International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 1
ISSN 2229-5518
Soile, O.O.B.*1 and Owoyokun, T. O.1
1Department of Chemistry and Biochemistry, Caleb University, Imota. Lagos. Nigeria.
*Corresponding author. bob.soile@yahoo.com, 234-8023107681.
Thermochemical liquefaction of Kraft lignin using three Ni catalysts at 300, 350 and 375oC at 24, 40 and
60 atmospheres using H2 and CO as reductants was done to produce liquid oil. The effects of catalysts, reductants, temperature, pressure, reaction times and feedstock on the product oil were studied using
%yield, %conversion, %CHR, HC and OC ratios as parameters. Mean and standard deviation as well as ANOVA and independent t-test at 0.05 significant levels were used to ascertain the statistical significant difference brought about by the varying variables on the parameters taken on the product oil. The difference in the values of the parameters was statistically significant (p<0.05) as result of varying catalysts, temperature, pressure, reductants, reaction time and feedstock. Liquefaction of Kraft lignin promises to be a good source of obtaining liquid oil using optimum process variables.
Keywords: Kraft lignin, liquefaction, reductants, ANOVA, t-test
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 2
ISSN 2229-5518
Biomass such as wood is a renewable and alternative source for the production of fuels and chemicals (Klass, 2004). Cellulose, hemicellulose and lignin portions are principal components of biomass with proportions of the lignin being one-third by weight (Mohan et. al.,2006). Lignin is a three- dimensional amorphous cross-linked biopolymer consists of phenyl-propane units,optionally substituted with methoxy and hydroxyl groups (Zakzeski et. al., 2010; Chakar and Ragauskas, 2004). It comprises 15–
30% by weight and up to 40% by energy of biomass (Perlack, 2005). It is located in the spaces between cellulose and hemicelluloses and holds the lignocellulosic matrix together and adding rigidity to plant material (Ritter, 2008). Quite a number of researchers have reported the potential of lignin as useful feedstock for the production of useful chemicals (Roberts et. al.,2011; Stark et. al.,2010; Zakzeskiet. al.,
2010; and Jongerius et.al., 2012). Lignin derived from abundant and renewable resources are nontoxic and extremely versatile in performance and qualities that have made them increasingly important in many industrial applications. (Bo Zhanget. al., 2008). It has been noted that lignin can be broken down to monomeric or low molecular weight compounds by a variety of routes, such as pyrolysis, thermochemical liquefaction, alkaline oxidation or hydrolysis, alkali fusion, alkaline demethylation, hydrogenolysis (Fengel and Wegener, 2003; De Wild et. al., 2009). High pressures and temperatures in addition to the use of catalyst are required for the optimum conversion of lignin to low molecular weight compounds in good yield (Meier et. al., 1994; Oasmaaet. al., 1993). Catalytic conversion of lignin feedstock to various bulk chemicals or fuel components using various catalysts has been reported. (Aho et. al., 2008 and Jongerius et. al., 2012). Liquefaction of lignin has been carried out using various catalysts such as copper chromite, rancy nickel, sulphur resistant catalysts such as molybdenum sulphide, thiomolybdates and thiotungstates (Goldstein, 1975) but the Ni catalysts used in this
study have rarely been used (Goldstein, 1975). It is against this background that this study
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 3
ISSN 2229-5518
investigated the effects of temperature, pressure, reaction time, nature of ambient atmosphere and catalyst type parameters or chars, gas, oil yield, elemental composition and conversion using three types nickel catalysts.
The feedstocks used in this study are:
i. Dark brown powdered Kraft lignin processed from straw was obtained from the Department of Biochemistry, University of Manchester, Institute of Science and Technology, Manchester M601QD, UK. It was the main feedstock was dark brown powdered Kraft lignin.
ii. Brown granular lignin, Batch Li/S/5 was obtained from the Biological Products Division of ICI Plc., Cleveland, England. This lignin has been produced from straw and wood wastes and based on a new recycling system using a high speed catalyst.
