International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1355
ISSN 2229-5518
Anatomical, Histological and Histochemical Adaptations of the Avian Alimentary Canal to Their Food Habits: II- Elanus caeruleus
Hamida Hamdi1*, Abdel-Wahab El-Ghareeb1, Mostafa Zaher1, Fathia AbuAmod2
ABSTRACT— These This study was the second in a series of studies aiming at establishing a connection between the food habits of aves and the anatomical, histological and histochemical structures of their alimentary tract. In this study, the gross anatomy, histology and histochemistry of the digestive system of the black -winged kite, Elanus caeruleus, a carnivorous bird, have been investigated. This study has revealed that, the oesophagus was relatively long with poorly developed crop; the simple stomach is differentiated into a glandular proventriculus and a muscular ventritculus or gizzard. The small intestine is divided into duo- denum, and ileum. The large intestine contains a very small pair of ceca called, lymphoid ceca and a short rectum. The internal mucous glands of the oesophagus keep the surface of the tube moist and slimy and thereby facilitate the passage of the food. The differences in the proventriculus structure may also be related to diet. The present study revealed that the alimentary tract shows the usual four laminae; serosa, musculosa, submucosa and mucosa. The oesophageal mucosa is thrown into numerous longitudinal folds. The mucosa of the oesophagus is lined with stratified squamous keratinized epithelium. The oesphageal glands were of the simple tubule alveolar type. The proventricular mucosa is thrown into numerous folds of different depths which are lined by simple columnar epithelium. The proventricular glands were simple tubular to simple branched tubular glands. A thin layer of gastric keratinoid material is found in the ventriculus. The goblet cells gradually increase in frequency from the duodenum to the rectum. The lamina propria contains diffuse lymphatic tissue and the muscularis mucosa is well de- veloped, and extends into the stroma of the villi. Also, the histochemical studies revealed that PAS positive matter was found in the entire alimentary tract. The oesophageal glands have acid mucopolysaccharide secretions. While gastric glands of stomach, the thin koilin layer, the goblet cells and crypts of Lieberkühn have acid and neutral mucopolysaccharide secretions. Proteins and nucleic acid was observed in the different regions of the alimentary canal.
INDEX TERMS— Anatomical, histological, histochemical, alimentary canal, birds.
—————————— ——————————
1 INTRODUCTION

He avian gastroimtestinal tract (GIT) has undergone a multitude of physiological structure when compared to other animal orders. On one hand it has evolved to take advantage of the physical and chemical characteristics of a wide variety of food types (1). On the other hand, it had to do so within the limitations of the requirements for flight (2). To this end, birds have evolved a light weight beak and muscular ventriculus, which replaces the heavy bone, muscular and dental structure characteristic of reptiles and mammals. The ventriculus and the small intestine are the heaviest structures within the gastrointestinal tract and are located near the bird’s centre of gravity within the abdomen. The overall length of the GIT is also less than that of a comparable mammal, another weight-saving flight adaptation. Interestingly, these character- istics are still shared with the flightless species such as ratites and penguins. In addition, the actual digestive process needs to be rapid to support the high metabolic rate typical of flight-
ed birds (3).
Raptors serve an important role in the ecosystems in which
they are found. These animals are built to function in the top
of the food web, and as such have developed a gastrointestinal
————————————————
• 1Department of Zoology, Faculty of Science, Cairo University, Egypt
,. E-mail: fab200656@yahoo.com
• 2Department of Zoology, Faculty of Science, El Margab University,
Libya
tract suitable for a carnivorous diet (4).
The purpose of this study is to report the special anatomi- cal, histological and histochemical structures of the alimentary canal of the black winged kite, Elanus caeruleus, a carnivorous bird, and correlating them with the type of food
2 MATERIALS AND METHODS
Healthy ten specimens of the black- winged kite, Elanus caer- uleus (Order: Accipitriformes, Family: Accipitridae), trapped alive from El-Mansouria, Giza Governorate; Egypt is used as a model of carnivorous birds (Fig.1).The specimens were anaes- thetized by chloroform, and then dissected carefully by mak- ing a longitudinal incision at the midventral surface.
For gross anatomy, photographs were taken for the digestive system within the body of the animal and also for the alimen- tary canal taken out of the body. In addition, in two speci- mens, the alimentary canal was cut longitudinally to describe the structure of the internal surface as the folds, the villi and valves.
For the general histological studies, the contents of the alimen- tary canal were drained by saline solution, small pieces of the various segments were fixed in aqueous Bouin solution, after
IJSER © 2013
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1356
ISSN 2229-5518
fiaxation, parts of the alimentary canal were dehydrated, em- bedded in paraffin wax and then transversely sectioned 6μ thick .Sections were stained with differential double stained Mayer's haemotoxylin and eosin (5).
For the histotochemical studies, the following techiques were implemented:
1- General carbohydrates were illustrated using the periodic acid Schiff (PAS) technique (6). In this procedure, sections were placed in 0.5% periodic acid for the liberation of alde- hydes, and then treated with Schiff's reagent for 2 minutes. A positive reaction is indicated by the appearance of magenta colouration resulting from the reaction between aldehydes and the decolourized solution (leucofuchsin) of Schiff's rea- gent.
2- Acid and neutral mucopolysaccharides were demonstrated by the Alcian blue-PAS method (7). By this method, acid mu- cins exhibit blue stainabilities whereas neutral mucins take a reddish colouration, and the mixtures of both mucins acquire a purple stainability.
3- For displaying the total proteins, the mercuric bromophenol blue method was employed (8). The existence of a dark blue stainability denotes the occurrence of total proteins.
4- Nucleic acids (DNA and RNA) were demonstrated by the methyl green pyronin method (9), while the application of Feulgen reaction was used for demonstration of DNA only (10).
3 Results
3.1 Gross anatomy
The exclusively meat diet of the black-winged kite requires a relatively unspecialized alimentary tract for digestion. The oesophagus leads into a poorly developed crop. The food passes from the crop to a simple muscular stomach (proven- triculus and ventriculus), which is highly distensible. In the proventriculus, chemical digestion commences and the food is passed as a yellowish chyme past the pyloric sphincter into the small intestine (consisting of duodenum and ileum), which leads into the large intestine, where there are two ceca, which are very small as in most carnivorous birds. The large intestine is short and terminates in the cloaca, which opens to the out- side by the cloacal opening (Figs. 2&3).
3.1.1 The oesophagus
The oesophagus was long, quite wide and highly distensible. The oesophagus distensibility is facilitated by a number of longitudinal folds, in order to accommodate the large amounts of food. These folds are large and extensive. The oesophagus of the black winged kite occupies the same position referred to in the quail. A spindle-shaped genuine crop is either hardly recognizable when not filled or very distinct, but always present.
3.1.2 The stomach
The stomach was large and thin walled compared to seed eat- ing bird species. A more simple muscular stomach is found.
The muscular stomach aids in the production of the compact pellet which is regurgitated after digestion is complete. The proventricular ventricular isthmus is not present so that the two organs form one large pear-shaped cavity. This lack of an isthmus and the shape of the black winged kite stomach give a space for larger pieces of prey. The black winged kite proven- triculus is consistently more developed than the ventriculus when compared with the quail, often quite large and distensi- ble. The proventriculus is thick-walled and spindle or cone- shaped.
3.1.2 The small intestine
The small intestine was relatively shorter in the black - winged kite. It begins immediately after the pyloric portion of the stomach and it is differentiated into the duodenum and the ileum. The duodenum was relatively long .The duodenum formed a characteristic `U'-shaped loop, consists of descend- ing and ascending limbs. The two loops of the duodenum were held together by a narrow fold of mesentery. The transi- tion between the jejunum and ileum could not be determined. Meckel‘s diverticulum were not observed. There is no sharp limit between the duodenum and ileum except for a slight difference in their diameter.
3.1.3 The rectum
The rectum of the black-winged kite was extending from the end of the small intestine until it opens distally in the cloaca in the form of a short and straight tube. Also, the large intestine contains a pair of out pocketing, ceca that project from the proximal part of rectum at its junction with the small intestine (ileocolic junction). These ceca were small finger-like struc- tures of approximately equal length called lymphoid ceca, they were not important in digestion but contain lymphocytes.
3.2 Histological studies
3.2.1 The oesophagus
The oesophagus consists of four distinct functional tunicae namely; mucosa, submucosa, musculosa and the outermost serosa (Fig. 4a). The oesophagus was lined throughout its length with keratinised stratified squamous epithelium. The lamina propria mucosae consist of connective tissue with dif- fuse lymphatic tissue scattered throughout. Oesophageal glands were abundant within the lamina propria mucosae. These were small tubule alveolar glands with separate excre- tory ducts penetrating the stratified squamous epithelium. A well-developed muscularis mucosa separates the lamina pro- pria mucosae from the underlying submucosa, which consists of more dense connective tissue with numerous profiles of vessels and nerves (Fig.4b). The musculosa was relatively thick and consists of two layers of smooth muscle cells: an in- ner longitudinal layer and an outer circular layer. The muscu- losa is surrounded by the tunica adventitia at the cervical part of the oesophagus and crop, and by the tunica serosa at the
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1357

