International Journal of Scientific & Engineering Research Volume 2, Issue 9, September-2011 1
ISSN 2229-5518
Antagonistic activity evaluation of Eucalyptus camaldulensis essential oil in control of algae and associated microorganisms
Ali M. Najem*, Abdul Latif M. Jawad* and Ayyad W. Raof
Abstract—This study includes isolation, purification, and identification of algae from different environmental places (Tigris River and canal around Al- Jadriah) during February 2008. Nine unialgal cultures were obtainedwhich included 4 species of blue-green algae, 4 species of green algae, and 1 species of diatoms. However, contaminant bacteria and fungi were isolated from 4 of these algal cultures which were Anabaena circinalis, Microcystis aeruginosa, Scenedesmus quardicauda and Chlorella vulgaris. The contaminant bacteria included nine different species which were Stenotrophomonas maltophilia and Bacillus polymyxa which isolated from all the selected algal cultures, while the rest Kocuria varians/rosa, Aerococcus viridians, Micrococcus sp., Staphylococcus auricularis, Staphylococcus capitis, Kocuria kristanae and Staphylococcus cohnii varied in their presence and absence in these algal cultures. The contaminant fungi of the four algal cultures were Aspergillus flavus which was isolated from M. aeruginosa culture, Asp.niger isolated from C. vulgaris culture , Cryptococcus albidus that isolated from S. quardicauda culture, whilst Penicillium sp. isolated from A. circinalis culture. However, Eucalyptus camaldulensis essential oil was extracted and subsequently showed highly activity when tested against six species of the isolated algae and against the isolated contaminant bacteria and fungi species. Chemical composition of the
extracted oil was analyzed by HPLC (High Performance Liquid Chromatography).
Index Terms— control algae,antagonistic activity ,eucalyptus camaldulensis
—————————— ——————————
1 INTRODUCTION
Algae are considered the main causes of plenty of problems in the aquatic ecosystems and they could be harmful by producing large populations in the aquatic environment. Large growths of some algae (e.g., the diatom Chaetoceros ) can clog the gills of fish, in addition to anoxic conditions can occur at the end of blooms of other algae (e.g. green algae) as the algae die and decompose that cause release toxins that sicken and kill other organisms that prey on these algae ( Hallegraeff et al., 2003). Many researches were carried out to control the harmful algal bloom which included mechanical, physical and chemical me- thods in addition to biomanipulation (Nicholas, 1973 and Sha- piro et al., 1975). All these approaches were not quite sufficient to solve the problem. Therefore, in the last few decades, a varie- ty of medicinal plants and plant extracts have been screened for their antimicrobial activity (Cowan, 1999). Essential oils, de- rived from aromatic and medicinal plants have been reported to be active against Gram-positive and Gram-negative bacteria as well as against yeasts, fungi, and viruses. They are mixtures of different lipophilic and volatile substances, such as monoter- penes, sesquiterpenes (Reichling, 1999). Yang et al.(2009) re- ported that essential oil from Chinease fir (Cunninghamia lan- ceolata) exhibited strong inhibitory action against one of the most harmful algal bloom causes by Alexandaria tamarense (dinoflagellate). However, all studies and applications which
————————————————
A*Biology Dep., College of science, Baghdad University.
deal with algae need to be these algal cultures free from other living organisms (axinic cultures). Thus, many studies had been done to eliminate the companion organisms especially bacteria and fungi from algal cultures included using of antibiotics, chemicals in addition to the physical methods such as use of the photo attraction which include over laying agar and unidirec- tional light methods
2 MATERIALS AND METHODS
2.1- Collection and identification of the studied plant
E. camaldulensis aerial parts were collected from the gardens of Baghdad University and identified. Leaves were air dried at room temperature ground to semi powdered state, the ground samples stored in and labeled in dark and clean containers.
2.2- Preparation of plant extract
Dried leaves of E. camaldulensis were chopped into small piec- es and subjected to hydro distillation using the Clevenger appa- ratus for 5 hours according to British Pharmacopeia (1963).
2.3- Sampling of algae
Samples were collected from Tigris River and canal around Al- Jadriah during Febreuary 2008 by using sterile container (250
ml) and ransported to the lab immediately.
