International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 945
ISSN 2229-5518
Assessing Some Emerging Effects of Hexavalent Chromium on Leaf Physiological Performance in Sunflower (Helianthus annuus L.)
Muhammad Imran Atta, Tasveer Zahra Bokhari, Saeed Ahmad Malik, Abdul Wahid, Sadia Saeed and Allah Bakhsh Gulshan
Abstract— Sunflower plants of two cultivars (Hysun-33 & SF-5009) were treated with varying Cr6+levels (mg kg-1) and leaf performance for photosynthetic rate (CO 2 exchange) and total chlorophyll contents was assessed. Post flowering data showed inhibitory effects of Cr application on photosynthetic rate and foliar chlorophyll contents. Limitations for intracellular CO 2 assimilation, transpiration rate and stomatal conductance found associated with inhibition of photosynthesis. Increased water use efficiency and CO 2 use potential of plants indicated more water and CO 2 needto plants. Both factors predicted development of drought mechanism and stressed carbohydrate biosynthesis during photosynthesis in Cr affected plants, respectively. Cr load in roots was more than leaves due to root efficient absorption. Cr accumulation particularly in leaves interrupted chlorophyll photoactivity performance to inhibit photosynthesis. The rate of reduction increased from lower to higher Cr doses. Gradual response of plants towards low Cr doses was due to resistant mechanism of plants than at higher Cr doses. Cr application 50 mg/kg developed positive effects on leaf performance of both test varieties revealing phytoactive than phytotoxic. Sunflower variety SF-5009 was less affected by Cr than Hysun-33.
Index Terms— Hexavalent, Chromium, Sunflower, photosynthesis, chlorophyll, photoactivity, phytotoxic, phytoactive.
—————————— ——————————
1 INTRODUCTION
EAVY metals are the elements having density over
5g/cm3and pollute soil and water due to anthropogenic ic activities (Alloway, 1995; Rouphael et al., 2008). Rapid
id growth of human population and industrialization found to be a major cause of heavy metal pollution (Wahid et al.,
2000). Burning of fossil fuel, mining, smelting of metalloids,
fertilizers, pesticides, dust accumulation, leather & textile processes and waste water irrigation has increased this pollu- tion on biosphere by interacting biotic and abiotic environ- mental components (Dube et al., 2001; Younis et al. 2013 a & b).
In nature, chromium occurs as chromite (Fe2 Cr2 O4 ) and tarapacaite (K2 Cr2 O7 ) and forms complexes with other met-
als i.e. PbCrO4 (Babula et al., 2008). Cr has two stable oxida- tion states Cr6+ and Cr3+ where Cr6+ is considered more toxic,

less water soluble than Cr3+(Panda & Patra, 2000; Nieboer & Richardson,1980). In aquatic environment, human activities and weathering of Cr-enriched rocks causes oxidation, reduc- tion, adsorption, dissolution and precipitation of Cr. At high pH Cr3+ precipitates and becomes soluble at acidic pH while Cr6+ forms are soluble at all pH conditions (Kimbrough et al., 1999). Cr3+ is up taken by passive means while Cr6+ is ab- sorbed by active mechanism of plant membranes and com- petes with essential elements (Zayed & Terry, 2003; Kim et al., 2006).
• Muhamma Imran Atta, Lecturer Botany Department, Govt. Post Graduate College, Dera Gazi Khan, Pakistan. E-mail: imranbotany80@yahoo.com
• Dr. Tasveer Zahara Bokhari, Porfessoer Dr. Saeed Ahmad Malik, Dr. Abdul Wahid, Sadia Saeed, Institute of Pure and Applied Biology, (Botany Division) Bahauddin Zakariya University, Multan, Pakistan. E-mail: tzb_5@hotmail.com, saeedbota- ny@bzu.edu.pk, wahid_64us@yahoo.com, saadiabzu@yahoo.com
• Allah Bakhsh Gulshan, currently enrolled in PhD Program, Institute of Pure and Applied Biology, (Botany Division) Bahauddin Zakariya University, Multan, Pakistan. E-mail: abgulshan@hotmail.com
Soil and water contamination by Cr is of great concern which has negative effects on plant physiological processes like pho- tosynthesis, mineral nutrition, water relations, respiration and turns land barren by loss of vegetation (Azmat & Khanum, 2005; Clijsters & Van Assche, 1985; Dube et al.,
2003). Cr inhibited photosynthesis is confined to abnormal stomatal conductance, reduced intercellular spaces, chloro- plast structural alteration and oxidative stress in plants that ultimately reduces growth and yield (Vazquez et al., 1987; Arun et al.,2005). Heavy metals enriched plants also possess health risks to consumers (Stobrawa et al., 2008).
