International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 254
ISSN 2229-5518
Cross-sectional study of infertile males with toxoplasmosis in Baghdad province
Husam E. Abdulla1*, Nada M. Al-bashier1, Ula Al-kawaz2, Arwa M. Abdullah Al-Shuwaikh1, Ahmed S. Abood3
Abstract—Toxoplasmosis is a zoonotic disease caused by a protozoan parasite called Toxoplasma gondii which infect all mammals and birds species throughout the world. some studies shows that toxoplasmosis have adverse effect for male reproductive system, parasites are isolated from different parts of animals even from semen but there are little information about the effect of toxoplasmosis on fertility in animals and humans. The present investigation dealt with studying the prevalence of chronic toxoplasmosis with in infertility patients. A total number of 83 individual, in which 64 infertile male patients, Include 25 males with primary infertility and also
39 males with secondary infertility. Blood were collected in a period between April 2104 and August 2014. Serum samples were studied for determination of chronic infection with toxoplasmosis, by using ELISA technique. This study showed clearly that 23 (27.7%) of individuals participated in this study having IgG Toxoplasma Ab, among those 3 samples (15.8%) were control, 20 sample were infertile and the infertile group sub divided in to 6 (30%) primary infertility and (14 70%) secondary infertility. The Toxoplasma infection have no effect on male reproductive parameters.
Index Terms— ELISA, IgG, Male Infertility, Toxoplasmosis, Toxoplasma gondii.
—————————— ——————————
1 INTRODUCTION
oxoplasmosis is a very common parasitic infection in hu- mans and other warm-blooded animals, with approxi- mately a third of the world’s human population estimated
to have been exposed to the obligatory intracellular parasite (1). Toxoplasma gondii is a ubiquitous parasite whose defini- tive hosts are members of the Felidae (cat family). Cats shed millions of environmentally resistant oocysts in their feces after primary infection and are usually without clinical mani- festations of disease. Include almost all warm-blooded mam- mals and birds, even humans, who accumulate infectious, qui- escent stages (bradyzoites) of the parasite in their tissues, par- ticularly in skeletal muscle and the brain. The Intermediate hosts may acquire infection by consuming raw or under- cooked flesh from other intermediate hosts, or by ingesting oocysts from the environment. Environmental sources of T. gondii (oocysts) are include soil, water, shellfish, fruits, and vegetables (2). Oocysts-acquired infections in humans are clin- ically more severe than tissue cyst-acquired infections (3). As tachyzoites are sensitive to environmental conditions they are usually killed rapidly outside the host and so are rarely in- volved in foodborne transmission of T. gondii (4). In acute infections, multiplying organisms spread from the gut through the lymphatics to regional lymph nodes and through the blood to the liver, lungs, heart, brain and other organs (5). Infertility is defined as the inability of a sexually active non- contracepting couple to achieve pregnancy in one year (6). Most couples achieve conception within one year and approx- imately 15% of couples are unable to do so , Approximately
20% of infertility cases are caused by male factors, with an additional (30-40) % of cases involving both male and female factors; therefore, a male factor is present in half of infer- tile couples (7).

1 College of Medicine- Al-Nahrain University.
2 High Institute Of Infertility Diagnosis And ARTs- Al-Nahrain University.
3 College of Education- Al-Iraqia University.
* Corresponding Author: husam.aljumaily@yahoo.com.
2. AIMS OF THE STUDY:
To investigate the distribution of toxoplasmosis within infer- tile males.
3 MATERIALS & METHODS:
3.1 Subjects
Patients: Selection of sixty four man who suffering from infer- tility for this study. Include twenty five males with primary infertility and also thirty nine males with secondary infertility. Controls: Selection of nineteen healthy fertile male as control, to compare with our patients in the same parameters of this study, sera were collected from those fertile males. From the total number fourty four were referred to Al-nahrain Universi- ty/ high institute of infertility diagnosis and assisted repro- ductive technologies according to the physician’s report, and the rest from private laboratory in metropolis Baghdad from Al-taji city in a period between April 2104 and August 2014.
A questionnaire sheet, was filled out for each individual in- volved in the study. In addition, ethical approval were ob- tained.