2.4- Isolation and purification of algae
Chu-10 medium solidified by 2% agar-agar and sterilized with autoclave, then poured in petri-dishes which left to solidify. Then the surface of each plate was inoculated with water sam- ple and incubated in a cooled illuminated incubator with light intensity about 200 µE/m²/s and 26± 2 C˚ for 7- 10 days. Each subculture was examined by using a compound microscope
IJSER © 2011
http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 9, September-2011 2
ISSN 2229-5518
(Stein, 1973). The isolated algae identified according to the keys (Desikachary, 1959; Prescott, 1973) for Cyanophyta and Chlorophyta respectively.
2.5- Algal lawns preparation
Appropriate media for algal growth were prepared in flat bot- tom flask which were either Chu-10 (Kassim et al., 1999) or Allen’s (Allen,1968) depending on algal species, agar- agar 2% was added for solidification, then sterilized by using the autoc- lave.The media cooled down to 40-45 C˚ algal culture was added to the agar media, shaked well and poured in petri dishes immediately. Petri dishes were incubated in an illuminated in- cubator with 200 µE/m²/Sec and 26± 2 C˚ for 2-3 days until the
plates turn into greenish color (Jawad, 1982).
2.6- Algal antagonistic activity evaluation
Certain numbers of wells were prepared on algal lawns with the help of sterile cork borer (8 mm in diameter), 0.2 ml of the oil concentrations (0.05, 0.1, 0.2, 0.3, 0.5, 1, 3, 5, 10, 15%) were introduced into the wells. Controls were made by using the solvent (ethylene glycol) which was used to dissolve oil. The plates incubated in an illuminated incubator for 24 hrs.. Inhibi- tion zones were determined by measuring their diameters. Rep- licates were made and the mean values were recorded (Jawad,
1982).
2.7- Isolation and identification of contaminant bacteria and fungi
Enrichment culture media for bacteria (Nutrient agar and Muel- ler Hinton agar) and (Sabouraud dextrose agar) for fungi were prepared. These media inoculated with 1ml of selected algal culture, then plates for bacterial enhancement were incubated for 24-48 hr at 26± 2 C˚ and 72 hr. at 28 C˚ to those for fungal growth improvement. colonies appeared after incubation were sub cultured continuously till pure isolated colony was ob- tained.
A- Identification of the isolated bacteria
Gram's Stain was used as primarily identification of bacterial isolates (Benson, 2002). In addition, analytical profile index was used for the identification of bacterial isolates, and allow-
ing fast, easy and accurate identification of bacteria, four types of api (Api 20E, Api 20NE, Api 20Strep, Api Staph ) were used in this study according to Benson ( 2002).
B- Identification of the isolated fungi
Shapes, colors and textures of some isolated colonies of fungi were primarily used to identify their types. Then, a small part from a fresh fungal isolate was transferred on a slide and a drop of lacto phenol dye was added and pressed together gently with
a cover slide, then examined under (10x) and (40x) of a com- pound microscope (Miline, 1996). While api Candida was used to find out the type of yeast isolate.
2.8- Evaluation of anti-bacterial activity of plants extracts
The anti-bacterial activity of the extracted oils from the studied plants was tested against the isolated bacteria from selected algal cultures by using agar-well diffusion method ( Perez et al.,
1990).
2.9- Evaluation of anti-fungal activity of the extracted oils
A- The anti fungal activity for molds precisely was evaluated
by measuring the fungal growth inhibition assay method using the following formula (Wang et al., 2005)
Growth inhibition% = [[Growth in control - Growth in treatment] /Growth in control] × 100
b- Agar well diffusion method was used to evaluate the anti fungal activity of plant extract against yeast isolates (Perez et al., 1990).
10-Statistical analysis
Complete Randomized Design (C.R.D.) was used as an exper- mintal design. Data were analyzed by using statistical analysis system- SAS (2004) to study the effect of different factors on
the diameters of inhibition zones. Least significant difference (LSD) was used to compare the significant difference between means at P≤ o.o5.
