International Journal of Scientific & Engineering Research Volume 2, Issue 5, May-2011 1
ISSN 2229-5518
A Study of Carcinogenic Components
Removal in Chemical Industry
R.Uma Mythili, K.Kanthavel, R.Krishna Raj
Abstract-The chemical industries are involving with various surfaces coating process which develops pollutions like Carcinogenic components. In industry and environment inhalation of high doses have the potential to induce lung tumors in human and animals. The process of Carcinogenic components removal is a major real time problem in environmental toxicology in chemical processing industry. The standard analysis method determines and calculates the constituent of hexavalent chromium and trivalent chromium compounds in carcinogenic fumes. The research identifies that the presence of Carcinogenic components leads to the low generations of gastric juice, epithelial lining fluids to the human beings and animals. The segregation and analysis of Carcinogenic components was attracted several researcher for the past three decades. This paper discusses with Biological, Chemical and Mechanical analysis methods of identifying Carcinogenic components present in the effluent of the chemical industry in the form of sludge.
Index Terms— Environmental Production Agency, Hexavalent chromium, Scrubber, Trivalent chromium
1 INTRODUCTION
—————————— • ——————————
N experiment was conducted by Abbasi and Soni(1984) thirty adult channel fish exposed to waterborne hexavalent chromium concentration of 50 to
100ppm and hardness of 3ppm resulted in alterations in swimming and balancing behaviors including loss of balance erratic and rapid twisting. Further research was proposed by James R Kastner et al (2002) for the determination of chromium compounds using Polarography.He embraced EPA method which is also named as ion chromatography to determine the dissolved hexavalent chromium (CrO42-) in ambient water .This method was developed by integrating analytical procedures.The promulgation of odour was controlled and determined using mass spectrometry and wet scrubber including the reducing agents [7].
John C.Chang et al (2003) inquiers Ontario Hydro analysis technique coupled with FGD scrubber in laboratory scale inorder to remove the effulent by 70% using [10]. More over Hyeon – Yeong Kim et al (2004) conducted experiment for Sprague Dawley rat, 90 day repeated dose inhalation toxicity study was carried out resulted in 0.5-5µm decrease of activity,nasal hemorrhage.Later Chih-Cheng Wu et al(2004) absorbed the effects of hexavalent chromium in municipal and hazardous waste and he adopted packed tower scrubber for the elimination[3]. Present investigation is aims at reduction in high percentage of trivalent chromium and hexavalent chromium accompanies with chemical using packed scrubber and spray technology at the exit.
2 THEORITICAL MODELS
Theoritical model was first adopted by S.Sakar et al (2007) for dust particle collection.In this model there are various paramaters which is used to calculate the percentage of the hexavalent chromium removal [15]. It includes two major factors to be found, they are collection
efficiency of the particle and the mass balance of the liquid drop.
.
2.1 Collection Efficiency
Dust collection efficiency is frequently expressed in terms of penetration which was adopted by Lim K.S et al (2006). Penetration is defined as the fraction of particles that passes through the scrubber uncollected.
Penetration is the opposite of the fraction of particles collected and is expressed as
Pt = 1 - 11 - (1)
Wet scrubbers usually have an efficiency curve that fits the relationship of
11= 1-e-f system - (2)
By substituting for efficiency, penetration can be expressed as:
Pt = 1 - 11
= 1-(1-e-f system )
Pt=e-fsystem - (3)
The major equation to be included is Souder’s– Brown equation. They are as follows
2.2 The Souder’s–Brown equation
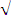
Trond Austrheim.et al., (2008) used the expression for sizing of gas scrubbers [16].This was the expression developed and given below as
K= ( 4gdd)/ ( 3Cd) - (4) Equation (4) involves an empirically quantified
factor known as the Souder’s–Brown value, the K-value,
or the Gas Load Factor. The basis of the Souder’s–Brown expression is a force balance resolved in the vertical direction on a spherical droplet in an upward flowing gas in a gravity field.