The Kraft lignin was used mainly for the study with the ICU lignin just providing a basis for feedstock comparison. Moreover, tetralin (1, 2, 3, 4 tetrahydronaphthalene) supplied by Huls Ltd, England was used as the main suspension medium in this study. Distilled water was only used for comparison.
Three types of Ni supported catalysts which are ideal for hydrogenation and supplied by
Johnson Mathey Research Centre, England were investigated a) Ni-0104P
b) Ni-6458P
c) Ni-552/820
Reducing gases used are
a) 99.5% BOC commercial grade Carbon Monoxide.
b) 99.5% BOC commercial grade electrolytic hydrogen gas
Moisture content of the feedstock was conducted after which the elemental analysis was carried out. Owing to the fact that the use of autoclaves involves many risks; hence,
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 4
ISSN 2229-5518
pressure testing of the autoclave was carried out to avoid pressure explosions, loss of toxic and inflammable gaseous products to the atmosphere.
The autoclave was charged with feed, reducing gas, suspension medium and catalysts with continual stirring to ensure slurry. After charging, the control panel was switched on followed by the agitation of the autoclave by rocking. The electric furnace was switched on and allowed to warm up the autoclave and its contents. At the end of the reaction time, the furnace was switched off and the autoclave was its contents were allowed to cool gradually overnight with continual agitation.
The product gas was a mixture of gases and volatile organic compounds generated by the reaction. These gases were vented at room temperature into a gas storage system. When the gaseous product had been completely vented out of the autoclave into the storage bulbs, the autoclave was opened and is contents consisting of liquid and solid products were transformed into a flanged top round bottom flask in a fume cupboard because of the very offensive odor of these products. The contents of the flask were then subjected to atmospheric and vacuum distillation using standard laboratory equipment. The principal aim of the atmospheric distillation was to recover the slurry solvent. The residue from the vacuum distillation which is a mixture of oily and chary materials was refluxed in acetone in order to solubilise as much residue as possible. After cooling, the content of the flask was centrifuged at a speed of
2000rpm to separate most and if not all the char. The acetone soluble portion was decanted
and further filtered. The combined char from the bottom of the centrifuge bottle and the filter paper was dried in an oven at 1000C overnight. After cooling in desiccators, its weight which included the spent or recovered catalyst was determined by difference. The acetone soluble material referred to as the oil was recovered by stripping the acetone and leaving a black and very viscous product referred to as the oil.
A mass balance was carried out for each reaction in order to establish whether mass was effectively conserved so as to be able to assess the economic availability of otherwise, the
process. The mass balance provided the information:
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 5
ISSN 2229-5518
i. Recovery from the materials charged to the autoclave ii. Weight of the gases produced
iii. Weight of organic liquids produced