3. Results and discussion
3.1- Isolation and identification of algae
Nine different taxa of algae were isolated, purified, and identi- fied from Tigris River and canal around Al-Jadriah, as they are shown in Table (1).
Table (1) The isolated algae in this study and their classification
Isolated al- gae | Division | Class |
Microcystis aeruginosa | Cyanophyta | Cyanophyceae |
Calothrix braunii | Cyanophyta | Cyanophyceae |
Anabaena circinalis | Cyanophyta | Cyanophyceae |
Nostoc com- mune | Cyanophyta | Cyanophyceae |
Chlorella vulgaris | Chlorophyta | Chlorophyceae |
Kirchinella sp. | Chlorophyta | Chlorophyceae |
Pediastrum boryanum | Chlorophyta | Chlorophyceae |
Scenedesmus quadricauda | Chlorophyta | Chlorophyceae |
Navicula anglica | Chrysophyta | Bacillariopha- ceae |
The algal isolates which obtained did not represent the whole algae in the collected samples in this study, this was due to the growth media used and other environmental factors adjusted for their growth. However, some algae need typical media with typical environmental factors to be grown which were different from those used in this study (Abedin and Taha, 2008). How-
IJSER © 2011
http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 9, September-2011 3
ISSN 2229-5518
ever, the density of algae during February is very low due to algal seasonal life.
3-2 Oil yield of the extracted oil
Results were showed that E. camaldulensis essential oil yield
was 2%, which is approximately the same that extracted by
Pappas and Sheppard- Hanger (1999) which was 2.3%.
Table (2) Yields of the extracted oils from plants involved in this study.
which showed significant inhibitory effects against number of both green and blue- green algae.
Table (3) Variations in the algicidal effects of the extracted oil concentrations against selected algae according to the diameters of the inhibition zones (mm). Controls showed no inhibition.
3-3 Evaluation of inhibitory effects of the ex- tracted oils against algae
Inhibitory effects of E. camaldulensis essential oil were tested
against 6 taxa of the isolated algae (N. commune, A. circinalis, M. aeruginosa, C. vulgaris, S. quardicauda and K. sp.) depend- ing on the diameters (mm) of inhibition zones according to agar-well diffusion method. The selection of those algal taxa depend on their ecological role, A. circinalis, M. aeruginosa and N. commune are responsible for producing a common toxin (microcystin) which considered as a threat to the aquatic organ- isms ( Sivonen and Jones, 1999). A. circinalis and M. aerugino- sa were known as harmful algal bloom formers, but Nostoc strains can also occur as a minor component of cyanobacterial blooms but rarely form mass occurrences ( Sivonen and Jones,
1999). Cooradi et al. (1999) mentioned that Scenedesmus acu- tus filterate had toxic effects on Daphnia magna. However, Rise (1984) reported that C. vulgaris produces substances that inhibit the growth of other algal species. Results in Table (3) showed that E. camaldulensis oil had a wide range of concentrations which showed a significant inhibitory action against the tested algae, the highest inhibitory effect of E. camaldulensis oil was (35 mm) against M. aeruginosa at 15% as showed in Figure (1), while (2) showed the moderate effect and the lowest effect was (9.5 mm) against both of A. circinalis and M. aeruginosa at
0.1%, figure (3) pertaining to control plate, the most susceptible alga to attack by E. camaldulensis oil was M. aeruginosa, and K. sp. was the most resistance. The highest antialgal activity of E. camaldulensis oil could be explain with the help of recorded results of profile HPLC, which showed that the total concentra- tions of phenolic compounds, terpens and alkaloids that sur- veyed were high in E. camaldulensis oil. Nakai et al. (2001) referred that phenols are very active antialgal substances. Nakai et al. (2004) found that the active antialgal allelochemicals which they isolated from Myriophyllum spicatum were ellagic, gallic and pyrogallic acids and catechin which are simple and polyphenols. Greca et al. (1998) investigated the antialgal sub- stances from Zantedeschia aethiopica which were phenols and polyphenols. Kobaisy et al. (2001) examined the essential oil of Hibiscus cannabinus leaves against Oscillatoria perornata which showed slightly inhibitory effect while it had no effect against one type of green algae (data not published), no one of these components (terpens) as they shown in the results is match to any of terpens which recorded in the oils in this study
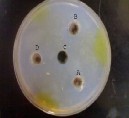
Figure (1) High inhibitory effects of E. camaldulensis essential oil concentrations against M. aeruginosa. (A) 15%, (B) 10, (D)
5%, (C) Control.