When the droplet is held stationary,
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 5, May-2011 2
ISSN 2229-5518
2g(p -p ) - (5)
vertical direction in this slice below which all drops will
Fr=0.5CdAdpgU2g,set=Gd=(rr/6)dd i g
In practice Cd varies with the droplet Reynolds number,
Re=pgUg,setdd/µ - (6) Except for high values of Re, where Newton’s law
states that it is constant and about equal to 0.43.
At low Re, the well-known relation of Stokes states that
Cd=24/Re - (7)
reach the floor of the chamber all such drops will be collected [15].
£t= (h/Uter) = (£x/Ugas) - (8)


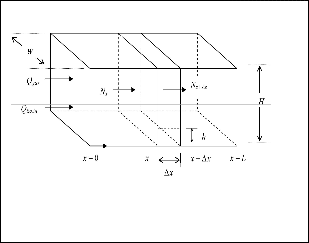
dN = - Uter dx - (9) N HUgas
Fig. 2 Horizontal co-current gas–liquid scrubber
Fig. 1 Upward flowing spherical droplet
Svrcek and Monnery said that decreasing the K- value with 25% for 85 bars pressure. They said while designing a column we have to avoid the upwards velocity entrains droplets and the recommended K-
value is K < 0.1 m/s for low-pressure applications often a safety margin of 50% is added for vessels without internals. Yarong Li,et al., (2002) said that for increasing pressures the critical K-value has been seen to decline. Increasing pressure in oil/gas applications is often accompanied by a decrease in interfacial tension and thereby a decrease in the droplet sizes [16].
2.3 Mass balance of liquid drops
Sarkar.S.et al., (2007) considered the schematic of a horizontal co-current gas–liquid scrubber (Fig 2). In this scrubber air stream containing a trace amount of trivalent chromium. This trivalent chromium enters horizontally at the left face (x = 0) with a velocity Ugas along with a spray of water droplets whose number concentration at the inlet is Nin. Trivalent chromium and hexavalent chromium was removed from the gas stream by absorption of droplets as both travels through the chamber from left to right. They assumed that individual drop moves horizontally with a velocity equal to Ugas and falls vertically at its terminal settling velocity Uter.
They assumed that mixed flow in chamber has mixing in the vertical direction while no mixing in the horizontal direction. Consider the slice or control volume of thickness £x. There will be a certain height h along the
Integrating Eq. from the inlet to any location x gives

N=Ninexp(-Uterx) - (10) HUgas
Nin, Nx, and Nx +£x be the number concentration of water drops at the inlet (x = 0) and at locations x and
x +£x.


<I= Nin -Nout = 1-exp (- Uter x) - (11) Nin HUgas
3 EXPERIMENTAL METHODS
Experimental methods have lots of categories. The major three main categories of experimental methods are Biological, Chemical and Mechanical methods. Each category has sub category based on the instruments and experiments.
3.1 Biological Technology
Graciela Rojas, Jorge Silva, Jaime A. et al., (2005) experimented using Flame atomic absorption spectrometry and colorimetric to determine Chromium in solution along with diphenylcarbazide. Cross-inked chitosan is efficient in removing hexavalent chromium from aqueous solutions and at the same time less efficient in eliminating trivalent chromium owing to protonated active sites interacting mainly with metallic anions at acidic pH. He absorbed that the maximum adsorption capacity achieved in this experiment, as high as 215
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 5, May-2011 3
ISSN 2229-5518
mg/g, is among the highest reported elsewhere [5].