ISSN 2229-5518
thoracic part of the oesophagus.
tricular gland capsule septa of thin connective tissue are
3.2.2 The stomach
Histologically, the stomach wall is formed of serosa, mu losa, submucosa, muscularis mucosa and mucosa. The se is made up of a layer of simple squamous epithelium.
The proventriculus
The musculosa of the proventriculus consist of a thick l
of circular muscle fibres exterior to which was a thin laye
longitudinal muscle fibres. The submucosa was a thin loose
areolar connective tissue containing a number of fine blood capillaries and nerve endings (Fig.5a). Its inner glands are ar- ranged into four to five longitudinal bulges.The lumen of pro- ventriculus was greatly reduced, being taken by its considera- bly thickened glandular walls. Two types of glands exist in the wall of the proventriculus: (1) tubular glands for the secretion of mucus and (2) gastric glands that secrete hydrochloric acid and pepsinogen,the glandular stomach w highly acidic, it dis- solves bones (Fig.5b).
The mucosal layer is formed of a lamina propria of dense con-
nective tissue that extends to hold two types of gastric glands; the deep and the superficial gastric glands. The deep gastric glands are compound-branched alveolar type lined with sim- ple cuboidal epithelial cells (Fig.5c). While the superficial glands were of the simple tubular type and appear in the form of numerous folds of mucosal epithelium. Among the proven-
present, and they extend to the musculosa.
The tunica mucosa (tunica mucosa gastris) is folded, some-
thing presenting anastomosis of the folds and the lining epi- thelium was simple columnar with clear cytoplasm. The cells of the basal region of the folds were low and much more stained than those of the superficial region (Fig.5d). Extending to the lamina propria was the muscularis mucosa which is made up of smooth muscle fibers manifesting a network ap- pearance and surrounding the bodies of the deep gastric glands. It almost reaches the bases of the superficial gastric glands where it ramifies and sends solitary and /or loosly packed smooth muscle fibres to support the mucosal epitheli- um of these glands.These exuberant branched tubular type gland are cuboid epithelial cells that are intensely eosinophilic and those situated at a basal position to give a serrated ap- pearance. All of the branched tubular glands open in the main unique tubule also lined up by cuboid cells, which leads to the
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1358
ISSN 2229-5518
proventricular luminal surface.
3.2.3The ventriculus
The mucous membrane of the ventriculus is thrown into well developed narrow and deep folds, lined with a thin tough keratin-like layer known as cutica gastrica. The mucosa of the ventriculus of the stomach has compound tubular epithelial cells with basal nuclei. In the lamina propria, we found simple tubular glands whose glandular cells were similar to those of the superficial lining epithelium. The submucosa is a thin lay- er of loose connective tissue containing a number of blood vessels. Musculosa consists of two layers of smooth muscle fibers, the inner thick circular muscle layer and the outer thin longitudinal muscle layer (Figs. 6a&b).
3.2.4The small intestine
The small intestine is differentiated into two main regions namely, the duodenum and the ileum. The small intestine shows the usual tunicae namely; mucosa, submucosa, muscu- losa and serosa. The mucosa of the intestine is thrown into simple longitudinal villi.These were deep, narrow and numer- ous in the duodenum but more or less finger-like, while in the
ileum, the villi are short and less numerous(Figs.7a&8a).
The lamina propria of the mucosa consists of loose connec- tive tissue and is lined with an epithelial membrane character- ized by its long narrow finger-like wavy projections forming the villi. The lining epithelium of these villi is formed mainly of simple columnar cells interspersed with goblet cells; being more numerous in the ileum than in the duodenum.The sub- mucosa is formed of thin loose connective tissue containing a number of blood vessels.
The muscularis mucosa is represented by a narrow part of longitudinally arranged smooth muscle fibers towards the side of the submucosa, but on the side of the lamina propria, it is represented by vertically arranged smooth muscle fibre strands. The mucosa is invaginated at the bases of the villi into straight tubular glands (crypts of Leiberkühn) which are con- tinuous with the columnar epithelium linning the villi. The lamina propria extends between the crypts of Leiberkühn and forms the underlying layer of the epithelial lining of the villi together with the vertical strands of the muscularis mucosa which extend along the midline of the villi (Figs.7b&8b).The musculosa consists of two layers of muscle fibres; outer longi-
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1359
ISSN 2229-5518
tudinal and inner circular muscle layers. The musculosa in the duodenum is thinner than the circular musculosa of the ileum. The serosa was the outermost layer made up of flattened sim- ple squamous epithelium with flattened nuclei.
3.2.5The large intestine
Although the rectum is essentially similar in structure to that of the small intestine, yet its muscular coat is considerably higher in thickness (Fig.9). The mucosa of the rectum is thrown into long and branched leaf- like villi lined by simple columnar epithelium containing goblet cells. The goblet cells are numerous in number and open into the lumen. At the base of the mucosal folds, rectal glands (simple tubular) are no- ticed. These glands are lined with simple columnar epithelium and goblet cells. The submucosa consists of loose connective tissue holding blood vessels. The muscularis mucosa is com- posed of longitudinal muscle fibers. This layer extends inside the mucosal folds as vertical muscle fiber strands.The muscu- losa layer is made up of two muscle layers; an outer thin longi- tudinal and a thick circular one. The lamina propria is invaded by small darkly stained lymphocytes whereas the submucosa holds the bodies of tubular glands. The serosa is a thin layer composed of simple squamous epithelium with flattened nu- clei. The mucosa of the large intestine has villi, though not to the degree of the small intestine, and is an important site for water reabsorption from both faeces and urinary products.
3.2.6The rectal caeca
The histological structure of the caecum was the same struc- ture of the rectum. But the caecum is characterized by the nu- merous numbers of lymphocytes that are located in the sub- mucosa and mucosa. The lumen is very narrow due to the large number of the lymphocytes and the mucosal folds are hardly observed.
3.3 -Histochemical studies
Carbohydrates (PAS-positive material):
3.3.1The oesophagus
The oesophageal glands are composed of typical mucous al- veoli. These glands were loaded with positively stained mate- rial (fig.10).
3.3.2The stomach
In the proventriculus, the cells of the surface lining epithelium of the mucosal folds shows PAS- positive mucin granules that were found to occupy the supra nuclear area of the cells (Fig.11). The ductular cells that lined the ducts of the submu- cosal glands showed PAS positive reaction in their apical ends.
In the ventriculus, the secretory material within the lumen of the glandular tubules showed positive reaction with PAS stain. The koilin showed a positive reaction to PAS stain.
3.3.3The ileum
The mucosa of the ileum revealed a strong magenta coloura- tion with PAS reaction in the goblet cells of both the villi and the crypts of Leiberkühn as well as the apical plasma mem- branes of simple columnar epithelial cells. However, the ground cytoplasm of the columnar epithelial cells exhibits a weak PAS reactivity (Fig.12a&b).
3.3.4 The rectum
General carbohydrates are localized in the rectal gland, goblet cells and in the surface mucous epithelium indicated by PAS technique in the form of magenta colouration (Fig.13a&b).
3.4 Mucopolysaccharides:
3.4.1The oesophagus
The Alcian blue - PAS method, showed that the nature of the oesophageal glands is like the nature of oesophageal glands of quail since it is in the form of acid mucopolysaccharides (the blue colour, Fig.14)
3.4.2 The stomach
In the proventriculus, the cells of the surface lining epithelium of the mucosal folds secrete acid and neutral mucopolysaccha- rides since they give blue and red colour with Alcian PAS stain (Fig.15). On the other hand, the ductular cells that lined the ducts of the submucosal glands give red colour with Alci- an PAS stain due to the presence of neutral mucopolysaccha- rides. The secretory cells were negatively stained with Alcian blue-PAS technique. In the ventriculus, the mucosa had gastric pits or crypts leading to gizzard tubular glands lined by co- lumnar epithelium stained positively to both PAS and Alcian blue indicating the presence of both neutral and acid mucin, similar to the proventriculus. The secretory material within the lumen of the glandular tubules showed a positive reaction for PAS and Alcian blue techniques. The koilin showed a positive reaction for PAS and Alcian blue indicating the presence of neutral and acidic mucin within its content (Fig.16a&b).
3.4.3The small intestine (the duodenum and the ileum)
Sections subjected to the Alcian blue-PAS technique have dis- played an intense reactivity for acid mucopolysaccharides in the goblet cells indicated by strong bluish colouration. Moreo- ver, red stained neutral mucoids in the basal regions of these cells have been encountered (Figs.17a, b&18).
3.4.4The rectum
Acid and neutral mucopolysaccharides are demonstrated by using Alcian blue-PAS technique, strong bluish colouration is observed in the goblet cells, reddish colouration in the basal regions of these cells, and a red- blue colouration are noted in between. Netural mucins are detected in the columnar epithe-
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1360
ISSN 2229-5518
lial cells of both folds and crypts (Fig .19a&b).
3.5 Total proteins
3.5.1 The oesophagus