3.2 Sample collection:
From each male (patient and control) venous blood (5 ml) were collected. The blood was placed in a plain tube and left to stand for one hour at room temperature for clot formation. For serum collection, the tube was centrifuged for 10 minutes at 4ºC at 450 XG. The serum was then aspirated using a Pas- teur pipette and dispensed into sterile appendorf tubes (0.5 ml in each) and stored at -20 ºC until used.
3.3 Quantitative ELISA:
Kit for detection of IgG antibodies against T. gondii antigens in serum (HUMAN Gesellschaft for biomedical and diagnosti- cal mbH) was used according to manufacturer`s instructions.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 255
ISSN 2229-5518
The absorbance of the low positive control was measured, and its value represents the cut-off value of the assay. Then, the absorbance of the test sample was substrated by the cut-off value to decide if the sample is positive or negative. Such deci- sion was made according to the following:
Positive sample: absorbance cut-off value ≥ 0.15
Negative sample: absorbance cut-off value < 0.15
3.4 Statistical analysis:
The data were statistically analyzed depending on the nature of the character, according to Snedecor and Cochran (1981) using computer software SPSS version 22.
4 RESULTS:
4.1 Distribution of studied individuals according to age groups frequencies:
The total subjects included in the presented study were eighty- three personnel, which distributed into two main groups: con- trol fertile and infertile group. The infertile group include primary and secondary infertility subgroups. The most fre- quent age group for the studied cohort was (31-40) years (See figure 1). The age mean for the control fertile group was (31.32
±5.09), and for the infertile group was (31.48 ±7.73). Figure (1): Age groups frequencies for the study group.
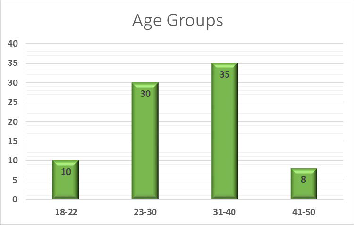
4.1 Toxoplasmosis positivity:
Immunoassay for Toxoplasma IgG for the subjects involved in this study show twenty three positive cases with mean of (38.97±33.745) and sixty negative cases with (1.735±0.734). There were high significant differences between positive and negative means (p value ≤0.001) (See Table 1).
According to toxoplasmosis positivity, the most frequent age group founded was (23-30). See figure (2). ANOVA test was used to investigate the significance in the toxoplasma IgG titer within the study groups (control fertile, primary and second- ary). We found out that there were no significant differences among these groups (p value=0.105) (See Table 2).
Table (1): Toxoplasmosis IgG Descriptive data.
*Titer=IU/ml
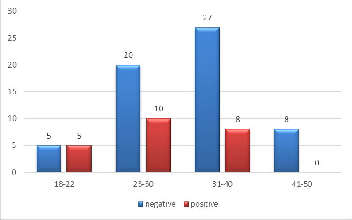
Figure (2) Age groups frequencies according to toxoplasmosis
positivity
Table (2) Toxoplasmosis IgG seroprevalence among study group.
| N | Mean & Std. Dev. | P value |
Control fertile | 19 | 7.442±13.049 | |
Primary | 25 | 4.964±8.806 | 0.104 |
Secondary | 39 | 17.227±32.216 | 0.104 |
Total | 83 | 11.389±24.148 | 0.104 |
Table (3) shows the significance of association between tox- oplasmosis seropositivity and different epidemiological pa- rameters. Seropositivity of the toxoplasmosis was higher in infertile group than in the control fertile (31.3% and 15.8% re- spectively). Although, there was no significant differences between the two groups (p value = 0.151). Frequency of toxo- plasmosis seropositivity within the infertile group show that the secondary infertility group have a higher frequency than that of the primary (70% and 30% respectively). Moreover, there was no significant differences between the primary and secondary subgroups (p value = 0.187).
Seropositivity of the toxoplasmosis was higher in urban cases than in the rural (70% and 30% respectively). Although, there was no significant differences between the two groups (p value = 0.145). After categorization of the infertile group ac- cording to the occupation (unemployed, employed and work- er), we found out that the highest frequency of toxoplasmosis seropositivity was in the employed subgroup (45%). Alt- hough, there was no significant differences between the sub- groups (p value = 0.753).