iv. Weight of aqueous material produced v. Recovery from the input feed
Standard run carried out in this work has the following characteristics:
i. 50g of feed stock ii. 1g of catalyst
iii. 400g of solvent
iv. 40 atmosphere reductant v. 3500C temperature
vi. 2hours reaction time
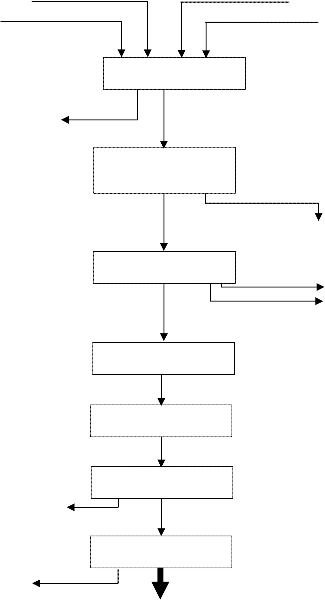
The schematic diagram of the liquefaction of Kraft lignin is depicted in figure 1.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 6
ISSN 2229-5518

Tetralin
Feed
Gas Catalyst
Reactor
Product gas
Atmospheric
Distillation
Vacuum Distillation
Aqueous and organic fractions
Cold trap fractions
Tetralin
Refluxing
Centrifuge
Char + catalyst
Filtration
Vacuum Distillation
Acetone
Figure 1: Schematic Diagram of the Liquefaction of Kraft-Lignin (Soile, 1987)
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 7
ISSN 2229-5518
The results of the elemental analysis and moisture content determination are stated below:
Feed stock | Moisture content | %C | %H | %N | %O* | %S | %Ash | H/C | O/C |
Kraft Lignin | 4.0 | 58.8 | 5.7 | 1.4 | 28.5 | 2.4 | 3.2 | 1.16 | 0.36 |
ICI lignin | 6.7 | 53.6 | 5.8 | 1.1 | 25.2 | 0.2 | 14.1 | 1.29 | 0.35 |
*Estimated by difference
Feed stock | Na | K | Mg | Ca | Fe |
Kraft Lignin | 1.0 | 0.1 | 0.02 | 0.1 | 0.1 |
The mass balance results showed that the recovery from the autoclave, processing recovery and solvent recovery averaged about 98%, 97% and 96% respectively. The contribution of tetralin to the project was found to be between 2.6% - 6.5% and this could also account for some unrecovered solvent. Calculations, conversions, yield, carbon and hydrogen recovered as oil, H/C and O/C ratios were based on the elemental analyses of the char and oil. The yield given represents the percentage of carbon in the input material found in the oil. The water soluble unextracted products are not considered in the yield. However this could be approximated from the difference between conversion and yield of the product oil because very small quantities of gases are formed from the feedstock during the reaction. It is also to be pointed out, however that oxygen content was determined via difference and all the errors (if any) in the determination of carbon, hydrogen, nitrogen, ash and sulphur all accumulate in the oxygen results. The conversion of Kraft lignin has been investigated extensively over the 300 –
375oC range.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 8
ISSN 2229-5518
Effect of Catalysts at Varying Temperature at Constant 40atm H2 Pressure
Catalyst | Temp(0C) | %Y | %X | % CHR | H/C | O/C |
Ni 6458P | 300 | 94.2 ±0.2 | 67.9 ±1.0 | 60.4± 1.0 | 1.09 ±0.01 | 0.14±0.02 |
Ni 6458P | 350 | 72.9±0.1 | 72.9±0.5 | 46.9±0.1 | 1.12±0.02 | 0.15±0.01 |
Ni 6458P | 375 | 96.0±.0 | 82.0±1.0 | 61.8±0.3 | 1.12±0.01 | 0.09±0.03 |
Ni-S52/820 | 300 | 61.3 ±0.3 | 45.0±2.0 | 36.9±2.0 | 1.11±0.03 | 0.16±0.04 |
Ni-S52/820 | 350 | 48.9±1.0 | 60.0±1.0 | 31.4±0.4 | 1.14±0.03 | 0.15±0.04 |
Ni-S52/820 | 375 | 76.5±1.0 | 70.1±0.4 | 49.3±0.3 | 1.19±0.02 | 0.11±0.06 |
Ni-0104P | 300 | 71.2±2.0 | 62.2±0.2 | 45.4±1.0 | 1.10±0.05 | 0.15±0.04 |
Ni-0104P | 350 | 68.0±0.5 | 64.7±1.0 | 43.6±0.1 | 1.01±0.01 | 0.19±0.02 |
Ni-0104P | 375 | 71.5±0.5 | 70.3±0.2 | 46.0±2.0 | 1.11±0.02 | 0.19±0.04 |
Y=Yield; X=Conversion; CHR = C+H recovered as oil