Application of mercuric bromophenol blue method on the oesophagus of the black-winged kite proved the presence of an exaggerated amount of proteinic elements of the cytoplasm of its stratified squamous epithelium. On the other hand, the oesophageal glands showed a negative response to the above mentioned method (Fig. 20).
by (Duke
3.5.2 The stomach


In the proventriculus, the cytoplasm of cells of the surface lin- ing epithelium of the mucosal folds and the ductular cells that lined the ducts of the submucosal glands showed modrate reation with the mercuric bromophenol blue method (Fig.56). While that tubular koilin and folds of the ventriculus showed positive reaction with the above mentioned method (Fig.21).
3.5.3The small intestine (the duodenum and the ileum)

Bromophenol blue stain reacts positively with the absorptive columnar cells of the mucosal folds .A similar reaction is also noted in lamina propria of the small intestine, however a weak reaction was also, observed in the goblet cells (Figs.22&23)
3.5.4 The rectum

Application of mercuric bromophenol blue method, indicated the absence of total proteins in the goblet cells, however, it is localized in the absorptive cells of the mucosal folds and rectal gland (Fig. 24).
3.5.5 Nucleic acids:


Histochemical demonstration of DNA revealed appearance of a dense product in the nuclei of the oesophageal, gastric and intestinal mucosal cells. Such a positive staining product was present in the place of the chromatin substances containing DNA (Figs.25&26). Application of methyl green pyronin methods proved the existence of a considerable amount of RNA inside the cytoplasm of the columnar epithelial cells in the different gut regions of the black winged kite (Fig.27).
4. DISCUSSION
In reviewing the knowledge of the anatomical, histological and histochemical structures of the alimentary canal in rela- tion to the food habits of the black- winged kite, it was neces- sary to compare it with other members with different mode of nutrition. Birds eating high-protein diets generally have less complicated digestive systems than those eating complex car- bohydrates (11).
The anatomical observation of the alimentary canal of
Elanus caeruleus, detects that it is a double-ended open tube,
which begins at the beak and ends at the vent. It is composed
of a mouth, oesophagus, crop, proventriculus, ventriculus (gizzard), intestine, caeca, rectum and cloaca. These results are in agreement with those obtained by Klasing (1),Calhoun (12, Farner (13), Selander (14), Toner (15) &Helander (16).
The size of the different portions of digestive tract of birds
varies according to the type of dietary habits (13, 17, 18& 19).
The oesophagus of the black winged kite, was quite wide, it accommodates with swallowing large preys. This finding agrees with (20, 21, 22& 23). The crop was poorly developed; it is in the form of a spindle –shaped enlargement of the oesoph- agus. These results were in agreement with those obtained (24), King & McLelland (25) & Ford (4).


In The black winged kite, the mucosal folds of the oesophagus are lined by stratified squamous epithelium interrupted by the ducts of the mucosal glands. This observation is similar to that found in the fowl (26, 27) and in Tyto alba (22). The stomach of the black winged kite is differentiated into proventriculus and ventriculus (gizzard). Similar findings were observed by Klas- ing (1) & (28)Vukicevic et al.(28)). In The black winged kite, the proventricular ventricular isthmus was not present so that the two organs form one large pear-shaped cavity. This is in accordance with the results of Ford (4). The muscular stomach appears simple, without thin and thick pairs of muscles. This is in accordance with the results of McLelland (17) and kute (11) in herons and penguins, respestively. The gizzard is sepa- rated from the small intestine by a small pyloric fold that regu- lates the passage of food into the small intestine by slowing the movement of large particles (29). The small intestine of Elanus caeruleus is differentiated into the duodenum and ile- um. These results agree with Klasing (1) & Abo-Shaeir (23).The present study revealed that, the relative length of the small intestine is generally shorter in carnivorous birds, than that of granivorous species. This is in agreement with Olsen et al. (30) The rectum of the black winged kite extends from the end of the small intestine until it opens distally in the cloaca in the form of a short and straight tube. This is in agreement with Duke (11). The mucosa of the large intestine has villi, though not to the degree of the small intestine, (1 and 25). In The black winged kite, there are two small rectal caeca observed. This is in agreement with Leake (31) and Clench and Mathias (32). These caeca are of lymphoid type. Similar finding were achieved by Mitchell (33) & Maumus (34). The liver of Elanus caeruleus is bilobed. It is composed of right and left lobes. These results are in agreement with those observed by Klasing (1), Abo-Shaeir (23), King & McLelland (25) and Leake (31), This study confirmed that the pancreas of the black winged kite is composed of dorsal, ventral and splenic lobes, like that of the three lobed structure in ducks. Pancreatic and bile ducts empty into the ascending loop of the duodenum (25).
The histological studies of the present investigation revealed
that the alimentary canal wall of the black winged kite is dif-
ferentiated into the same basic four layers of other birds. These
layers are; serosa, musculosa, submucosa, and mucosa. Similar
findings were achieved by Abou-Dief & El-Akkad (35). The black winged kite oesophageal wall consists of a mucosa,
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1361
ISSN 2229-5518
submucosa, musculosa and a serosal layer. It generally con- tains only smooth muscle cells with the circular muscle layer predominating (17)
The current study revealed that the oesophagus of the black
winged kite was lined by a keratinized stratified squamous
epithelium. The lamina propria of the oesophagus and crop
consists of a loose connective tissue. This layer contains tu- bule- alveolar glands although the number, type and location of the glands vary. The submucosa is a loose connective tissue containing vessels and nerves. The tunica muscularosa of the oesophagus consists of smooth muscle. Its fibres were ar- ranged mainly in a circular orientation. However, the orienta- tion of fibres in some parts of the tunica musculosa was oblique. The tunica musculosa consists of two layers; inner circular and outer longitudinal, similar to that observed in the oesophagus of domestic fowl (34). The presence of these mu- cous glands could be considered as another kind of oesopha- geal adaptation with the nature of food items. Our histological results disclosed that the structures of the proventriculus and of the gizzard are similar to those of other birds. So, we could observe that the mucous membrane of the proventriculus and of the gizzard presents with several folds, in accordance to what is described by several authors (12,17,36&37). Similar to our conclusion, some authors have found that the proventricu- lus simple tubular glands are lined by simple columnar epi- thelium (37&38). The compound tubule-alveolar proventricu- lar glands formed the greatest thickness of the proventricular wall supporting results of others (39,40&41). Cells lining the tubulo-alveolar units of the proventricular glands showed a dentate appearance; similar findings were achieved by Kad- him et al. (42). The glands consist of lobules, each of which has numerous tubules radiating from a central cavity into which they discharge. In agreement to other reports, the current study showed that the gizzard wall in the black winged kite consists of mucosa, submucosa, musculosa and serosa (43,44). The ventriculus, or muscular stomach of birds, is lined on its mucosal surface by a cuticle layer, the koilin. The structure of both the ventriculus and the koilin varies according to diet between species of birds. The koilin serves as a grinding sur- face, enabling the ventriculus to mechanically digest food as it contracts. Insectivorous, herbivorous, and granivorous birds possess a well-developed, muscular ventriculus with a thick, abrasive koilin layer. In contrast, birds that eat foods not re- quiring significant mechanical digestion (i.e., carnivores and piscivores) have an almost rudimentary ventriculus and soft koilin (2,25,45&46). The histological studies showed that, the mucosal layer of intestine is invaginated at the bases of the villi into straight tubular glands (crypts of Leiberkühn or rec- tal glands) which are continuous with the columnar epitheli- um lining the villi. The crypts of Leiberkühn are embedded in the connective tissue of the lamina propria and represent a further increase of the mucosal surface. These crypts of Leiberkühn have been described in other avian species (22,23,25,27,47,48&49). These observations were recorded in Fulica atra (50), Egretta ibis (35) and Tyto lba (22).
The musculosa consists of two layers of muscle fibres;
outer longitudinal and inner circular muscle layers. This find- ing is in accordance with that of Abd El-Aziz (20) in Ardeola ibis ibis. These findings could be an adaptation of this layer to the nature of the bird's food items. The epithelial cells of the villi increasing the absorbing surface area. This agrees with (Klasing (1). Also, goblet cells in the ileum which has slender bases secrete mucous that protects the intestinal epithelium. Leznicka (50) mentioned that these cells (goblet cells) are greatly correlated with the consistency of the bird's food items. The submucosa consists of loose connective tissue holding blood vessels. The muscularis mucosa is composed of longitu- dinal muscle fibers. This layer extends inside the mucosal folds. The rectal villi are shorter than those of the small intes- tine.The mucosa of the rectum is thrown up into numerous leaf-like villi, all covered by simple columnar epithelium con- taining goblet cells. The goblet cells are numerous in number and open into the lumen. This agrees with Abd El-Aziz (20) Rectal glands are noticed at the base of the mucosal folds. This observation was recorded by Abo-Shaeir (23) in Tyto alba. Histochemically, the present study revealed that a strong PAS- positive reaction was given by the oesophageal glands. These results agree with El- Banhawy et al. (48). Moreover, Leznicka (50) reported that a meat diet had a decreasing effect on the number of these glands.
The mucosal folds of the stomach and the mucosa of the
small and large intestine showed PAS-positive reaction. These