Further Jinwook Chung,et al., (2010) adopted hydrogen based membrane biofilm reactor along with reducing bacteriafor reduction of hexavalent and trivalent chromium.Reduction occurred rapidly under normal membrane biofilm reactor denitrifying condition and Reduction is optimum near 7pH. The results show that the hydrogen based membrane biofilm reactor is a treatment technology for treating hexavalent chromium in wastewater.For effective hexavalent chromium removal, the critical operational parameters for maximizing reduction hexavalent chromium are hydrogen concentration, nitrate concentration and pH [9].Later Trond Austrheim.et al., (2008) Trametes Versicolor Polyporus fungi .The Absorption capacity of this trametes versicolor polyporus fungi was 125.0 mg/g. The maximum uptake of hexavalent chromium ions was occurred at pH 4.By increasing the amount of the biosorbent which increase the percentage of metal ions removal [16].
Surfactant cetyltrimethylammonium bromide was used as an adsorbent by Sadaoui.Z.et al., (2009) to remove the hexavalent chromium from wastewaters.The adsorption performances of cetyltrimethylammonium bromide was studied.The adsorption capacity of hexavalent chromium was increased with initial metal concentration and in a lesser extent with solution pH. However, it decreases with initial cetyltrimethylammonium bromide mass and with concentration of other ions present in the solution.The maximum capacities of metal adsorption as calculated as
17.89 mgg-1 and 13.85 mgg-1 using Langmuir adsorption isotherm at 450C [13] .
Alok Prasad Das,Susmita Mishra, (2010) implemented Microbial remediation Peptone Yeast Exatract and Brevibacterium Casei for hexavalent chromium removal.These bacterium detoxify the chromate present in contaminated wastes of industries and minings. This Brevibacterium Casei can effectively degrade hexavalent chromium upto 99% in 12hr at neutral pH and temperature of 30% [1].
Futher Activated sludge with powdered Activated carbon technique was adopted by Ferro Orozco A.M. et al., (2010) [4] .They observed that the hexavalent chromium removal in the combined activated sludge with powdered activated carbon system in comparison is high than individual Activated sludge treatments.Chromate removal was improved by increasing powdered activated carbon concentrations in both PAC and activated sludge with powdered activated carbon systems.
3.2 Chemical Technology:
Ramajeevan Ganeshjeevan,et al., (2003) investicates hexavalent chromium detection in the presence of a high
load of colourants using an on-line dialysis technique for ion chromatography.This method has been developed to remove water-soluble anionic dyes and particulate colourants and other substances to facilitate hexavalent chromium quantification. They found that this method has a detection limit of 5 mg/ l, recovery rate of 100% and analysis time less than 20 min [11].
Later Venkata Subbaiah.M.et al., (2006) inquiries coupled plasma mass spectrometry along with dynamic reaction cell with ammonia for detection. This method is used for determining ultra-trace levels of hexavalent chromium in ambient air. The method involves a 24-h sampling of air into potassium hydroxide solution, followed by silica gel column separation of hexavalent chromium, then preconcentration by complexation and solvent extraction. The hexavalent chromium complex was dissolved in nitric acid. The resultant chromium ions were determined by inductively coupled plasma mass spectrometry using a dynamic reaction cell with ammonia
9.3% of trivalent chromium converted to hexavalent chromium within 6 hrs [17].
Complex matrices such as crude oil and sludge was adopted by Safavi.A,Maleki.N., (2006). They implemented sensitive method for determination of chromium ion (VI) in complex matrices such as crude oil and sludge was presented based on the decreasing effect of hexavalent chromium on cathodic adsorptive stripping peak height of Cu–adenine complex. Under the optimum experimental conditions, a linear decrease of the peak current of Cu–adenine was observed, when the chromium (VI) concentration was increased from 5 µgL-1 to 120µgL-1. Trivalent chromium at concentrations10–
100fold excess of hexavalent chromium [14].