There was no significant differences between the smokers and non-smokers infertile subgroups (p value = 0.540). And there was no significant differences in the infertile group be- tween subgroups classified according to source of drinking
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 256
ISSN 2229-5518
water (p value = 0.278).
The infertile cases with no history of surgical operation
shows the highest frequency of toxoplasmosis seropositivity
(90%). Again with no significant differences between the sub-
categories (p value=0.106).The infertile cases with no history
of abortion with their wives shows the highest frequency of
toxoplasmosis seropositivity (90%). And again with no signifi- cant differences between the subcategories (p value=0.621).
When the infertile group subdivided according to blood groups, we found out that the most frequent toxoplasmosis seropositive blood group was the O+ blood group (26.1%). moreover, there was no significant differences between the subcategories (p value=0.257) .
The infertile cases with no history of contraceptive with their wives shows the highest frequency of toxoplasmosis seroposi- tivity (87%). In addition, with no significant differences be- tween the subcategories (p value=0.145).
Table (3) Epidemiological parameters association with Toxo- plasmosis.
Parameters | p value |
Control fertile and Infertile | 0.151 |
within infertile | 0.187 |
Address | 0.145 |
Job | 0.753 |
Smoking | 0.540 |
drinking water | 0.278 |
Surgery | 0.106 |
Abortion | 0.621 |
Blood Group | 0.275 |
History of contraceptive | 0.145 |
5 DISCUSSION:
In Iraq, it is the first time to conduct a research project with such design which investigate the toxoplasmosis seropositivi- ty with infertility in males.
T. gondii infects a large proportion of the world's popula- tion but uncommonly causes clinically significant disease. However, certain individuals are at high risk for severe or lifethreatening disease due to this parasite. Individuals at risk include fetuses, newborns, and immunocompromised patients (8).
The frequency of age group was higher (35 year) in the range (31-40) and the mean age of the persons (control and infertile) at presentation of this study were (31.32 ±5.09,
31.48±7.73 respectively). That may be due to population dis- tribution of males and the delayed knowledge of infertility. This finding agreed with the finding of Gardi (2005) (9) who reported that most common age presentation of male infertili- ty is around (25-35) years. It has been suggested that the aging male has reduced fertility that begins in his late thirties and early forties (10). Others found that in general cumulative probabilities of conception decline with age (11). So another study concluded that increased male age is associated with a decline in semen volume, sperm motility and sperm morphol- ogy (12).
In the current study, tried to estimate the actual percentage
of toxoplasmosis frequency among men of this study cohort, and observed their effect on male infertility, by using ELISA technique for detection of specific IgG in serum. When catego- rized the selected persons according to the positivity of toxo- plasmosis (negative and positive groups) were found to be (23 positive out of a total of 83 cases), the statistical analysis showed highly significant differences between descriptive data (p value ≤ 0.001) see table (1).
This result agreed with Ruqia (2010) (13), which showed the results of 91 samples were 23 % samples positive for anti- T. gondii IgG antibodies, also the result were in-line with the results obtained by researcher in Iraq showed that out of 100 positive chronic infection 49 % in male with toxoplasmosis (14). In other world countries (IRAN), the prevalence of toxo- plasmosis infection among were 35.57 % from sera of 1,026 men in Arak City showed 365 of them had anti-toxoplasma antibody about 35.57% from total patients (15).
When selected persons classified according to toxoplasmo- sis positivity and noticing more frequent age for infection ob- served (23-30) have more frequent to infection see figure (2). The rate of acquisition of infection in relation to age varies according to the country and socioeconomic level of selected samples. This compatible with Al-Saadii (2013) when showed that the highest percentages of toxoplasmosis infections were age group (26-33) while, the age group of (50-57) years showed the lowest (16), also this result were coincided with other results obtained by Dargham (2011) in Iraq who they concluded that the main age group range of seropositive toxo- plasmosis was between (20-30) years (17), this result were dis- agreed with Jassam (2010) who recorded that the age group (30-39), (40-49) and (≥ 50) with the highest rates (48.6%), (44%) and (58.1%) respectively (14).