findings are in agreement with El-Banhawy et al. 48 and El-
Sayyad (49).
The proventriculus mucosa shows folds lined by simple
columnar cells containing PAS and Alcian blue positive mucin
granules (neutral and acid mucin, respectively) as reported by
Hodges (36) in domestic fowl and Shyla et al. (51) in
ducks.The positive reaction of the surface epithelium for both


acid and neutral mucin was in contrary to the finding of Pas- tor et al.( 52) in chicken who found that the neutral mucin and acid mucin positive cells occur independently in some places. The presence of neutral and acid mucin protects the mucosal surface and forms a resistant mucosal barrier in birds (53). in birds.The ductular cells that lined the ducts of the submucosal glands showed PAS positive reaction in their apical ends. The secretory, oxyntico-peptic cells stained negatively for PAS and Alcian blue indicating that these cells may not be of mucous secretory function, but they certainly secrete HCL and pepsin analogues to that of mammalian stomach secretion (36). The gizzard mucosa is covered by a thin layer of koilin stained positive for PAS and Alcian blue indicating the presence of neutral and acidic mucin within its contents; in agreement with reports of other authors (54). The surface epithelium of ventriculus was PAS positive. They showed predominance of neutral mucin whereas, Suganuma et al. 55 reported that it was predominated by acid mucins in birds. The goblet cells and the crypts of Leiberkühn have acid and neutral mucopol- ysaccharide; these findings are in agreement with 48El- Banhawy et al. (48) & El-Sayyad (49).
The present data showed that in the black winged kite, the distribution of proteins in the cytoplasm of their gut mu-
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1362
ISSN 2229-5518

cosal cells is more or less identical. It is of interest to mention that the histochemical pattern of the proteins in the gut muco- sa of the described animal is closely similar to the previously investigated birds (48&49).
The present work showed also a proportional correlation be-
tween the RNA content and the proteonic amount of the cyto-