Turkish brown coals (Ilg n, Beys_ehir, and Ermenek) have been established for treating chromium by Gulsin Arslan, Erol Pehlivan, (2007). This method is simple, effective and economical means of wastewater treatment. The mechanism of hexavalent chromium ion binding to brown coals may include ion exchange, surface adsorption, chemisorption, complexation, and adsorption–complexation. Maximum adsorption capacity of 11.2 mM of hexavalent chromium /g for Ilg n, 12.4 mM of Hexavalent chromium /g for Beys_ehir, 7.4 mM of hexavalent chromium /g for Ermenek and 6.8 mM of Hexavalent chromium /g for activated carbon was achieved at pH of 3.0[6].
Tri-chamber bath system, titanium plated ruthenium anode,nafion quaternary cation has been adopted by JIANG Xiaojun et al., (2009) . This technique has higher current efficiency 34.7% and no health damage to the operators [8].
3.3 Mechanical Technology
Mass spectrometry analysis and wet scrubber has been adopted for carcenogenic component removal by
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 5, May-2011 4
ISSN 2229-5518
James R.Kastner and K.C.Das, (2002).Emission rate of
Sulfur compounds is reduced nearly 90 % [7].
Wet Scrubber Simulator System and Ontario Hydro Analysis were used as technique for examination by John C.S Chang,S.Behrooz Ghorishi , (2003). The model can be used to predict Hg0 increase across wet FGD scrubbers when the Hg2+ reduction and Hg0 emission in the scrubber is dominated by the mechanism.
Nout
5 CONCLUSION
Number concentration of liquid drops at the outlet (x = L) of the scrubber
Pilot- and full-scale tests are needed to validate the results from this current work which was conducted under simulated bench-scale conditions. This reduce S (IV) concentration enhances Hg0 and Hg2+ emission [10].
Amornpon Chandsuphan et al., (2003) explorize oxidizing agent along with packed bed wet scrubberwas meant for treating the chemicals. 99.10% of carcenogenic components were removed. Surfactants with packed tower scrubber reduce C10E4 (Naphthalene) nearly 60%. By using Pilot Scale Packed Bed scrubber along with oxidant 99.5%H2S was removed [2].
Residence time distribution approach has been used by Sarkar.S.et al., (2007) for developing a theoretical model for predicting the SO2 removal efficiency in a horizontal co-current gas–liquid scrubber by water spray. Experimental investigation shows that a very high percentage removal of SO2 can be achieved from air–SO2 mixture without using any additives or pre-treatment. Removal of sulfur compound efficiency lies in the range of 60-97% [15].
4 LIST OF SYMBOLS
TABLE1
Symbol Quantity

Pt Penetration
11 Collection efficiency
e Exponential function Function of the scrubbing

Various biological, chemical and mechanical method list different percentages of hexavalent chromium and trivalent chromium removal, among these Microbial remediation and Brevibacterium Casei removes hexavalent chromium upto 99% in 12hr.This is the maximum percentages of chemical removal as examined among the biological techonolgy upto now,Maximum adsorption capacity of 11.2 mM of hexavalent chromium
/g for Ilg n , 12.4 mM of hexavalent chromium /g for Beys_ehir , 7.4 mM of hexavalent chromium /g for Ermenek and 6.8 mM of hexavalent chromium /g has been observed by Gulsin Arslan.Among the chemical technology this Turkish brown coals produces maximum removal of chemicals. 99.10% of mercury is removed in the mechanical technoly using packed bed wet scrubber. The future work concentrates in the increase of trivalent and hexavalent chromium removal percentage.
REFERENCES
[1] Alok Prasad Das,Susmita Mishra, Biodegradation of the metallic carcinogen hexavalent chromium Hexavalent chromium by an indigenously isolated bacterial strain,Vol.9,Journal of carcinogenesis,2010.
[2] Amornpon Chandsuphan,Somrat Kerdsuwan,Vladimir, Efficiency of Mercury Removal in Packed Bed Wet Scrubber from Infectious Waste Incinerator, Vol.13, No.4, Journal of KMITNB,2003.
