene groups in both alternate dianhydride and diamine components in PI-2. Because of low dielectric constant values of PI 2 even at high temperature, it can be the best candidate for use as insulator applications.
The authors are grateful to the Department of Science and
Technology, India for the financial support of this work (Grant
SR/NM/NS-112/2008).
1. Yin D. Li, Shao Y, Zhao Y, Yang X and Fan S L 2005
J. Fluorine Chem. 126 819–823.
2. Dusselberg D, Verreault D, Koelsch P and Staudt C 2011
J. Mater. Sci. DOI 10.1007/s10853-011-5399-6.
3. Faghihi K, Hajibeygi M and Shabanian M. 2010 J. Poly.
Res. 17 379–390.
4. Jintian Y, Wei H, Yongfeng Z and Deyue Y 2007 Front.
Chem. China. 2(2) 107–112
5. Yan S, Chen W, Yang W, Chen C, Huang M, Xu Z, Yeung
K. W. K and Yi C. 2010 Poly. Bull. DOI 10.1007/s00289-010-
0343-5.
6. Yang C. P, Chen R. S and Hsu M. F 2002 J. Poly. Res. 9
245–250.
7. Yang C. P and Chen R. S 2001 Colloid Polym. Sci. 279
736–744.
8. Yang C. P and Chiang H. C 2004 Colloid Pol. Sci. 282
1347–1358.
9. GuanJun C, ShiZhen T, Xuan L, Qiang W, Zhe X, Lin Z and
RunXiong L 2008 Sci. in China Series B: Chem. 51(3) 275–281.
10. Wang C, Zhao X, Li G and Jiang J 2009 Pol. Deg. and
Stab. 94 1526–1532.
11. Kapantaidakis G. C, Koops G. H and Wessling M 2002
Desalination. 145 353–357.
12. Ekinci E, Koytepe S, Pasahan A and Seckin T 2006 Turk.
J. Chem. 30 277–285.
13. Zhang X, Hu Z, Zhang S, Chen S, Chen S, Liu J and Wang
L 2011 J. App. Pol. Sci. 121 1707–1716.
14. Rahimpour A. 2011. Korean J. Chem. Eng. 28(1) 261-266.
15. Hsiao S. H and Huang T. L 2004 J. Pol. Res. 11 9–21.
16. Wang C, Zhao X, Li G and Jiang J 2009 Pol. Deg. and Stab.
17. Butt M. S, Akhter Z, Zaman M. Z and Siddiqi H. M 2008
Colloid Polym Sci 286 1455 –1461.
18. Dixit B. C, Dixit R. B and Desai D. J 2009 J. Pol. Res. DOI
10.1007/s10965-009–9334-3.
19. Won D. S, Lee G. Y and Lee J. Y 2008 Poly. Bull. 61 43-51.
20. Mohsin M. A, Akhter Z, Bolte M, Butt M. S, Khan M. S. U
and Siddiqi H. M 2009 J. Mater. Sci. 44 4796–4805.
21. Faghihi K, Shabanian M and Shabani F 2010 J. Polym. Res.
DOI 10.1007/s10965-010-9458-5.
22. Kumar S. V, Yu H. C, Choi J, Kudo K, Jang Y. H, Chung C.
M 2010 J. Polym. Res. DOI 10.1007/s10965-010-9513-2.
23. Purushothaman R, Bilal I. M and Palanichamy M 2010
J. Polym. Res. DOI 10.1007/s10965-011-9564-z
24. Lazareva Y. N, Vidyakin M. N, Yampolskii Y. P, Alentiev
Y, Yablokova M. Y, Semenova G. K, Kuznetsov A. A and
Likhachev D. Y 2006 Polym. Sci. Ser. A. Vol. 48 No. 10 pp.
1073–1079.
25. Chang W. Y, Fang T. H and Lin Y. C 2008 Appl. Phys. A.
26. Ding Y, Bikson B and Nelson J. K 2002 Macromolecules.
27. Deligoza H, Ozgumus S¸ Yalcınyuva T, Yıldırım S,
Degerb D and Ulutas K 2005 Polymer. 46 3720–3729.
28. Yang F. C. P and Hsiao Z 2003 J. Polym. Res. 10 181–193.
29. Hu B, Wei H, Han Y, Zhu G, Pei X, Zhu J and Fang X 2011
J. Mater. Sci. 46 1512–1522.
30. Yang C. P, Chen Y. C, Hsiao S. H, Guo W and Wang H. M
2010 J. Polym. Res. 17 779–788.
IJSER © 2012