plasm of the mucosal epithelial cells in the different gut re- gions. This feature confirms the findings of El-Sayyad (49).
In conclusion, the histological difference between simi- lar segments of the alimentary canal of various birds have, most probably, no taxonomical significance and are rather due
to the difference in the nature of food of various avian forms. Thus, it is obvious that the anatomy as well as the histology of the alimentary canal of various birds demonstrate certain spe- cific characteristics of functional adaptation as a reflection for the carnivorous mode of feeding of all birds.
REFERENCES
[1] K. C. Klasing, Avian gastrointestinal anatomy and physiology. Semin
Avian Exotic Pet Med 8:42-50. 1999.
[2] D. M. Denbow, Gastrointestinal anatomy and physiology. In: Avian Physiology, 5th edn (G. C.Whittow, Ed.). San Diego, California: Aca- demic Press, 299–325. 2000.
[3] S. Gelis, Evaluating and Treating the Gastrointestinal System in Har- rison, G. J., and Lightfoot, T. L. (Eds): Clinical avian medicine. Palm
Beach, FL: Spix Publishing Inc. 412- 440. 2005.
[4] S. Ford, Raptor Gastroenterology: Topics in Medicine and Sur- gery.Journal of Exotic Pet Medicine 19(2):140-150, 2010.
[5] N. M. Castro, & J. S. Camargo, Coloração policrômica de Cortes his-
tológicos. An. Fac. Farm. Odontol. Univ. São Paulo., 9: 211-215, 1951.
[6] A. G. E. Pearse, Histochemistry; theoretical and applied. Churchill
Livingstone, London. 1968.
[7] R.Y. Mowry, Alcian blue techniques for the histochemical study of acidic carbohydrates. J. Histochem. Cytochem., 4:407, 1956.
[8] D. Mazia, P. A. Brewer, & M. Alfert,: The cytochemical staining and measurement of protein with mercuric bromophenol blue. Bull., 104:
57-67, 1953.
[9] N.B. Kurnick, Pyronin Y in methyl green pyronin histological stain.
Stain Technol., 30: 213-217, 1955.
[10] R.Stowel, Feulgen reaction for thymonucleic acid, Stain Technol., 20-
45. 1945.
[11] G.E. Duke, Gastrointestinal physiology and nutrition in wild birds.
Proc. Nutr. Soc. 1049-1056, 1997.
[12] Calhoun, M. L. 1954: Microscopic anatomy of the digestive system of the chicken. Ames, Iowa State College Press. 108 .
[13] Farner, D. S. 1960: Digestion and the digestive system. In: Biology and Comparative Physiology of Birds (A. J.Marshalled.).New York: Academic Press,1: 114–449.
[14] U. Selander, Fine structure of the oxyntic cells in the chicken proven- triculus. Acta Anat.55: 299–310. 1963.
[15] P. G. Toner, The fine structure of resting and active cells in the sub- mucosal glands of the fowl proventriculus. J. Anat.97: 575–583. 1963.
[16] H.F. Helander, The cells of the gastric mucosa. Int. Rev. Cit., 70:273-
89. 1981.
[17] J. Mclelland, Digestive System. InKing, A.S and McLelland, J. (eds.): Formand Function in Birds. Academic Press, London, 1:69-181. 1979.
[18] F. P. Kehoe, & C. D. Ankner, Variation in digestive organ size among five sbecies of diving ducks(Aytha spp.). Can. J. Zool. 63:2339-2342.
1985.
[19] P. Jordano, Frugivory, external morphology and digestive system in
mediterranean sylvid warbens Sylvia ssp. Ibis, 129 (2):175-89. 1987.
[20] I. I. S. Abd El-Aziz, Comparative macroscopic and microscopic anat- omy on the digestive system of some vertebrate animals. Ph. D. The- sis Zoology Department, Faculty of Science, Al-Azhar Univ., Cairo.
1984:
[21] S.B. Salem, Comparative anatomy and histology of the alimentary canal of some birds. Ph. D. Thesis, Faculty of science, Comenus Univ., Bratislava. 1984:
[22] S. Ismail, Comparative Macroscopic and Microscopic studies on some Egyptian vertebrates. M. Sc. Thesis, Fac. Sci., Al-Azhar Univ., Cairo, Egypt. 2000.
[23] W. A. M. Abo-Shaeir, Macroscopic and microscopic comparative studies on some vertebrates in Egypt. M. Sc. Thesis, Fac. Sci., Al-
Azhar Univ., Cairo, Egypt. 2001.
[24] G.E. Duke. Reptor physiology. Pages 225-231 in M.E.Fowler (ED).
Zoo and wild animal medicin. Ist Ed. W.G.Saunders. Co.philadelphia. pA U.S.A. 1978.
[25] A. S. King, & J. Mclelland, Birds, their structure and function.
BaillierTindall, London.84-109. 1984.
[26] O. C. Bradley, The structure of the fowl. Black Co., London, U.K.
1915.
[27] M. L. Calhoun, The microscopic anatomy of the digestive tract of
Gallus domesticus. Iowa State Coll. J. Sci., 7(3): 61- 81. 1933.
[28] T. T.Vukicevic, K., Babic, D. Mihelic, & V. G. Kantura, The anatomy of the digestive system of the ostrich (Struthiocamelus). Proceedings