Sunflower (Helianthus annuus L.) is an important seed oil crop in the world and in Pakistan. Its seeds contain 40% edible oil (PARC, 2007). As physiological attributes of leaf has strong relation with growth and yield of plant, so we focused these attributes in sunflower cultivars and various effects from Cr metal were assessed.
2 Materials and Methods
2.1 Experimental Trail
For two sunflower hybrids i.e. Hysun-33 (variety- A) andSF-
5009 (variety-B), a pot experiment was conducted in Bio-Park of Bahauddin Zakariya University, Multan (Pakistan) during February-June, 2012 and data was observed at post flowering stage. A crystalline K2Cr2O7 salt (Merck-Germany) was used as source chemical for hexavalent chromium. Various Cr6+ doses (50, 100, 150, 250, 350, 400 & 500 mg kg-1 of pot soil) were prepared and mixed with the pot soil. Salt weighing was executed with digital balance (MK-200B, Chyo-Japan). Each treatment including control was comprised with seven replicates and a total of 112 pots were arranged in a completely randomized block design (CRBD).Sandy loam, humified soil with pH 7.21, EC 1.73 ds/cm and organic matter
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 946
ISSN 2229-5518
7.12% was filled in well set earthen pots of 16 inch diameter. In each pot, six certified and uniform sized seeds were sown at equal distance and depth. Tap water was used to irrigate pots and thinning was performed at day 20 when seedlings attained an approximate size of 6 inch. For either variety two plants/pot were left as harvest.
2.2 Measuring Parameters
2.2.1 Rate of Photosynthesis
At day 90 (90-DAS) plant physiological analysis was carried out using a portable infrared gas analyzer (IRGA, ADC- LCA4/ Analytical Development Company, England). At each day, analysis conceded out during full sunny days from 9:00-
11:00 (AM). Three young similar aged leaves were analyzed
through leaf chamber and mean values were used to manipu-
late data. For physiological study; intracellularCO2 assimila- tion (Ci = Vpm), rate of photosynthesis (A = µM m-2 s-1), tran- spiration rate (E = m. mol m-2 s-1), stomatal conductance (gs = mol m-2 s-1),water utilizing efficiency (WUE = A/E) and CO2 use potential(CUP = Ci/A) of plants were measured. Leaf chamber was specified with temperature range 28-32.4ºC, am- bient pressure 98 k Pa and PAR at leaf surface (Qleaf ) was kept
800-900 µmol m-2 s-1.
2.2.2 Total chlorophyll contents
Total chlorophyll contents were measured by using a chloro- phyll meter SPAD-502 (Konica-Japan) having SPAD-unit accu- racy ± 1. The selected plant leaf was inserted into leaf chamber followed by a gentle press and chlorophyll reading was noted on screen.
2.2.3 Cr metal uptake study
Cr heavy metal in roots and leaves was estimated by combus- tion &digestion method (Panichev et al., 2005). 1g oven dried homogenized plant sample was ashed in muffled electric fur- nace at 650 ºC, cooled in glass desiccator and dissolved in a mixture of dilute HNO3 & HCl. The volume of solution was then increased up to 25ml by adding distilled water. At 357.9 nm, chromium metal was estimated using Perkin-Elmer atom- ic absorption spectrometer.
2.2.4 Statistical analysis of Data
The data was analyzed by one-way ANOVA, Duncan’s Multi- ple Range Tests (Steel & Torrie, 1984) at α-0.05 using SPSS-
17.0statistical software. Standard deviation was also applied.
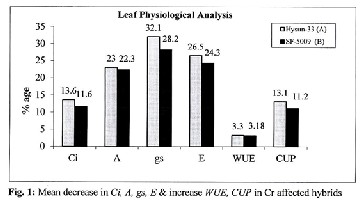
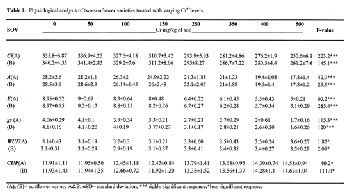
43.14% and 25.25-58.09%,respectively.Cr dose 50 mg/kg ex- posed positive emerging effects on photosynthetic rate (A) and its associated attributes. Water use efficiency (WUE)and CO2 use potential (CUP) of plants increased along increasing Cr concentration gradient up to 3.3&13.1%, showing signifi- cance behavior of Cr upon leaf physiological attributes
in both varieties.