At 300oC, the difference in %Y, %X and %CHR using different Ni catalysts was significant (p<0.05), Ni-6458P has highest %Y, %X and %CHR followed by Ni-0104P with Ni-S52/820 having least values of these parameters. The difference in HC and OC ratio was not significant (p>0.05). At 350OC and 375oC, the difference in %Y, %X and %CHR and HC ratio was significant (p<0.05), Ni-6458P has highest %Y, %X and %CHR followed by Ni-0104P with Ni-S52/820 having least values of the parameters. Ni-S52/820 has highest value of HC ratio followed by Ni-6458P with Ni-0104P having least HC ratio both at 350oC and 375oC. The difference in OC ratio was not
significant (p>0.05) both at 350oC and 375oC.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 9
ISSN 2229-5518
Effect of Temperature Variation when CO was used as the Reductant at 40atm. Pressure
Catalyst | Temp(0C) | %Y | %X | %CHR | H/C | O/C |
Ni-6458P | 300 | 98.9±0.05 | 66.4±0.3 | 63.3±0.2 | 1.06±0.02 | 0.15±0.02 |
Ni-6458P | 350 | 40.6±0.2 | 51.9±0.05 | 26.0±0.5 | 1.07±0.02 | 0.16±0.03 |
Ni-6458P | 375 | 99.8±0.2 | 76.3±0.2 | 65.4±0.1 | 1.13±0.04 | 0.14±0.01 |
Ni-S52/820 | 300 | 67.6±0.1 | 54.3±0.1 | 43.4±0.3 | 1.13±0.01 | 0.16±0.03 |
Ni-S52/820 | 350 | 67.9±0.1 | 61.6±0.3 | 43.6±0.3 | 1.10±0.05 | 0.16±0.02 |
Ni-S52/820 | 375 | 57.1±0.4 | 69.5±0.3 | 36.9±0.3 | 1.15±0.03 | 0.6±0.01 |
For both Ni-6458P and Ni-S52/820 catalysts using CO as the reductant at 40 atmosphere pressure, the difference in %Y, %X, and %CHR is significant at varying temperature (p<0.05). Values of %Y, %X and %CHR are highest at 375oC followed by the values at 300oC with the values at 350oC being the least. In HC ratio, the difference is significant at varying temperature (p<0.05) for Ni-S52/820 catalyst only, the highest HC ratio was obtained at 375oC and lowest at
300oC; while the difference in OC ratio is significant for Ni-S52/820 catalyst only, highest value
was obtained at 375oC with the value being constant at 300oC and 350oC . For values of %Y, %X and %CHR are highest at 375oC followed by the values at 300oC with the values at 350oC being the least.
The difference in %Y, %X, %CHR and HC ratio obtained at constant temperature (300oC)
using CO as the reductant at 40 atmosphere pressure is significant (p<0.05). The mean value of
%Y (98.9), %X (66.4) and %CHR (63.3) obtained with Ni-6458P catalyst is significantly greater than %Y (67.6), %X (54.3) and %CHR (43.4) obtained with Ni-S52/820 catalyst. The difference in HC ratio obtained with Ni-S52/820 catalyst (1.13) was significantly higher than that of Ni-6458P
catalyst (1.06). The difference in OC value was not significant between the catalysts. (p > 0.05).
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 10
ISSN 2229-5518
Effect of H2 Pressure Variation of %Y, %X, %CHR, H/C and O/C using Ni-458P Catalysts
Pressure (atm.) | %Y | %X | %CHR | H/C | O/C |
20 | 48.5±0.5 | 64.2±0.2 | 31.4±0.4 | 1.11±0.04 | 0.14±0.01 |
40 | 72.9±0.4 | 72.0±0.5 | 46.9±0.3 | 1.12±0.02 | 0.15±0.02 |
60 | 95.1±0.1 | 73.0±0.25 | 61.0±0.2 | 1.10±0.03 | 0.13±0.04 |
The difference in %Y, %X and %CHR using varying H2 pressure under the catalytic action of Ni-
458P was significant (p<0.05). Highest values of %Y, %X and %CHR were obtained at 60 atm. followed by 40 atm. With the least values of these parameters obtained at 20 atm. H2 pressure. The difference in HC and OC ratios was not significant (p > 0.05).