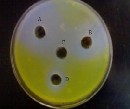
Figure (2) moderate inhibitory effects of E. camaldulensis es-
IJSER © 2011
http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 9, September-2011 4
ISSN 2229-5518
sential oil concentrations against N. commune. (A) 3%, (B) 1%, (D) 0.5%, (C) Control
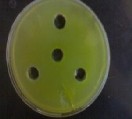
Figure (3) No inhibitory effect of the extracted oil against algae.
3-4 Isolation, purification and identification of contaminant bacteria and fungi from selected algal cultures
Four algal cultures included A. circinalis, M. aeruginosa, S. quardicauda and C. vulgaris were chosen for this pur- pose. However, 36 pure bacterial isolates were obtained, the detected bacterial isolates were 6 genera included Ste- notrophomonas, Bacillus, Kocuria, Aerococcus, Micrococ- cus and Staphylococcus.Those genera included 9 different species which varied in their existence with the selected algal cultures.
Table (4) Contaminat bacteria which isolated from se- lected algal cultures.
Alga /Bact eria | Ste no. Mal to- phi- lia | K. va ria ns/ Ro sa | | | | K. kri sta nae | Sta ph. coh nii | | Sta ph. Au- ricu la- ris | | St a p h. ca pi tis |
Alga /Bact eria | Ste no. Mal to- phi- lia | K. va ria ns/ Ro sa | | A. viri- dan s | | B. po- ly- my xa | Mi- cro- coc- cus sp. | K. kri sta nae | Sta ph. coh nii | | Sta ph. Au- ricu la- ris | | St a p h. ca pi tis |
Alga /Bact eria | Ste no. Mal to- phi- lia | K. va ria ns/ Ro sa | | | B. po- ly- my xa | Mi- cro- coc- cus sp. | K. kri sta nae | Sta ph. coh nii | | Sta ph. Au- ricu la- ris | | St a p h. ca pi tis |
Alga /Bact eria | Ste no. Mal to- phi- lia | K. va ria ns/ Ro sa | | | | K. kri sta nae | Sta ph. coh nii | | | | St a p h. ca pi tis |
1. C. vulga- ris | + | - | - | + | - | + | - | - | + |
2. M. aeru- ginosa | + | + | + | + | - | - | - | - | + |
3. A. circi- nalis | + | - | - | + | - | - | + | - | - |
4. S. quadri cauda | + | _ | _ | + | + | _ | _ | + | - |
Results revealed that the detected fungal isolates included 3 types of molds which were Aspergillus niger, Aspergillus flavus and Penicillium sp., in addition to one type of yeast that was Cryptococcus albidus. Results showed that the Asp.flavus present in M. aeruginosa culture only, Asp.niger present only in C. vulgaris culture , Cryptococcus albidus present in S. quardi- cauda culture, whilst Penicillium sp. present in A. circinalis
culture.