3.2 Total chlorophyll contents in leaves
Foliar chlorophyll contents decreased pretentiously (P≤0.05) along increasing Cr application. For both test varieties, rate of chlorophyll reduction was 6.99-11.44% and 18.80-30.88% at
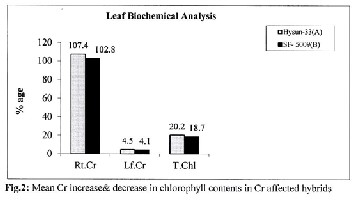
100-150mg/kg & 250-500 mg/kg of Cr, respectively. The re- duction line increased from low to high Cr dose level. At 50 mg/kg of Cr, both sunflower cultivars showed a positive re- sponse to hexavalent chromium where total chlorophyll con- tents were better than control (Table-2). Mean reduction val- ues for physiological attributes also revealed Cr significant effects (Fig.1&2).
3 Results
3.1Rate of photosynthesis
Table-1shows significant effects (P≤0.05) of Cr application on various leaf physiological aspects in sunflower plants from both test varieties. Intracellular CO2 assimilation (Ci), rate of photosynthesis (A), transpiration rate (E) and stomatal con- ductance (gs) were strongly affected by Cr as compared to control. The reduction line was consistent at 100-150 mg/kg Cr dose i.e. 2.69-7.47%, 6.60-11.39%, 0.66-10.55% and 2.42-
16.25%, respectively. At 250-500mg/kg Cr; leaf performance
was rapidly declined by 13.28-23.68%, 21.14-40.34%, 27.72-
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 947
ISSN 2229-5518
3.3 Chromium uptake study
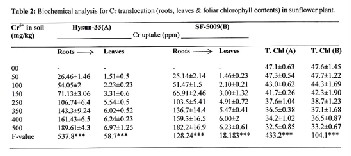
At Cr dose 50-500mg/kg of soil, Cr contents increased in roots from 25.14-189.61 ppm. In leaves, Cr contents were 1.46-6.97 ppm. Increase in Cr contents was directly related to its appli- cation in soil. Fig.2. Rate of Cr accumulation was more in Hysun-33(A) than SF-5009(B).
4 Discussion
At 100 & 400 µM Cr6+, spinach (Spinaceae oleracea L.) has showed lower leaf water potential, transpiration rate and al- teration in plant water use efficiency (Gopal, 2009). In rice plants, Cr6+ affects 62% rate of photosynthesis and 66% sto- matal conductance (Ahmad et al., 2011). Net photosynthetic rate, transpiration rate, stomatal conductance and internal CO2 concentration changed significantly as compared to con- trol at Cr6+ doses 10-6 M and 10-4 M (Liu et al., 2008). Low rate of photosynthesis due to inhibition of electron transport sys- tems, abnormal photochemical process, low chlorophyll con- tents which resulted in stunted plant growth is due to acceler- ated Cr accumulation in plants (Van Assche & Clijsters, 1983; Singh & Agarwal, 2007; Tang et al., 2012). Photosynthetic pigments are strongly damaged by Cr induced O2 radicals having redox potential of 1.38 eV (Pinto et al., 2000; Vernay et al., 2007).
Our investigation from present study also agreed with these
result lines as overall leaf physiology altered by Cr6+ applica-
tion. A minimal effect of Cr was observed at lower Cr doses
(100-150 mg/kg) than higher doses 250-500mg/kg). Leaf phys- iological performance for various attributes reduced remarka- bly up to 60%.Water use efficiency and carbon use potential of
plants increased by 3.3 & 13.1%, respectively. Results obtained at 50 mg/kg Cr revealed leaf physiology and chlorophyll con- tents improved likewise control and this Cr dose found to be beneficial for test varieties remained unreported by earlier researchers. Increased water use efficiency indicated more water requirement to Cr stressed plants found associated with reduced transpiration rate and less rate of stomatal conduct- ance following less CO2 intake and water uptake to inhibit rate of photosynthesis. Increased values of carbon use poten- tial were due to less CO2 assimilation during photosynthesis predicted inefficiency of carbon fixing enzymes and carbohy- drate biosynthesis (Table-1).
Alteration in foliar chlorophyll contents increased with in- creasing Cr application and pretentiously reflected metal tox- icity in treated plants (Table-2).
Chromium destroys animo-levulinic acid dehydratase (ALAD), a precursor enzyme for chlorophyll biosynthesis and affects amino-levulinic acid (ALA) to decrease chlorophyll level (Vajpayee et al., 2001). Bertrand & Poirier (2005) reported chlorophyll degradation and enhanced membrane permeabil- ity damaged at high chromium concentration. Chlorophyll–a
& b decreased up to 47&43% while carotenoids were affected
50% in Cr stressed rice plants (Ahmad & Wahid et al., 2011).