When selected persons classified according to infertility groups into (control, primary and secondary) and observed no significant differences (p value = 0.104) according to toxo- plasmosis positivity see table (2) between study groups and toxoplasmosis, this result related to number of samples, resi- dence of infertile samples, and duration of infection with tox- oplasmosis. there were not enough studies about the frequen- cies of toxoplasmosis between fertile and infertile groups, but in the female El-Tantawy (2014) recorded a statistically signifi- cant higher prevalence (p < 0.01) of T. gondii infection in infer- tile female patients (61.85%) in Dakhalia governorate, Egypt in comparison with the control group (18), Others agreed with those of Li, et al. (2011) who reported a high prevalence (15.9%) of anti T. gondii IgG antibodies using ELISA among female infertility patients in comparison to 5.6% among pregnant–puerperant women (19). These differences could be due to developing laboratory means and good health educa- tion in our society and or because of small sample size. Also when there were no significant differences (p value = 0.151) between control group and infertile groups, this result were incompatible with (Zhou et.,al 2002) in China who found that Toxoplasmosis infection in infertile human couples was higher than fertile couples (20).
Also, present study disagreed with (al-saadii 2014) which
showed that fertile male infected with chronic toxoplasmosis
had a lowest percentge of anti-Toxoplasma IgG antibodies 31
(30.69%) while infertile males showed the higher 70 (69.30%)
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 257
ISSN 2229-5518
(16),this differences in results may be due to number of select- ed persons, laboratory techniques that uses in the studies, there are very little studies about the frequencies of toxoplas- mosis positivity between infertile groups (primary and sec- ondary) and the presented study showing no significant dif- ferences (p value= 0.187) table (3), this result may be related to duration of infection with toxoplasmosis ,samples numbers and the time of treatment for toxoplasmosis before and after investigation of infertility. The current study was observed that toxoplasmosis did not have significant differences (p val- ue = 0.145) table (3) according to residence (rural and urban), that’s related to socioeconomic level of selected samples, and may be elevated of health awareness from infection, so the selected samples in this study were nearly equal according to residence, this results were corresponding with several previ- ous studies shows no significant differences of toxoplasmosis infection according to residence by Al-Najjar 2005 (21), Al- Wattari 2005 (22), and Karem 2007 (23).
Another authors Jassam 2010 (14) showed that among posi- tively rate of anti-toxoplasma IgG antibodies selected explana- tory variable among schizophrenic group was no significant among rural (49.4%) compared to urban residence (47.1%). This result was disagreed with Data obtained from (Al- Saadii
2014) (16) revealed significantly high percentage (P<0.01) of toxoplasmosis in males inhabited rural area where
111(91.73%) compared to urban residents 10 (8.26%), the pre- sent study also disagreed with previous study (Kawashima,
2000) (24) where they indicated significantly higher (p<0.001) seropositive in rural than urban. The lack of statistical signifi- cant analysis in this study probable due to rising health care between men in rural area, also may be men have low contact with soil, water and meat contaminated with oocyst of toxo- plasmosis. In this study positive toxoplasmosis occupies pri- vate worker (worker and unemployed) revealed percentage
55% from total positive toxoplasmosis) than those having offi- cial works 45% in the presence of anti-Toxoplasma IgG anti- bodies. There were no significant differences between these groups (p value = 0.753) table (3). In Iraq (Al-Jubori, 2005) was agreed with this study reported all seropositive rate was (33.42%), high prevalence rate in barbers (72.73%) followed by food handlers (41.18%) housewives (38.9%) donated blood (24.30%) and medical staff (21.43%) (25). another study was harmonize with this study (Hamza, 2006) revealed that farm- ers had highest anti-Toxoplasma antibodies rate (47.4%) com- pared with other occupations (clerks 31%, teachers 42.1%, stu- dents 21.2%, workers 37.5%) by ELISA which was further supported by Logistic regression analysis (26). The explana- tion of these results may related to the fact that farmers were likely to be of low education or their contact with to soil and other risk factors were more than others.