Effect of Reductant Variation of %Y, %X, %CHR, H/C and O/C using Ni-458P Catalysts at 300oC
Parameters | Reductant | Mean | Standard deviation | P value |
%Y | H2 | 94.2000 | .20000 | 0.000 |
%Y | CO | 98.9000 | .05000 | 0.000 |
%X | H2 | 67.9000 | 1.00000 | 0.068 |
%X | CO | 66.4000 | .30000 | 0.068 |
%CHR | H2 | 60.4000 | 1.00000 | 0.008 |
%CHR | CO | 63.3000 | .20000 | 0.008 |
HC ratio | H2 | 1.0900 | .01000 | 0.081 |
HC ratio | CO | 1.0600 | .02000 | 0.081 |
OC ratio | H2 | .1400 | .02000 | 0.573 |
OC ratio | CO | .1500 | .02000 | 0.573 |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 11
ISSN 2229-5518
The difference in %Y and %CHR using different reductants (CO and H2) with the catalytic action of Ni-6458P at 300oC was significant (p<0.05). The values of %Y and %CHR obtained were higher when CO used than when H2 was used as reductant (p<0.05). However, there was no significant difference in %X, HC and OC ratios obtained between when H2 and CO was used. This implies that use of reductant also slightly affect the product oil qualities obtained in the liquefaction of Kraft lignin at 300oC; with CO enhance the quality of the product oil more than H2.
Effect of Reductant Variation of %Y, %X, %CHR, H/C and O/C using Ni-458P Catalysts at 350oC
Parameters | Reductant | Mean | Standard deviation | P value |
%Y | H2 | 72.9000 | .10000 | 0.000 |
%Y | CO | 40.6000 | .20000 | 0.000 |
%X | H2 | 72.9000 | .50000 | 0.000 |
%X | CO | 51.9000 | .05000 | 0.000 |
%CHR | H2 | 46.9000 | .10000 | 0.000 |
%CHR | CO | 26.0000 | .50000 | 0.000 |
HC ratio | H2 | 1.1200 | .02000 | 0.038 |
HC ratio | CO | 1.0700 | .02000 | 0.038 |
OC ratio | H2 | .1500 | .01000 | 0.613 |
OC ratio | CO | .1600 | .03000 | 0.613 |
The difference in %Y, %X and %CHR and HC ratio using different reductants (CO and H2) with the catalytic action of Ni-6458P at 350oC was significant (p<0.05). The values of %Y, %X, %CHR and HC ratio obtained were higher when H2 used than when CO was used as reductant (p<0.05). However, there was no significant difference in OC ratio obtained between when H2 and CO
was used. This implies that use of reductant also slightly affect the product oil qualities
obtained in the liquefaction of Kraft lignin at 300oC; with H2 enhancing the quality of the product oil more than CO at 350oC.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 12
ISSN 2229-5518
Effect of Reductant Variation of %Y, %X, %CHR, H/C and O/C using Ni-458P Catalysts at 375oC
Parameters | Reductant | Mean | Standard deviation | P value |
%Y | H2 | 96.0000 | 1.00000 | 0.003 |
%Y | CO | 99.8000 | .20000 | 0.003 |
%X | H2 | 82.0000 | 1.00000 | 0.001 |
%X | CO | 76.3000 | .20000 | 0.001 |
%CHR | H2 | 61.8000 | .30000 | 0.000 |
%CHR | CO | 65.4000 | .10000 | 0.000 |
HC ratio | H2 | 1.1200 | .01000 | 0.696 |
HC ratio | CO | 1.1300 | .04000 | 0.696 |
OC ratio | H2 | .0900 | .03000 | 0.030 |
OC ratio | CO | .1500 | .01000 | 0.030 |
The difference in %Y,%X, %CHR and OC ratio using different reductants (CO and H2) with the catalytic action of Ni-6458P at 350oC was significant (p<0.05). The values of %Y, %CHR and HC ratio obtained were higher when CO used than when H2 was used as reductant (p<0.05) while
%X obtained was higher when H2 used than when CO was used as reductant (p<0.05). However,
there was no significant difference in OC ratio obtained between when H2 and CO was used. This implies that use of reductant also affect the product oil qualities obtained in the liquefaction of Kraft lignin. At 375oC, different parameters were enhanced using different
reductant.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 13
ISSN 2229-5518