of the 11th Ostrich World Congress Island Great Brijun Croatia, 15-17
October-2004, 66-69. 2004.
[29] P.Vergara, C. Ferrando, & M.Jimenez, et al. Factors determining gas- trointestinal transit time of several markers in the domestic fowl. Quart.J. Exp. Physiol., 74:867- 874, 1989.
[30] M. A.Olsen, R.Myklebust, T. Kaino, V.S. Elbrod , & S. D. Mathiesen, The gastrointestinal tract of Adelie penguins – morphology and func- tion. Polar Biol, 25:641–649, 2002.
[31] L. D. Leake, Comparative histology, an introduction to the micro- scopic structure of animals, Academic Press INC. (London) LTD, 21-
97. 1975.
[32] M. H. Clench, and J. R. Mathias, the avian caecum; Review.Wilson
Bull. 107(1): 93-121, 1995:
[33] P. C. Mitchell, On the intestinal tract of birds with remarks on valua- tions and nomenclature of zoological characters. Trans.Linn. Soc.
London Zool., 3: 73-75. 1901.
[34] J. Maumus, Les caecums des oiseaux. Ann. Sci. Nat., Zool. et Paleo.
15:1-148. 1902.
[35] F. Abou-Dief, & M. El-Akkad, Histological and ultra structural stud- ies on the ileum of the Cattle Egret, Egretta ibis ibis (Ardei, Ciconi-
formes). J. Egypt. Ger. Soc. Zool., 30(C): 79-98. 1999.
[36] R. D. Hodges, The Histology of the Fowl. London: Academic Press,
35–88. 1974.
[37] W. J. Fieri, Aspectos anatômicos e histológicos do tubo digestivo da codorna Nothura maculosa maculosa, (TEMMINCK, 1815).109 pp
[(Tese doutor.)Univ. Mackenzie, 1984:
[38] L.V. Espinola, & E.A. Galliussi, Estudio anátomohistológico del trac- to digestivo de Fulica armillata (VELELLOT, 1817) Aves (Gruiformes, Rallidae) Iheringia Sér. Zool., 70:93-108, 1990.
[39] T. Okamoto, & J. Yamada, Light and electron microscopic studies on the endocrine cells in the duck proventriculus. Nippon JuigakuZas- shi, 43: 863-870, 1981.
[40] R.V. Prasad, & K. Kakade, Histology andhistochemistry of proven- triculus of domestic duck (Anasplatyrhynchos Linnaeus). Mysore. J.
Agric.Sci., 24: 506-511. 1990.
[41] M.Imai, , T.Shibata, , K. Moriguchi, , M.Yamamoto, & H. Hayama, Proventricular glands in fowl. Okajimas Folia Anat. Jpn., 68: 155-160.
1991.
[42] K. K., Kadhim, A. B. Z. Zuki, M. M. Noordin, & S. M. Babjee, Histo- morphology o f the Stomach, Pro-ventriculus and,Ventriculus of the
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 1363
ISSN 2229-5518
Red Jungle Fowl Anatomia, Histologia, Embryologia, 40( 3): 226–233.
[43] Gabella, G. 1985: Chicken gizzard. The muscle, the tendon and their attachment.Anat. Embryol.171, 151–162, 2011.
[44] T. A.Bailey, E. P. Mensah-Brown, J. H. Samour, J. Naldo, P. Law- rence & A.Garner Comparative morphology of the alimentary tract and its glandular derivatives of captive bustards. J. Anat., 191: 387-
348, 1997.
[45] A. R. Akester, Structure of the glandular layer and koilin membrane in the gizzard of the adult domestic fowl (Gallus gallusdomesticus).
J. Anat.147, 1–25, 1986.
[46] J. T. Lumeij, Gastroenterology. In: Ritchie BW, HarrisonGJ, Harrison LR, eds. Avian Medicine: Principles and Application. Lake Worth, FL: Wingers; 483–521. 1994.
[47] M. El-Banhawy, M. E. Mohallal, , T. R. Rahmy, & T. I. Moawad, A comparative histochemical study on the proventriculus and ileum of two birds with different feeding habits. J. Egypt. Ger. Soc. Zool.,
11(C): 155-174, 1993.
[48] M. El-Banhawy, M. E. Mohallal, T. R. Rahmy, & T. I. Moawad, a: A comparative histochemical study on the proventriculus and ileum of two birds with different feeding habits. J. Egypt. Ger. Soc. Zool.,
11(C): 155-174, 1993
[49] H. I. H. El-Sayyad, Structural analysis of the alimentary canal of- hatchingyoungs of the owl Tytoalbaalba.J. Egypt. Ger. Soc. Zool.,
16(C): 185-202, 1995.
[50] B. Leznicka, The effect of diet on the histological structure of the oesophagus and glandular stomach in the coot (Fulica atra). Zool.
Poioniae, 3(21): 263-280, 1971.
[51] P.Shyla, , P.A. Ommer, & P.Lucy, Structure and post-natal develop- ment of the proventriculus in the duck. Ind. J. Poult. Sci.27: 10–14.
1992.
[52] L. M.Pastor, J. Ballasta, J. F. Madrid, R. Perez-Tomas, & F. Hernan- dez, A histochemical study ofthemucins in the digestive tract of the chicken. Acta.Histochem., 83: 91-97, 1988:
[53] G.M. Mogilnaia, and M. BogatyrLia, Histochemical characteristics of the epitheliocytes of the avian glandular stomach. Arkh.Anat.Gistol.
Embriol., 84:62-70, 1983.
[54] R.Y. Mowry, Alcian blue techniques for the histochemical study of acidic carbohydrates. J. Histochem. Cytochem., 4:407, 1956.