This is the first study discuss the effect of smoking habit on the infection with toxoplasmosis, when classified toxoplasmo- sis positivity according to smoking habit there were no signifi- cant differences (p value = 0.540), (table 3), this result depend on socioeconomic level and health awareness between peoples and no studies founded in Iraq or in other world countries to compare with this result. when categorized the toxoplasmosis positivity according to source of drinking water failed to ob- tain significant differences (p value = 0.278), table (3) may be
due to elevated health awareness between people to avoiding diseases, also number of selected sample play important role in determination of significant differences, the current result disagreed with (Chiang et.,al 2012) (27), when revealed signifi- cant differences ( p value = 0.36) between toxoplasma positivi- ty according to source of drinking water, other study was in- compatible result when (Sroka et.,al 2006) (28) showed a total number of 114 drinking water samples were taken, 80 samples from shallow household wells with a windlass, 16 from deep wells with a pump, and 18 from the water supply system. In microscopic and PCR examinations of 114 water samples, T. gondii was found in 15 (13.2%) and 31 (27.2%) of samples, re- spectively.
Comparison studies about association between frequencies of toxoplasmosis positivity with surgical operation (kidney transplantation and varicocele) has not been obtained. So there is no significant differences in this study (p value = 0.106) see table (3) between toxoplasmosis positivity and any surgical operations, this relation can Verified by demonstrated the bi- opsy from testicular tissues to diagnose the toxoplasma tachyzoite. Several reports express association of male genital tract impairment with special feature of testicular toxoplasmo- sis (29), Toxoplasma orchitis (30). Evidences from Animal Models, Lopes et al (2009) suggest that acute Toxoplasmosis in male mice can induce pathological changes in different repro- ductive organs such as testes, epididymis, vas deferens and prostate. Also, observed in larger animals such as sheep exper- imentally infected with T. gondii, and can cause various histo- pathological changes in testicles, prostate and seminal vesicles in infected animals observed (31).
There was no obvious significant differences between toxo- plasmosis positivity and history of abortion occurrence in their wives (p value = 0.621) see table (3), there are several causative factors responsible for both habitual and sporadic abortions. However, toxoplasmosis prevalence in women with bad ob- stetrics history is known to be significantly higher than in normal, the seroprevalence in pregnant women on worldwide scale varies from (7- 51.3) % and in women with abnormal pregnancies and abortions; the seroprevalence varies from (17.5- 53.3) % (32). This result were disagreed with (AL- Timimi 2004) observed, by using latex and IFAT methods, among 168 women with a history of abortion in Baghdad the rates were (44 – 29) % respectively (33).
In Mosul (34), in addition to (AL-Ubaydi ,2004) compared
the seropositivity by applying three different laboratory
methods on a total of 406 sera; latex test showed 79 % fol-
lowed by active infection 46 % using Dye test (DT), while us-
ing ELISA technique gave 37 % (35).
Mohanad M. et al., 2013, also explain the overall percentage
of positive reaction to T. gondii in both couples was 38.4
(35/91), while for wife infected it was only 30.7% (28/91)
and husband infected was only 13.1% (12/91) (36). so did not
get research that care with investigate the toxoplasmosis be-
tween wives of positive husbands before the occurring of
pregnancy and in the first, second and trimester period of pregnancy. Most the studies shows the relation between the time of infection with toxoplasmosis with the occurring of congenital anomalies, abortion and still birth. The frequency of
T.gondii infection among women with abortion was found to
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 258
ISSN 2229-5518
be (19.17) % by using ELISA test. However, the frequency was found to be (21.66) % by using Immunohistochemical analysis. This high frequency may be due to the sample size, sample selection, and to the multiple source of infection (37). These variable results may be due to the differences in the specimens used by each researcher and their variable condition and data of studies, strain of T. gondii, and genetic variations.