Effect of Reductant Variation of %Y, %X, %CHR, H/C and O/C using Ni-S52/820 Catalyst at 300oC
Parameters | Reductant | Mean | Standard deviation | P value |
%Y | H2 | 61.3000 | .30000 | 0.000 |
%Y | CO | 67.6000 | .10000 | 0.000 |
%X | H2 | 45.0000 | 2.00000 | 0.001 |
%X | CO | 54.3000 | .10000 | 0.001 |
%CHR | H2 | 36.9000 | 2.00000 | 0.005 |
%CHR | CO | 43.4000 | .30000 | 0.005 |
HC ratio | H2 | 1.1100 | .03000 | 0.325 |
HC ratio | CO | 1.1300 | .01000 | 0.325 |
OC ratio | H2 | .1600 | .04000 | 1.000 |
OC ratio | CO | .1600 | .03000 | 1.000 |
The difference in %Y, %X and %CHR using different reductants (CO and H2) with the catalytic action of Ni-S52/820 at 300oC was significant (p<0.05). The values of %Y, %X and %CHR obtained were higher when CO used than when H2 was used as reductant (p<0.05). However, there was no significant difference in HC and OC ratios obtained between when H2 and CO was used. This implies that use of reductant also slightly affect the product oil qualities obtained in the liquefaction of Kraft lignin at 300oC; with CO enhance the quality of the product oil more than
H2.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 14
ISSN 2229-5518
Effect of Reductant Variation of %Y, %X, %CHR, H/C and O/C using Ni-S52/820 Catalyst at 350oC
Parameters | Reductant | Mean | Standard deviation | P value |
%Y | H2 | 48.9000 | 1.00000 | 0.000 |
%Y | CO | 67.9000 | .10000 | 0.000 |
%X | H2 | 60.0000 | 1.00000 | 0.001 |
%X | CO | 61.6000 | .30000 | 0.001 |
%CHR | H2 | 31.4000 | .40000 | 0.050 |
%CHR | CO | 43.6000 | .30000 | 0.050 |
HC ratio | H2 | 1.1400 | .04000 | 0.340 |
HC ratio | CO | 1.1000 | .05000 | 0.340 |
OC ratio | H2 | .1500 | .03000 | 0.656 |
OC ratio | CO | .1600 | .02000 | 0.656 |
The difference in %Y, %X and %CHR ratio using different reductants (CO and H2) with the catalytic action of Ni-S52/820 at 350oC was significant (p<0.05). The values of %Y, %X and %CHR obtained were higher when CO used than when H2 was used as reductant (p<0.05). However, there was no significant difference in HC and OC ratios obtained between when H2 and CO was used. This implies that use of reductant also slightly affect the product oil qualities obtained in the liquefaction of Kraft lignin at 350oC; with CO enhancing the quality of the product oil more than
H2 at 350oC.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 15
ISSN 2229-5518
Effect of Reductant Variation of %Y, %X, %CHR, H/C and O/C using Ni-S52/820 Catalyst at 375oC
Parameters | Reductant | Mean | Standard deviation | P value |
%Y | H2 | 76.5000 | 1.00000 | 0.000 |
%Y | CO | 57.1000 | .40000 | 0.000 |
%X | H2 | 70.1000 | .40000 | 0.106 |
%X | CO | 69.5000 | .30000 | 0.106 |
%CHR | H2 | 49.3000 | .30000 | 0.000 |
%CHR | CO | 36.9000 | .30000 | 0.000 |
HC ratio | H2 | 1.1900 | .02000 | 0.127 |
HC ratio | CO | 1.1500 | .03000 | 0.127 |
OC ratio | H2 | .1100 | .06000 | 0.000 |
OC ratio | CO | .6000 | .01000 | 0.000 |
The difference in %Y, %C and OC ratio using different reductants (CO and H2) with the catalytic action of Ni-S2/820 at 375oC was significant (p<0.05). The values of %Y, %CHR and OC ratio obtained were higher when H2 used than when CO was used as reductant (p<0.05). However, there was no significant difference in %x and HC ratio obtained between when H2 and CO was used. This implies that use of reductant also affect the product oil qualities obtained in the
liquefaction of Kraft lignin. At 375oC, H2 enhanced the qualities of the product oil more than CO.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 16
ISSN 2229-5518
Effect of Time Variation of %Y, %X, %CHR, H/C and O/C at 3500C using Ni-458P Catalysts
Time (hr) | %Y | %X | %CHR | H/C | O/C |