[55] P. S. Selvan, S. Ushakumary, & G. Ramesh, Studies on the histochem- istry of the proventriculus and gizzard of post-hatch Guinea fowl (Numidameleagris). Int. J. Poult. Sci.7: 1112– 1116. 2008.

[56] T.Suganuma, , T. Katsuyama, , M.Tsukahara, , M., Tatematsu, Y.

Sakakura, & F.Murata, Comparative histochemical study of alimen- tary tracts with special reference to the mucous neck cells of the stomach. Am. J. Anat.161: 219–238, 1981.
LEGENDS OF FIGURES
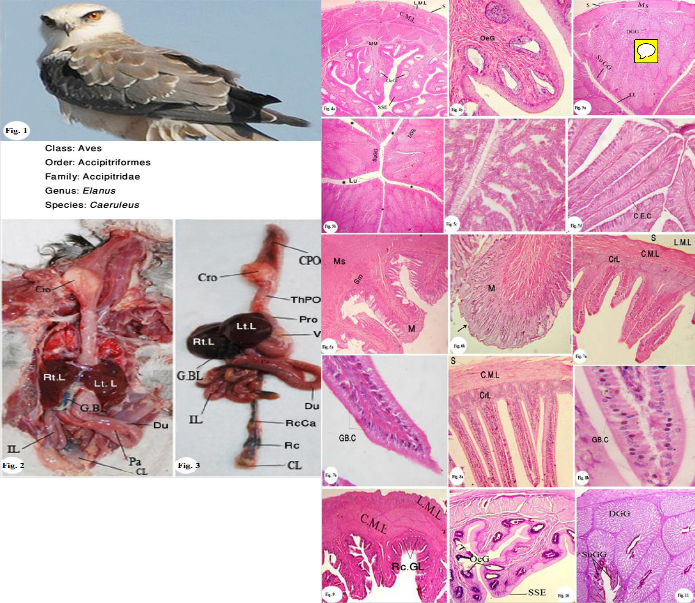
Fig. 1: Photograph of the black winged kite.
Fig. 2: Photograph of the dissection of the alimentary canal of Elanus caeruleus showing crop (Cro), duodenum (Du), ileum (IL), cloaca (CL), liver (L), gall bladder (G.BL) and pancreas (pa).
Fig. 3: Photograph of a fresh isolated alimentary canal showing the cervi-
cal part of oeosphagus (CPOe), thoracic part of oesophagus (ThPOe),
proventrculus (Pro), ventrculus (Ven), rectal caeca (RcCa) rectum (Rc), and cloaca (CL).
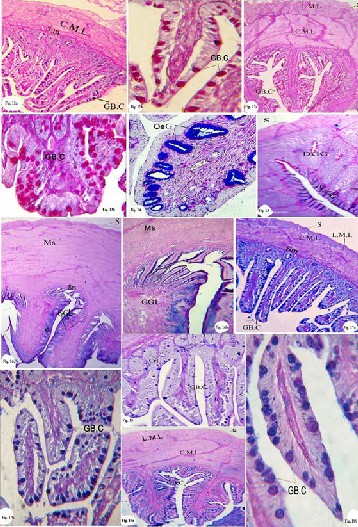
Fig. 4a: Photomicrograph of a transverse section of the oesophagus show- ing the oesophageal gland (OeG), stratified squamous epithelium (SSE), musculosa, circular and longitudinal layers (C.M.L&L.M.L) and serosa (S). H &E stain, X100.
Fig. 4b: Photomicrograph of enlarged portion of the oesophagus showing
the oesophageal gland (OeG) H&E stain, X400.
Fig. 5a: Photomicrograph of a transverse section of the proventriculus
showing the serosa(S), musculosa (Ms), deep gastric gland (DGG), super- ficial gastric gland (SuGG), and lumen (LU).H&E stain, X 100.
Fig. 5b: Photomicrograph of enlarged portion of the proventriculus show- ing the superficial gastric gland (SuGG), and five longitudinal (*) bulges. H&E stain, X200.
Fig. 5c: Photomicrograph of enlarged portion of the proventriculus of showing the deep gastric glands are compound-branched alveolar lined with simple cuboidal epithelial cells (arrow). H&E stain, X 600.
Fig. 5d: Photomicrograph of enlarged portion of the proventriculus, show-
ing the folds and the lining epithelium is simple columnar. H&E stain, X
400.
Fig. 6a: Photomicrograph of a transverse section of the ventriculus, show-
ing the mucosa (Mu), sub mucosa (Sm), and musculosa (Ms), H&E stain, X100.
Fig. 6b: Photomicrograph of enlarged portion of the ventriculus, showing mucosa folds and koilin thin layer H&E stain, X 600.
Fig. 7a: Photomicrograph of a transverse section of the duodenum, show-
ing serosa(S), musculosa (circular and longitudinal layers, C.M.L&L.M.L), and crypts of leiberkühn (CrL) H&E stain, X60.
Fig. 7b: Photomicrograph of enlarged portion of the duodenum, showing goblet cells (GB.C), in the mucosal layer H&E stain, X400.
Fig. 8a: Photomicrograph of a transverse section of the ileum, showing the
H&E stain, X100.
Fig. 8b: Photomicrograph of enlarged portion of ileum, showing goblet
cells (GB.C), in the mucosal layer H&E stain, X400.
Fig. 9: Photomicrograph of a transverse section of the rectum, showing the
rectal gland (Rc.Gl) H&E stain, X100.
Fig. 10: Photomicrograph of a transverse section of the oesophagus, show-
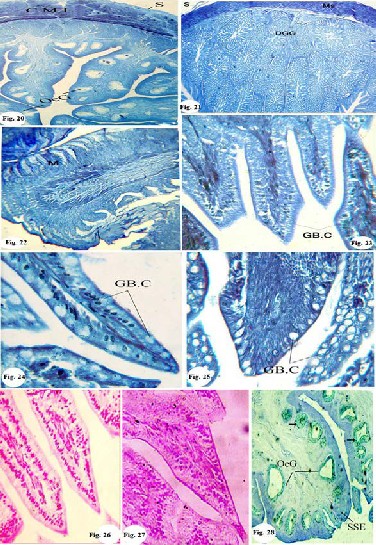
ing the carbohydrate content (PAS- positive stain) X40.
