International Journal of Scientific & Engineering Research Volume 2, Issue 7, July-2011 1
ISSN 2229-5518
Adsorption of carbon from Liquid Hydrocarbons
Parantap Nandi
—————————— • ——————————
Colored salts like NiSO4, K2Cr2O7, K2CrO4, PbCrO4 etc. can extract carbon from hydrocarbons like hexane, heptane, octane in which the salt acts as catalyst. It provides the surface on which carbon gets adsorbed. The salt does not undergo any change chemically which proves that it acts only as a catalyst and provides a surface for adsorption of carbon.
When a colored salt like NiSO4 is soaked in a hydrocarbon like hexane (C6H14), octane (C8H18) and heated, it turns black due to the deposition of carbon on its surface. On excess heating it turns in to an ash colored substance which shows carbon is charred. In case of PbCrO4 the carbon particles smolder on the surface of the salt. K2Cr2O7 & K2CrO4 turn black. Pb(NO3)2 first turns yellow due to decomposition of the salt into PbO and then turn black .Colorless salts e.g. NaCl, KCl (though they appear white) can not extract the whole carbon so they turn partially black and sometimes do not turn black at all. So they must not be used as the effect can not be noticed. However yellow NaCl which can be produced by heating NaCl in an atmosphere of Na vapor can definitely extract carbon from hydrocarbons and thereby turn black.
a. NiSO4 & hexane mixture when heated in a sealed tube turns ash.
b.PbCrO4 & heptane mixture smoulders on heating.
On heating the black substance with conc.H2SO4 or HNO3, CO2 is liberated along with formation of the corresponding sulfate or nitrate salts. SO2 is liberated in
case of H2SO4 and NO2 in case of HNO3.This proves that the salts merely act as catalysts providing a surface for adsorption of carbon.
C + 2H2SO4 � CO2 + 2SO2 + 2H2O
C + 4HNO3 � CO2 + 4NO2 + 2H2O
Colored salts e.g. NiSO4, K2Cr2O7, K2CrO4, PbCrO4 etc. can act as catalysts and extract carbon from liquid hydrocarbons when heated.
NiSO4 + C8H18 � 8C (deposited on surface) + 9H2O K2Cr2O7 + C8H18 � 8C (deposited on surface) + 9H2O +
K2CrO4 (formed by decomposition of the salt)
K2CrO4 + C8H18 � 8C (deposited on surface) + 9H2O
PbCrO4 + C8H18 � 8C (deposited on surface) + 9H2O +
H2SO4 � CO2 + SO2 (due to decomposition of H2SO4) +
H2O + PbSO4 (formed by reaction between H2SO4 &
PbCrO4)
These reactions prove the formation of carbon and
catalytic action of the salts.
Liquid hydrocarbons can be converted to carbon by heating them with colored salts. Large amounts of carbon can be obtained by heating the hydrocarbon-salt mixture in a closed vessel. Hexane, heptane, octane, dodecane etc. when heated with Ni2+, Pb2+,Cr3+etc. get converted into amorphous carbon.
N.B. Simply heating hydrocarbons without salts lead to
mere evaporation and no carbon is formed.
It can be used to extract carbon from liquid hydrocarbons. The black substance can be dissolved in water. The salt will become soluble and the carbon particles may be filtered out. Also it can distinguish between liquid
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 7, July-2011 2
ISSN 2229-5518
hydrocarbons and substances like CH3COCH3 which do not deposit carbon on the salts. When heated in a closed chamber the hydrocarbon salt mixture can yield large
amounts of carbon. It is also a new method of producing carbon from hydrocarbons besides incomplete combustion.
Salt | Color | Color after heating with hydrocarbon |
NiSO4 | Green | Black |
K2Cr2O7 | Orange | Black |
PbCrO4 | Yellow | Black |
K2CrO4 | Yellow | Black |
Colored salts of transition metals which undergo d-d transitions can extract carbon from liquid hydrocarbons like hexane, heptane etc. in amorphous form.
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 7, July-2011 3
ISSN 2229-5518
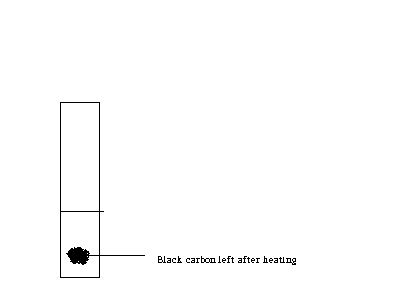
Figure 2 Black carbon adsorbed on the salt
IJSER © 2011 http://www.ijser.org