3-5 Control of contaminant bacteria
Plant extract concentrations which did not have any inhibitory
actions against the tested algae were used to control the isolated bacteria from those algae (Table 3). Results showed that E. ca- maldulensis oil concentrations had high inhibitory actions against all the bacterial isolates in this study, E. camaldulensis oil major concentration was 3% which caused (32 mm) inhibi- tion zone against Staph. capitis (Figure 4), while the minor was
0.05% which caused (14 mm) against K. varians, B. polymyxa and Micrococcus sp (Figure 5). Diameters of inhibition zones in mm. Tested oil in this study showed active inhibitory effects against bacteria which isolated and identified in this study as in figure (4), except Aerococcus viridians which was the most resistant. All the contaminant bacteria which isolated in this study were positive to gram stain except Stenotrophomonas maltophilia. These bacteria isolated at 26±1 C˚ which is opti- mum temperature to algal growth and the temperature at which the isolated bacteria should be vital, but during the study it was found that isolated bacteria at 26±1 C˚ have the ability to grow at 37 C˚ . E.camaldulensis essential oil was the most effective against all contaminant bacteria which isolated in this study. Ghalem and Mohamad (2008) showed the antibacterial activity of E.camaldulensis essential oil against gram positive bacteria especially Staphylococcus aureus and low activity against gram negative bacteria. Akin et al. (2009) also reported the signifi- cant antibacterial activity of E.camaldulensis essential oil against gram positive bacteria. Adeniyi and Ayepola (2008) experminted E.camaldulensis essential oil and crude extract against 8 enteric pathogenic bacteria, the results exhibited high antibacterial activity against tested bacteria, α-pinenes, fight against pathogenic bacteria and all kinds of fungi. They are able to eliminate the microorganisms or inhibit their growth as well as intervene on their metabolism (Alma et al., 2004), α-pinenes was recorded as one of the terpen components in all extracted oils in this study. Generally the essential oils mechanism of action against both bacteria and yeast represented by its ability to lyse their cell walls and also it role in metabolic activities weaken inside cell by interfering with cytoplasmic membrane functions especially the inhibition of protein synthesis which result in block the active transport of ions through the cellular membrane (Al-Chaesi, 2008).
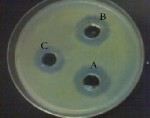
Figure (4) Variation in the inhibition zones of Staph. capitis due to the inhibitory effect of E. camaledulensis concentrations (A)
3%, (B) 1%, (C) 0.5%
IJSER © 2011
http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 9, September-2011 5
ISSN 2229-5518
3-6 Control of contaminant fungi
Antifungal activity of the extracted oils was tested against the
isolated fungi from the selected algal cultures in order to ride off those fungi from algal cultures and get axenic cultures of algae. However, this achieved by fungal growth inhibition assay (for molds) and agar-well diffusion method (for yeast isolate). E. camaldulensis oil showed strong inhibitory effect against Aspergillus niger, Aspergillus flavus Penicillium sp. and Cryp- tococcus albidus as they are clearly appeared in table (6) and figures (5-8). It was found that the isolated fungi which actually are contaminant of four algal cultures were not belong to aqua- tic fungi which complete their life cycle in water only and that may be due to the growth media which used were not specific to these fungi. Begum et al. (2005) examined the antifungal activity of E. camaldulensis essential oil against Aspergillus sp., Penicillium sp., Fusarium solane and Verticillus sp. which
cause inhibition to the mycillium of these fungi. monoterpenes increase cytoplasmic membrane fluidity and permeability, dis- turb the order of membrane embedded proteins, inhibit cell respiration, and alter ion transport processes (Reichling et al.
2006).
Table (6) The antagonistic activity of the extracted oil against three strains of molds measuring by growth inhibition (%), and one strain of yeast measuring by diameters of inhibition zone in mm.

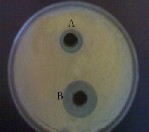
Figure (5) Highly inhibition of Asp. flavus due to the inhibitory effect of E. camaldulensis 3%
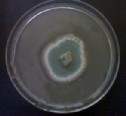
Figure (6) Highly growth inhibition of Penicillium sp. due to the inhibitory effect of E. camaldulensis 3% essential oil.
Figure (8) The inhibitory effects of (A) 3% (B)
1% E. camaldulensis oil against Cryptococcus al- bidus.