Fig. 11: Photomicrograph of a transverse section of the proventriculus, showing the carbohydrate content: (PAS positive stain) X40.
Fig. 12a: Photomicrograph of a transverse section of the ileum, showing the carbohydrate content. (PAS positive stain) X 40.
Fig. 12b: Photomicrograph of enlarged portion of ileum, showing the car- bohydrate content of the goblet cell (PAS-positive stain) X 656.
Fig. 13a: Photomicrograph of a transverse section of the rectum, showing
the carbohydrate content of the goblet cell (PAS-positive stain) X100.
Fig. 13b: Photomicrograph of enlarged portion of the rectum, showing the
carbohydrate content: (PAS positive stain) X600.
Fig. 14: Photomicrograph of a transverse section of the oesophagus, show-
ing the mucopolysaccharide content (PAS-Alcian blue stain) X 600.
Fig. 15: Photomicrograph of a transverse section of the proventriculus, showing the mucopolysaccharide content (PAS-Alcian blue
showing the mucopolysaccharide content (PAS-Alcian blue stain) X stain) X 164.
Fig. 16a: Photomicrograph of a transverse section of the ventriculus, 150. Fig. 16b: Photomicrograph of enlarged portion of ventriculus showing the mucopolysaccharide content (PAS-Alcian blue stain) X 400.
Fig. 17a: Photomicrograph of a transverse section of the ileum, showing the mucopolysaccharide content.(PAS-Alcian blue stain) X 150.
Fig. 17b: Photomicrograph of enlarged portion of the ileum, showing the mucopolysaccharide content. (PAS-Alcian blue stain) X 600.
Fig. 18: Photomicrograph of enlarged portion of the duodenum, showing the mucopolysaccharide content. (PAS Alcian blue stain) X 400.
Fig. 19a: Photomicrograph of a transverse section of the rectum, showing
the mucopolysaccharide content (PAS-Alcian blue stain) X100.
Fig. 19b: Photomicrograph of enlarged portion of the rectum, showing the
mucopolysaccharide content. (PAS-Alcian blue stain) X 400.
Fig. 20: Photomicrograph of a transverse section of the oesophagus, show-
ing the protein content (Bromophenol blue stain) X 60.
Fig. 21: Photomicrograph of a transverse section of the proventriculus,
showing the protein content (Bromophenol blue stain) X 60.
Fig. 22: Photomicrograph of a transverse section of the ventriculus, show- ing the protein content (Bromophenol blue stain) X 600.
Fig. 23: Photomicrograph of a transverse section of the duodenum, show- ing the protein content (Bromophenol blue stain) X 600.
Fig. 24: Photomicrograph of a transverse section of the ileum, showing the
protein content (Bromophenol blue stain) X 600.
Fig. 25: Photomicrograph of a transverse section of the rectum, showing
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013
ISSN 2229-5518
1364
the protein content (Bromophenol blue stain) X 600.
Fig. 26: Photomicrograph of a transverse section of the duodenum, show
ing the DNA content. ( Feulgentechnique;) X 560
Fig. 27: Photomicrograph of a transverse section of the ileum, showing the
DNA content. ( Feulgentechnique;) X 560
Fig. 28: Photomicrograph of a transverse section of the oesophagus, show
ing the DNA and RNA contents (Methyl green-pyronine stain) X560.
IJSER lb) 2013
http://www.ijserorq