They also investigated plants for NPK required for different
metabolic activities and observed remarkable reduction line
up to 82, 37 & 42%, respectively. Cr accumulation showed
plants having no transport systems for Cr, and imbalanced
chlorophyll and nutrient contents in affected parts (Diwan et al., 2012). The same findings were in this experiment and low Cr translocation from root to leaves was significantly ob- served, but Cr contents in plant organs increased along its
application in soil. In roots we observed more Cr uptake 189.6 ppm than in leaves i.e. 6.96 ppm (Table-2). Due to Cr toxicity in leaves, chlorophyll contents reduced up to 31% predicted chloroplast structural abnormalities with the rift in N, P, K & Mg ions to destroy porphyrin ring (Table-1). Affected rate of photosynthesis was due to unusual photoactivity of chloro- phyll under Cr stress.
5 Conclusion
From the present study, it can be concluded that use of hexa- valent chromium is harmful for both sunflower cultivars. Dif- ferent Cr doses (mg/kg) have affected physiological attributes of leaves. Same way, leaf chlorophyll contents also found to be affected by Cr toxicity. The stressed leaf physiological activity proposed to be associated with affected plant growth and yield due to possible less accumulation of metabolites. How- ever, some hopeful findings also obtained at lower Cr doses where injurious effects were consistent and affordable than at higher Cr doses. Some emerging effects of Cr obtained at 50 mg/kg were much hopeful. The plant responses were positive and better even than at control. Hence, this dose can be rec- ommended as a beneficial micronutrient for test hybrids. However, comparing physiological performance of two sun- flower hybrids, SF-5009found to be more Cr tolerant than Hysun-33.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 948
ISSN 2229-5518
6 References
Ahmad, M.,A. Wahid, S.S. Ahmad, Z. A. Butt and M. Tariq.
2011. Ecophysiological responses of rice (Oryza sativa
L.) to hexavalent chromium. Pak. J. Bot., 43(6): 2853-
2859.
Alloway, B.J. 1995. Heavy metals in soils.2nd edition.USA: Blackie Academic & Professional.
Arun, K., T. Shanker, C. Cervantes, H. Loza-Tavera and S.Avudainayagam.2005. Chromium toxicity in plants. Environment International, 31: 739-753.
Azmat, R and R. Khanum. 2005. Effect of Chromium on up- takes of minerals inbean plant. Pak.J.Biol.Sci.,
8(2):281-283.
Babula, P., A. Vojtech, R. Opatrilova, J. Zehnalek, L. Havel and R. Kizek. 2008. Uncommon heavy metals, metalloids and their plant toxicity: Environmental Chemistry Letters,volume6, Number 4, 189-213
Bertrand, M and I. Poinier. 2005. Photosynthetic organisms and excess of metals. Photsynthetica., 43: 345-353.
Clijsters, H and F. Van Assche. 1985. Inhibition of photosyn- thesis by heavy metals. 2: 263-66.
Diwan, H., A. Ahmad and M. Iqbal. 2012. Chromium induced alterations in photosynthesis and associated attributes in Indian mustard. J. Environ. Biol. 33, 239-244.
Dube, A., R. Zbytniewski and T. Kowalkowski. 2001. Adsorp- tion and migration of heavy metals in soil. Polish J. Environ. Studies, 10(1): 1-10.
Dube, B. K., K. Tewari, J.Chatterjee and C.Chaterejee. 2003.
Excess chromium alters uptake and translocation of
certain nutrients in Citrullus. Chemosphere 53: 1147-
1153.
Gopal, R and A. Hussain. 2009. Chromium alters iron nutri- tion and water relations of spinach. Journal of Plant Nutrition, 32: 1551-1559.
Kim,Y.J ., J.H. Kim, C.E. Lee, Y.G. Mok, J.S. Choi, H.S. Shin and S. Hwanga. 2006. Expression of yeast transcrip- tional activator MSN1 promotes accumulation of chromium and sulfur by enhancing sulfate trans- porter level in plants. FEBS. Letters.vol. 580(1), pp.
206–210.
Kimbrough, D.E.,Y. Cohen, A.M. Winer, L. Creelman and C.
Mabuni. 1999. A critical assessment of chromium in
the environment. Critical Reviews in Environmental
Science and Technology, vol. 29(1), pp.1–46.