3-7 Chemical constituents of the ex- tracted oils
The analysis of the secondary metabolic compounds precisely terpens, alkaloids and phenols revealed that major terpen constituent in the oil of E. camaldulensis was β- phellan- drene 160.2 (μg/ml) and Thujone 86.9 (μg/ml) was the minor and both are part of another detected terpens which were α- pi- nene 130.8 (μg/ml), camphene 95.3 (μg/ml),
myrcene 158.1(μg/ml),linalool 90.6 (μg/ml), cymene 94.4
(μg/ml) while their total concentration was 816.3 (μg/ml). the peaks showed in figure (9) and (10) which are shown the stan- dard terpens and those present in this target oil. Alkaloids present in the extracted oil were also identified by HPLC, and they were Atropine 84.2 (μg/ml), Hyocyamine 66.8 (μg/ml), 3- OH- tropane 65.2 (μg/ml), tropine 59.8 (μg/ml), scopolamine
101.6 (μg/ml) and 7-OH Hyocyamine 72.2 (μg/ml) and the total concentration of alkaloids was 449.8 (μg/ml) as peaks are shown in figures (11) and (12). Scopolamine 101.6 (μg/ml) was the major alkaloid, while Tropine 59.8 (μg/ml) was the minor in the E. camaldulensis oil. Phenolic compounds were the third secondary metabolic compounds in the studied oil which identi- fied. Gallic acid 793.4 (μg/ml) was the major phenolic com- pound, while Synirgic acid 12.6 (μg/ml) was the minor in the E. camaldulensis oil, in addition to other identified phenolic com- pounds in this oil which they were chlorogenic acid, caffeic acid, gallic acid, protocatcheic acid, syringic acid, OH –benzoic acid, D-coumaric acid, vanillic acid and catechol which their concentrations were 119, 28.5, 793.4, 212.3, 12.6, 24.9, 52.1,
178.26, 191.8 (μg/ml) while their total concentration was
1612.86 (μg/ml). As their peaks are shown in figures (13) and
(14).
IJSER © 2011
http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 9, September-2011 6
ISSN 2229-5518
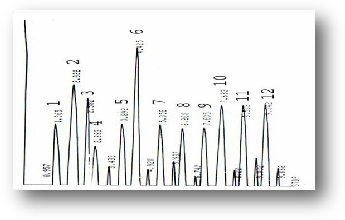
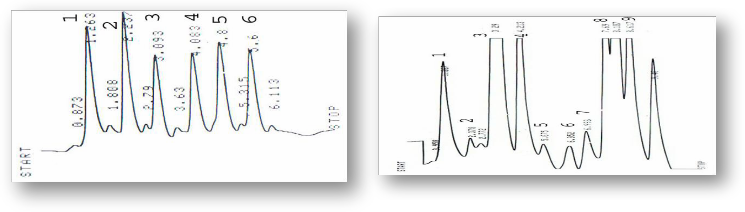
Figure (9) HPLC profile of Terpens standards (1) α- Pinene, (2) Camphene, (3) β- Pinene, (4) 3- Carene, (5) Myrecene(6) Thujone, (7) Linalool, (8) Cymene, (9) Terpinoline,(10) Terpine-4-ol, (11) Geranyl acetate, (12) β-Phellandrene.
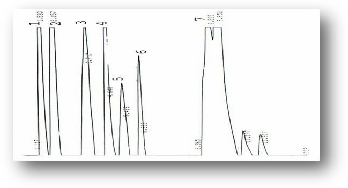
Figure (10) HPLC profile of E. camaldulensis essential oil Terpens (1) α- Pinene, (2) Camphene, (3) Myrecene(4) Thujone, (5) Linalool, (6) Cymene, (7) β-Phellandrene.
Figure (11) HPLC profile of alkaloids standards (1) Atropine, (2) Hyocyamine, (3) 3-OH tropane, (4) Tropine, (5) Scopala- mine, (6) 7-OH Hyyocyamine.
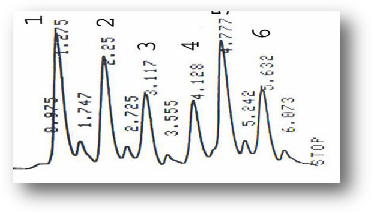
Figure (12) HPLC profile of Eucalyptus camaldulensis essential oil alkaloids (1) Atropine, (2) Hyocyamine, (3) 3-OH tropane, (4) Tropine, (5) Scopalamine, (6) 7-OH Hyyocyamine.
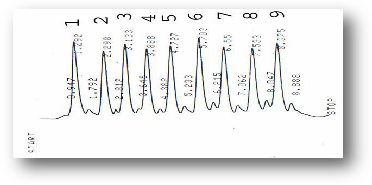
Figure (13) HPLC profile of phenols standards (1) Chlorogenic acid, (2) Caffeic acid, (3) Gallic acid, (4) Protocatcheic acid, (5) Synirgic acd, (6) OH-benzoic acid,(7) D-coumaric acid,(8)
Vanillic acid, (9) Catechol.