0 | 83.6±0.3 | 75.2±0.3 | 54.2±0.2 | 1.21±0.02 | 0.11±0.01 |
1 | 76.9±0.2 | 60.6±0.5 | 49.6±0.4 | 1.16±0.02 | 0.15±0.03 |
2 | 72.9±0.1 | 72.0±0.5 | 46.9±0.1 | 1.12±0.03 | 0.15±0.01 |
3 | 76.2±0.2 | 65.1±0.3 | 49.2±0.2 | 1.16±0.03 | 0.14±0.04 |
Highest values of %Y was obtained at less than 1 hour reaction time followed by values obtained after 1 hour followed by the that obtained after 3 hours with the least values obtained after 2 hours. Highest values of %X was obtained at less than 1 hour followed by values obtained after 2 hour followed by the that obtained after 3 hours with the least values obtained after 1 hour. Highest values of % CHR was obtained at less than 1 hour followed by values obtained at 1 hour followed by the that obtained after 3 hours with the least values obtained after 2 hour. The difference in HC and OC ratios was not significant (p > 0.05). This implies that products of the liquefaction process are obtainable maximally within an hour reaction time.
Comparison liquefaction of ICI and Kraft Lignin at 3500C and 40 atm. H2 using Ni 6458P catalyst
Feed | %Y | %X | %CHR | H/C | O/C |
Kraft | 72.9±0.4 | 72.9±0.2 | 46.9±0.4 | 1.12±0.04 | 0.15±0.01 |
ICI | 75.7±0.3 | 85.2±0.2 | 49.4±0.1 | 1.29±0.03 | 0.09±0.02 |
The difference in %Y, %X, %CHR, HC and OC ratios obtained from the liquefaction of Kraft and ICI lignin using varying 40 atm. H2 pressure under the catalytic action of Ni-458P was significant (p<0.05). The values of all the parameters obtained from ICI lignin is greater that those
obtained from Kraft lignin
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 17
ISSN 2229-5518
From the study, the following conclusions are reached:
i. Liquefaction of Kraft lignin is best carried out at higher temperature as the process at
375oC gave the best quality product oil for the three types of Ni catalysts.
ii. Higher pressure aided liquefaction gave highest values of the parameters taken on the product oil. Liquefaction at 60 atmosphere gave the product oil of best quality in comparison with one carried out with 20 and 40 atmospheres.
iii. Greatest values of the parameters taken on the product oil were obtained in the process of liquefaction achieved in less than an hour.
iv. Catalysts play a major role in the liquefaction process as the performance of the catalysts differs significantly. Of all the three catalysts used (Ni-64586P, Ni-S52/820 and Ni-0104P), Ni-64586P performed best when both when CO and H2 pressure were used.
v. The reductants used affected the quality of product oil. The parameters on the product oil obtained differ significantly at constant temperature using the same catalyst. For Ni-64586P catalyst, CO yielded product oil of better quality than H2 at 300 and vice versa at 350oC; at 375oC, some parameters well enhance by CO while others by H2. For Ni-S52/820 catalyst, CO yielded product oil of better quality than H2 at 300 and
350oC and vice versa at 375oC.
vi. Kraft lignin promises to be a good source of product oil as it achieved appreciable values of parameters for the product oil deemed to be of good quality as compared with the ICI lignin; although parameters from the product oil obtained from the ICI lignin were greater.
vii. Conclusively, product oil of best quality was obtained using Ni-64586P catalyst for the
liquefaction of Kraft lignin using 60 atmospheres H2 pressure as reductant at 375oC.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 18
ISSN 2229-5518
1. Aho,A., Kumar, N., Eraenen, K., Salmi, T., Hupa, M. and Murzin, D.Y. (2008): “Catalytic pyrolysis of woody biomass in a fluidized bed reactor: Influence of the zeolite structure,”Fuel,87, 2493–
2501.