The present study revealed high frequency of blood group
O⁺ associated with positive toxoplasmosis 26.1% while the lowest frequency in blood group AB⁺ was 0 % where there
were no significant difference between blood group (p value =
0.275) see table (3). This result agreed with (Joesph et.,al 2010)
revealed that infection were concentrated on blood group type
O⁺ with percentage of (43.47)%. This result do not support
with (Al-Saadii 2013) that investigated the blood group type
AB⁺ was higher in frequency 41.32 % while the lowest fre- quency was blood group type O⁺ (15.70) %, with high signifi-
cant differences (p value = 0.01) (16). A study among blood
donors in Russia (Zhiburt et.,al 1997) has reported similar findings, with toxoplasmosis seroprevalence being twice as high among subjects with blood group AB than among sub-
jects with blood group O⁺ (54, 27) % respectively (38). Howev-
er, this result is agreed with (Al-Kaysi and Ali 2010) whom
they showed that Toxoplasma infection was more prevalent
among O⁺ and AB⁺ blood group with the seroprevalence were
35.8% and 38% respectively (39). This result was disagree with
(Al-Shikhly 2010) who showed that the higher percentage re-
sult (30.6%) was occurred in female with AB+ blood group
(40). Finally in Brazil (Rodrigues ACF 2011) shows that The
ABO blood group system is not associated with the presence
or absence of anti-T. gondii antibodies in pregnant women in Brazil and, therefore, phenotypic variations resulting from this histo-blood group system do not seem relevant for T. gondii infection. The frequencies of the four ABO blood
group phenotypes, independent of the results of the ELISA, were 39.5% (397/1006) for group A , 11.2% (113/1006) for group B, 3.8% (38/1006) for group AB, and 45.5% (458/1006)
for group O⁺ (41). The disagreement among these results and
other studies may resulted from several factors. It is possible
that the molecular variability of strains in Iraqi patients, or use
only male patients in this study gave findings may therefore
differ from findings in other population. Or it is possible that
AB antigens exerts a large influence on the adherence of T. gondii to the gastrointestinal mucosa and its contribution is evident by the high prevalence of infection by these parasites in the Iraqi population. In the table (3) revealed no significant
differences (p value = 0.145) between the usage of contracep- tive methods in male positivity wives, The relation between toxoplasmosis positivity and usage of contraceptive methods did not look in Iraq previously, also in the world there were no clear research about this relation, that’s might be due to the belief that there is no correlation between positivity toxoplas- mosis and contraceptive methods.
6 CONCLUSIONS:
The prevalence of toxoplasmosis a among main study groups (control and infertile) in Baghdad province was 15.8% and 31.3% respectively ELISA IgG, with no significant differ-
ences. The main percentage of infection with toxoplasmosis was observed in the age between (23-30) years, while the age group of (41-50) year had the lower percentage. There were no relationship between toxoplasmosis seropositivity and epide- miological factors (habitation, occupation, job, smoker habit, source of drinking water and blood group). Toxoplasmosis seropositivity had no liaison with reproductive parameters (history of surgical operation, history of abortions, blood group, and history of contraceptive).
REFERENCES
[1] Hill DE, Sreekumar C, and Jones J, et.,al Toxoplasma gondii. Ch 12 In: Simjee S 2007 Foodborne diseases. Humana Press, Totowa, p. 337–353aa.
[2] Marawan A. Abu-Madi, and Jerzy M. et.,al Toxoplasma gondii Seropositivity
and Co-Infection with TORCH Pathogens in High-Risk Patients from Qatar.
2010. College of Arts and Sciences, Med. Hyg., 82(4), 2010, pp. 626–633 doi:10.4269/ajtmh.2010.09-0530.
[3] Dubey JP Toxoplasmosis - A waterborne zoonosis. 2004 Vet. Parasitol 126(1-
2):57–72.
[4] Bonfioli, A. A. and F. Orefice (2005). Toxoplasmosis. Semen. Opthalmol.
20:129-141.
[5] Dubey. J. The History of Toxoplasma gondii—The First 100 Years 2008 Jour- nal compilationr2008 by the International Society of Protistologists OI:
10.1111/ j.1550-7408.2008.00345.x/ J. Eukaryote. Microbial. 55(6), pp. 467–475. [6] Sinisi AA, Di Finizio B, and Pasquali D, et.,al: 2003. Prevalence of antisperm
antibodies by Sperm MAR test in subjects undergoing a routine sperm
analysis for infertility. Int J Androl 16: 311-314.