Figure (14) HPLC profile of E. camaldulensis essential oil phe- nols (1) Chlorogenic acid, (2) Caffeic acid, (3) Gallic acid, (4) Protocatcheic acid, (5) Synirgic acd, (6) OH-benzoic acid,(7) D-coumaric acid,(8) Vanillic acid, (9) Catechol.
IJSER © 2011
http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 9, September-2011 7
ISSN 2229-5518
REFERENCES
1- Abedin, R.M.A. and H.M.T. Taha. 2008. Anti- bacterial and antifungal activity of Cyanobacteria and green microalgae. Evaluation of medium components by Plackett- Burman design for anti- microbial activity of Spirulina platensis. Gobal J. Biotech. and Biochem. 3(1): 22- 31.
2- Adeniyi, B.A. and O.O. Ayepola. 2008. The Phytochemical Screening and Antimicrobial Ac- tivity of Leaf Extracts of Eucalyptus camaldulen- sis and Eucalyptus torelliana (Myrtaceae). Re- search Journal of Medicinal Plant. 2, pp: 34-38.
3- Akin, M.; A. Aktumsek and A. Nostro. 2009. Antibacterial activity and composition of the es- sential oils of Eucalyptus camaldulensis Dehn. and Myrtus communis L. growing in Northern Cyprus. African Journal of Biotechnology Vol. 9 (4), Pp.
531-535.
4- Al-Chaesi, S.A.S. 2008. Effect of Myrtus com- munis essential oil on growth and activity of sev- eral pathogenic bacterial types and Candida albi- cans yeast. Om Salma Journal for Siences, 5: 8-
12. (In Arabic)
5- Allen, M. 1968. Simple conditions for the growth of unicellular blue-green algae on plates. Journal of Phycology, 4:1-4.
6- Alma, M.H.; S. Nitz; H. Kollmannsberger ; M. Digrak ; F.T. Efe and N. Yilmaz . 2004. Chemical composition and antimicrobial activity of the es- sential oils from the gum of Turkish pistachio (Pistacia vera L.). J .Agric. Food Chem.
52(12):3911–4.
7- Begum, S.H.; S.M.M.H. Majumder; M.A. Ma- jid and M. M. Hossain. 2005. Isolation, Characte- rization and Study of the Physical, Chemical and Anti Microbial Properties of Fat Obtained from Eucalyptus (Eucalyptus camaldulensis Dehnh) Leaf. European Journal of Scientific Research, Vol 10, 3.
8- Benson, H.J. 2002. Microbiological Applica- tions Laboratory Manual in General Microbiol- ogy. New York. McGraw-Hill companies.
9- British Pharmacopoeia, 1963. Determination of Volatile Oil in Drugs. The Pharmaceutical Press, London.
10- Cooradi, M.G.; G. Orbi; H.M. Abdel Moneam; A. Torelli and M. Bassi. 1999. Exudates from the wild type and a CR-tolerant strain of Scenedesmus acutus influence differently CR (VI) toxicity to algae. Science Direct, vol 37, Pp: 3019-3025.
11- Cowan, M.M. 1999. Plant products as antimi- crobial agents. Clin. Microbiol. Rev. 12:564–582.
12- Desikachary, T.V. 1959. Cyanophyta. Aca- demic press. New York.
13- Ghalem, B.R and B. Mohamed. 2008. Anti- bacterial activity of leaf essential oils of Eucalyp- tus globulus and Eucalyptus camaldulensis. Afri- can Journal of Pharmacy and Pharmacology Vol.
2(10). Pp. 211-215.
14- Greca, M. D.; M. Ferrara; A. Fiorentino; P. Monaca, and L. Previtera.1998. Antialgal com- pounds from Zantedeschia aethiopica. Phytoche- mistry, 49: 1299-1304.
15- Hallegraeff, G. M.; D. M. Andersen and A.D. Cembella. 2003. Manual on Harmful Marine Mi- croalgae.UNESCO Publishing, Paris.