2. Bo Zhang, Hua-Jiang Huang and Shri Ramaswamy, (2008)“Reaction Kinetics of the Hydrothermal
Treatment of Lignin,” Applied Biochemistry and Biotechnology, Volume 147, Numbers 1-3 , 119-
131. Doi: 10.1007/s12010-007-8070-6)
3. Chakar, F.S. and Ragauskas, A.J., (2004):“Review of current and future softwood kraft lignin process chemistry, “Ind. Crops Prod., 20, 131–141.
4. De Wild P., Van der Laan R., Kloekhorst A., and Heeres H.J.(2009), “Lignin valorisation for chemicals and (transportation) fuels via (catalytic) pyrolysis and hydrodeoxygenation.” Environmental progress & Sustainable Energy, 28(3), 461 – 469.
5. Fengel D. and Wegener G., (2003): “Ultrastructure, Reactions. Kessel Verlag Remagen.” Wood: Chemistry (2003).
6. Goldstein, I.S.,(1975): Proceedings of the eight cellulose conference (Timell, T.E., Ed),
John Wiley and Sons, N.Y., p261 (1975)
7. Jongerius, Anna L., Jastrzebski, Robin, Bruijnincx, Pieter C.A. and Weckhuysen, Bert M,(2012) : “CoMo sulfide-catalyzed hydrodeoxygenation of lignin model compounds: An extended reaction network for the conversion of monomeric and dimeric substrates,” Journal of Catalysis, 285,
315–323.
8. Klass, D.L. and Cleveland, C.J. (Ed.) (2004): Encyclopaedia of Energy, vol. 1 Elsevier, Amsterdam.
9. Meier D., Berns J., Faix O., Balfanz U. and Baldauf W., (1994): “Hydrocracking of organocell lignin for phenol production,”Biomass & Bioenergy. 1994, 7, 99 – 105.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 19
ISSN 2229-5518
10. Mohan,D., Pittman Jr, C.U.and Steele,P.H., (2006): “Pyrolysis of Wood/Biomass for Bio- oil: A Critical Review,”Energy & Fuels2006, 20, 848–889.
11. Oasmaa A., Alen R. and Meier D., (1993) “Catalytic hydrotreatment of some technical lignins,”
Bioresource Technology. 45, 189 – 194.
12. Perlack,R.D., Wright, L.L., Turhollow, A.F., Graham, R.L., Stokes, B.J. and Erbach, D.C. (2005): “Biomass as Feedstock for a Bioenergy and Bioproducts Industry: The Technical Feasibility of a Billion-ton Annual Supply”, US Department of Energy.
13. Ritter,S.K.,Che (2008). “Calling All Chemists,” Eng. News, 86, 10–17.
14. Roberts, V.M., Stein, V., Reiner, T., Lemonidou, A., Li, X. and Lercher, J.A. (2011): “Towards quantitative catalytic lignin depolymerisation,” Chem. Eur. J. 17, 5939–5948.
15. Soile, O. O. (1987): “The Thermochemical Liquefaction of Kraft Lignin,” Published Ph. D Thesis, Department of Chemistry, Faculty of Technology, University of Manchester, UK, page 114.
16. Stark,K., Taccardi, N., Bosmann, A. and Wasserscheid, (2010): “Oxidative depolymerization of lignin in ionic liquids,”P.ChemSusChem, 3, 719–723.
17. Zakzeski, J., Bruijnincx, P.C.A., Jongerius, A.L. and Weckhuysen, B.M. (2010): “The catalytic
valorization of lignin for the production of renewable chemicals,”Chem. Rev. 110, 3552–3599.
IJSER © 2013 http://www.ijser.org