[7] Domagala, A. Kurpisz, M.: Identification of sperm immunoreactive antigens for immuncontraceptive purposes: 2004 a review. Reproductive Biology and Endocrinology .Vol.18.Pp:1-7.
[8] Hokelek M. and Safdar A. (2005). Toxoplasmosis. Retrieved November 15 ,
2005 from http://www.emedicine.com/med/topic 2294.
[9] Gardi, A. H. (2005). Assessment of Psychosocial aspect of infertile women in ErbilKurdistan region – Iraq. M.Sc. Thesis. University of Salahaddin, College of Nursing.
[10] Bayer, R.S.; Alper, M.M.; Penzias, A.S. (2007). "The Boston IVF Hand Book of
Infertility". 2nd ed. USA. The Pathenon Publishing groups.
[11] Gnoth, C.; Godehardi, E.; Herrmann, P.F.; Friol, K.; Tigges, J. ; Freundl, G. (2005). Debate- contiued: Definition and prevalence of subfertility and infertili- ty. Hum Reprod., 2(5), 1144-1147.
[12] Sharon, A.K.; Brenda, E.; Andrew, J.W. (2001). Effects of male age on semen quality and infertility. Fertil Steril., 75(2), 237- 48.
[13] Ruqia M. Ewadh. 2010. Seroepidemiological study of toxoplasmosis in blood donors in babylon province. Biology Dep., Collage of Science for Women, Babylon University, Iraq 409:3:9
[14] Jassam, F. S. (2010). Relationship between toxoplasmosis and testosterone hormone among schizophrenic patients in Baghdad. M. Sc. Thesis. College Council oF Health and Medical Technology. pp 81.
[15] Eslamirad Z, Hajihossein R, and Ghorbanzadeh B, et.,al. 2013. Effects of Tox- oplasma gondii Infection in Level of Serum Testosterone in Males with Chronic Toxoplasmosis. Iranian Society of Parasitology http:// isp.tums.ac.ir.
[16] Al-Saadii S. 2013. The Effect of Toxoplasmosis on The Level of Some Male Sex Hormones In Samples from National Blood Transfusion Cen- ter/Baghdad. B.Sc. in Biology / College of Science / University of Baghdad.
[17] Dargham, M. B. (2011).Prevalence of toxoplasmosis and laboratory serologi- cal diagoosis and some haematological and biochemical tests in fected wom- en in AL-Najaf province .M.SC. thesis. College of Health and Medical Tech- nology.pp106.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 1, January-2015 259
ISSN 2229-5518
[18] El-Tantawy N. Taman A. Shalaby H. 2014 Toxoplasmosis and Female Infer- tility: Is there a CoRelation? American Journal of Epidemiology and Infectious Disease, 2014, Vol. 2, No. 1, 29-32. DOI:10.12691/ajeid-2-1-6
[19] Li S, Cui L, and Zhao J,et.,al . 2011. Seroprevalence of Toxoplasma gondii infection in female sterility patients in China. The Journal of Parasitology, 97,
529-530..
[20] Zhou Y. Lu YJ, and Wang B. et.,al Survey of infection of Toxoplasma gondii in infertile couples in Suzhou countryside. Zhonghua Nan Ke Xue 2002; 8(5):
350-352.
[21] AL-Najjar, S. A. 2005. Detection of Anti-Toxoplasma antibodies among pa- tients with acute leukemia or lymphoma using Latex Agglutination and ELI- SA. M. Sc. Thesis. College of Medicine. University of Mosul. pp 78.
[22] AL-Wattari, I. I. 2005. Seroprevalence of Toxoplasma gondii antibodies among unmarried women of child bearing age in Mosul. M.Sc. Thesis. Col- lege of Medicine. University of Mosul. pp 77.
[23] Karem, L. O. 2007. Seroepidemiological study of Toxoplasma gondii for aborted women sera in Sulaimania city. M.Sc. Thesis, College of Science, Uni- versity of Baghdad.pp 124.