16- Jawad, A.L.M.1982. Interaction between cya- nobacteria and other micro-organisms. Ph.D.Thesis.Liverpool University. England.
17- Kassim, T.I.; H. AL-Saadi and N.A. Salman.
1999. Production of some phytoplankton and zooplankton and their use as live food for fish lar- va. Iraqi J. Agric. Proc. of 2nd Sci. Confer., 4(5):
188-201.
18- Kobaisy, M.; R. Mario; R. Tellez; C. L. Web- ber; F. E. Dayan; K. K. Schrader and D. E. Wedge. Phytotoxic and Fungitoxic Activities of the Essen-
IJSER © 2011
http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 9, September-2011 8
ISSN 2229-5518
tial Oil of Kenaf (Hibiscus cannabinus L.) Leaves and Its Composition. Journal of Agiculture and Food Chemistry, 49(8): 3768-3771.
19- Miline, L.J.R. 1996. Fungi In: Practical Medi- cal Microbiology edited by Collee, J.F. Churchill, Livingstone.
20- Nakai, S.; Y. Inoue and M. Hosomi. 2001. Al- gal growth inhibition effects and
inducement modes by plant-producing phenols. Water Res., 35 (7) 1855-1859.
21- Nakai, S.; Y. Shingo and H. Massaki. 2004. Anti-cyanobacterial fatty acids released from My- riophyllum spicatum.Hydrbiologia, 543:71-78.
22- Nicholas, S.A. 1973. The effects of harvesting aquatic macrophytes on algae. Transactions of the Wisconsin Academy of Sciences, Arts and Letters,
61: 165-172.
23- Perez, L.; M. Pauli and P.Bazequre.1990.Antibiotic assay by the agar well diffusion method. Journal of Actabiology, 15: 113-
115.
24- Prescott, G.W. 1973. Algae of the Western Great Lakes Area. OHO Koeltz. Science publish- ers. Germany.
25- Reichling, J. 1999. Plant-microbe interaction and secondary metabolites with antiviral, antibac- terial and antifungal properties; in Wink M (ed):
Functions of Plant Secondary Metabolites and
Their Exploitation in Biotechnology. Sheffield, Sheffield Academic Press, 1999, pp 187–273.
26- Reichling, J.; U. Suschke ; J. Schneele and H.K. Geiss. 2006. Antibacterial activity and irrita- tion potential of selected essential oil components
– structure-activity relationship. Nat.Prod. Com- mun., 1:1003–1012.
27- Rice, E. L. 1984. Allelopathy, 2nd ed. Aca- demic Press, Ltd., London, United Kingdom.
28- SAS. 2004. SAS / STAT Users Guide for Per- sonal Computers. Release 6.12. SAS Institute Inc., Cary, NC., USA. (SAS = Statistical Analysis Sys- tem).
29- Shapiro, J.; V. Lamarra and M. Lynch. 1975. Biomanipulation: An ecosystem approach to lake
restoration. In: Brezonick, P.I. and J.L. Fox (eds.), Water quality management through biological control. Department of Environmental Engineer- ing Scinces. University of Florida, Gaenisville, Pp: 85-96.
30- Sivonen, K. and G. Jones. 1999. Cyanobac- terial toxins, pp: 41–111. In : Chorus and J. Bar- tram (ed.), Toxic cyanobacteria in water. E&FN Spon,London, Great Britain.
31- Stein, J.R.1973. Handbook of phycological methods: Culture methods and growth measure- ments. Cambridge University Press. Cambridge.
32- Wang, S.Y.; C. Wu; F. Chu; S. Chien ; Y. Kuo
; L. Shyur; and S. Chang. 2005. Chemical compo- sition and antifungal activity of essential oil iso- lated from Chamaecyparis formosensis Matsum. Wood. Holzforschung, 59, Pp. 295–299.
33- Yang, W.; J.S. Lu; H.Y. Li; X.L. Zang and Y.Z. Oi. 2009. Inhibition of the Growth Alexadrium tamarense by Algicidal Substances in Chinese Fir (Cunninghamia lanceolata). Bull. Environ. Con- tam. Toxicol. 83: 537-541.
IJSER © 2011
http://www.ijser.org