Liu, D., J. Zou, M. Wang and W. Jiang.2008. Hexavalent chro- mium up take and its effects on mineral uptake, anti- oxidant defence system and photosynthesis in Ama- ranthus viridis L. Bioresource Technology, 99:2628-
2636.
Nieboer, E and D.H.S. Richardson. 1980. The replacement of the nondescript term ‘heavy metals’ by a biologically and chemically significant classification of metal ions. Environ. Pollut., 1(B): 3-26.
Panda, S.K and H.K. Patra. 2000. Nitrate and ammonium ions effect on the chromium toxicity in developing wheat seedlings. Plant National Academy of Science of In- dia, B70:75-80.
Panichev, N., K. Mandiwana, M. Kataevaand S. Siebert. 2005.
Determination of Cr(VI) in plants by electro thermal
atomic absorption spectrometry after leaching with
sodium carbonate.SpectrochimicaActa,PartB60:699-
703.
PARC.(2007). Sunflower cultivation in Pakistan, Pakistan Ag- riculture Research Council Islamabad. PP- 1-6.
Pinto, E., L. H. Catalani, N. P. Lopes, P. Di Mascio and P.
Colepicolo. 2000. Peridinin as the major biological ca-
rotenoid quencher of singlet oxygen in Gonyaulax
polyedra. Biochem, Biophys. Res. Commun. 268: 496-
500.
Rouphael, Y., M. Cardarelli, E. Reab, and G. Cola. 2008. Graft- ing of cucumber as a means to minimize copper tox- icity. Environment And Experimental Botany, 63: 49-
58.
Singh, R. Pand M. Agrawal.2007. Effects of sewage sludge amendment on heavy metal accumulation and conse- quent responses of Beta vulgaris plants. Chemo- sphere, 67: 2229-2240.
Steel, R.G.D and Torrie, J. H. (1984). Principles and Procedures of Statistics. New York: McGraw Hill Pub.
Stobrawa, K and P.G. Lorenc.2008. Thresholds of heavy-metal toxicity in cuttings of European black poplar (Populus nigraL.) determined according to antioxidant status of fine roots and morphometrical disorders. Science of the total environment390:86-96.
Tang, J., J. Xu, Y. Wu, Y.Li and Q.Tang. 2012. Effects of high concentration of chromium stress on physiological and biochemical characters and accumulation of chromium in tea plant (Camellia sinensisL.). African Journal of BiotechnologyVol.11(9),pp.2248-2255.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 8, August-2013 949
ISSN 2229-5518
Vajpayee, P., U.N. Rai, M.B. Ali, R.D. Tripathi, V. Yadav, S.
Sinha and S.N. Singh. 2001. Chromium induced phys-
iological changes in Vallisneria spiralis L. and its role
in phytoremediation of tannery effluents. Bull. Envi-
ron. Contam. Toxicol., 67, 246-256.
Van Assche, F and H. Clijsters.1983. Multiple effects of heavy metals on photosynthesis. The Hague Nijhoff/Junk.,371-382.
Vazquez, M.D., C. Poschenrieder and J. Barcelo. 1987. Chro- mium VI induced structural and ultrastructural changes in Bush bean plants. Annals of Bot., 59: 427-
438.
Vernay, P., C. Gauthier-Moussard, L. Jean, F.O. Bordas, F.
Faure,G. Ledoigt and A. Hitmi. 2008. Effect of chro- mium species on phytochemical and physiological parameters in Daturainnoxia.Chemosphere,72: 763-
771.
Wahid, A., M.G.A. Nasir and S.S. Ahmad. 2000. Effects of wa- ter pollution on growth and yield of soybean. Acta Scient.,10: 51-58.
Younis, U., Bokhari, T. Z., Shah, M. H. R., Mahmood, S. and Malik, S. A. 2013 a. Dust Interception Capacity and Alteration Of Various Biometric And Biochemical At- tributes In Cultivated Population of Ficus Carica L. IOSR Journal of Pharmacy and Biological Sciences (IOSR-JPBS). Volume 6, Issue 4: 35-42.
Younis, U., Bokhari, T. Z., Malik, S. A., Ahmad, S. & Raja, R.
2013 b. Variations in leaf dust accumulation, foliage
and pigment attributes in fruiting plant species ex-
posed to particulate pollution from Multan. Interna-
tional Journal of Agricultural Science and Research
(IJASR). Vol. 3, Issue 3: 1-12.
Zayed, A.M and N. Terry. 2003. Chromium in the environ- ment: factors affecting biological remediation. Plant and soil 249: 139-156.
IJSER © 2013 http://www.ijser.org