[24] Kawashima, T; W. khin-Sane; and M. Kawabata; et.,al. 2000. Prevalance of antibodies to Toxoplasma gondii among urban and rural residents in the Phil- ippines South East Asian. J. Trop.Med. Public Health; 31:742-746.
[25] AL-Jubori, A. R. M. (2005). Serological of toxoplasmosis in Kirkuk province.
M.Sc. Thesis. College of Health and Medical Technology.pp 82.
[26] Hamza, J. K. (2006). Seroepidemiological study of Toxoplasma antibodies among women in reproductive age in Hilla city.M.Sc. Thesis. College ofUni- vercity of Baghdad pp102.
[27] Chiang T. Hsieh H. and Chu Kuo M.2012. Seroepidemiology of Toxoplasma gondii Infection among Healthy Blood Donors in Taiwan Taiwan. PLoS ONE
7(10): e48139. doi:10.1371/journal.pone.0048139.
[28] Sroka J, Wójcik A, Dutkiewicz J.2006. Occurrence of Toxoplasma gondii in water from wells located on farms. Ann Agric Environ Med. 2006;13(1):169-
75.
[29] Martinez. F, Regadera J, and Mayer R, et.,al. 1996. Protozoan infections in the male genital tract. J Urol.; 156(2 Pt 1):340–9.
[30] Haskell L, Fusco MJ, and Ares L, et.,al 1989. Disseminated toxoplasmosis presenting as symptomatic orchitis and nephrotic syndrome. Am J Med Sci;
298(3):185–90.
[31] Lopes WD, Costa AJ, and Souza FA, et.,al 2009. Semen variables of sheep (Ovis aries) experimentally infected with Toxoplasma gondii. Anim Reprod Sci.2009; 111(2-4):312–9.26. Lopes WD, Santos TR, Luvizotto MC, Sakamoto CA, Oliveira GP.
[32] Talaro, Kathleen P. Barry chess. 2008. Foundations in microbiology, eighth edition, ISBN 978-0-07-337529-8. MHID 0-07-337529-2.
[33] Al-Timimi, L. 2004. Detection of toxoplasmosis among different groups of
aborted women during gestational age of pregnancy. Diploma, Thesis. Col- lege of Health and Medical Technology.pp77.
[34] AL-Ubaydi, G.T. 2004. Toxoplasmosis in pregnant women and its relation with some parameters. M. Sc. Thesis. College of Science. University of Mosul. pp 127.
[35] AL-Ubaydi, G.T. 2004. Toxoplasmosis in pregnant women and its relation with some parameters. M. Sc. Thesis. College of Science. University of Mosul. pp 127.
[36] Mohanad M., Shehab A., Abudalla H. 2013. Seroprevalence of Toxoplasma gondii between couples in Ramadi city using enzyme linked immunosorbent assay (ELISA) International Journal of Medicine and Medical Sciences Vol.
5(6), pp. 295-299, June 2013 DOI: 10.5897/ IJM MS11.101 /ISSN 2006-9723.
[37] Huppatz C, Durrheim DN and Levi C, et.,al Etiology of encephalitis in Aus- tralia, 2009 1990-2007. Emerging Infectious Diseases 15(9):1359–1365.
[38] Zhiburt, B.; A. Ionova; and K.Danilchen;et.,al (1997). The spread of antibodies
to cytomegalovirus and Toxoplasma among donors of blood componnents, microbiol. Epidemiol .Immunobiol . ; (1):59-61.
[39] Al-Kaysi, A. M and N. M. Ali, (2010). Serological and biochemical study of HB, HC, HIV and toxoplasmosis infection among blood donors in Iraq. Egypt J. Comp. Path. and Clinic, Path. 23(1):1-9.
[40] AL-Shikhly, A. 2010. Early detection of toxoplasmosis percentage in premari- tal females by immunological methods. M.Sc. Thesis. College of Science. Uni- versity of Baghdad. pp 147.
[41] Rodrigues, A.; S. Uezatos; and M. Vono et. al;.(2011). Association between antiToxoplasma gondii antibodies and ABO blood group system .J. Venom. Anim. Toxin.Trop. Dis.17 (2):184-189.
IJSER © 2015 http://www.ijser.org