[3] Chih-Cheng Wu and Whel May Grace Lee, Control of Vaporous Naphthalene by Scrubbing with Surfactants, Vol.140, No.112,Journal of Environmental Engineeering,2004.
[4] Ferro Orozco A.M,Contreras E.M , N.E. Zaritzky, Effects of
f system
system variables
combining biological treatment and activated carbon on hexavalent chromium reduction, Vol.102,No.2495-
Cd Drag coefficient
K Gas Load Factor g Gravity field
dd Droplet diameter
A Projected area of the
d droplet,(IT d 2 /4 )
dd Droplet diameter
Densities of the gas and
2502,Bioresource Technology,2010.
[5] Graciela Rojas, Jorge Silva, Jaime A. Flores, Ang´elica Rodriguez,Martha Ly, Holger Maldonado, Adsorption of chromium onto cross-linked chitosan, Vol.44 ,31–36, Separation and Purification Technology,2005.
[6] Gulsin Arslan , Erol Pehlivan ,Batch removal of chromium(VI)
from aqueous solution by Turkish brown coals, Vol.98 ,2836–
2845, Bioresource Technology,2007.
[7] James R.Kastner and K.C.Das, Wet Scrubber Analysis of
pi and pg
U g,set
liquid
Terminal velocity relative to the gas
Volatile Organic Compound Removal in the Rendering Industry, Vol.5, 459-469,Journal of the air and waste management association,2002.
Fr Flow force
G d Gravity force
[8] JIANG Xiaojun,CHEN Wenchao, XU Hongbo, Elimination of a
pollution associated with chromic acid during the electro-
IJSER © 2011 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 2, Issue 5, May-2011 5
ISSN 2229-5518
deposition of Trivalent chromium using appropriate anodic and membrane materials in a double film bath,No.52-55,Journal of Environmental Sciences Supplement,2009.
[9] Jinwook Chung, Robert Nerenberg,Bruce.E.Rittmann, Bio- reduction of soluble chromate using a hydrogen-based membrane biofilm reactor,Vol.41 Journal of water research,2010.
[10] John C.S Chang,S.Behrooz Ghorishi ,Simulatiom and Evaluation of Elemetal Mercury Concentration Increase in Flue Gas Across e Wet Scrubber, Vol.37, 5763-5766, Environ. Sci. Technology,2003.
[11] Ramajeevan Ganeshjeevan, Raghavan Chandrasekar, Subramanian Yuvaraj,Ganga Radhakrishnan ,Determination of hexavalent chromium by on-line dialysis ion chromatography in a matrix of strong colourants and trivalent chromium, Vol.988 151–159,Journal of Chromatography ,2003.
[12] Raj Mohan.B. Comprehensive analysis for prediction of dust removal efficiencyusing twin-fluid atomization in a spray scrubber, Vol.63 269–277, Separation and Purification Technology,2008.
[13] Sadaoui.Z. , S. Hemidouche, O. Allalou , Removal of hexavalent chromium from aqueous solutions by micellar compounds, Vol.249 768–773, Desalination,2009.
[14] Safavi.A,Maleki.N. H.R. Shahbaazi, Indirect determination of hexavalent chromium ion in complex matrices by adsorptive stripping voltammetry at a mercury electrode, Vol.68 1113–
1119,Talanta,2006.
[15] Sarkar.S. B.C.Meikap,S.G .Chatterjee,Modeling of Removal of Sulfur dioxide from Flue Gases in a Horizontal Cocurrent Gas –Liquid Scrubber, Vol.131 263–271, Chemical Engineering Journal,2007.
[16] Trond Austrheim,Lars H. Gjertsen,Alex C. Hoffmann,An experimental investigation of scrubber internals at conditions of low pressure, Vol.138, 95–102, Chemical Engineering
Journal,2008.
[17]
VenkataSubbaiah.MS.Kalyani,G.SankaraReddy,VeeraM.Boddu, A.Krishnaiah, Biosorption of Hexavalent chromium from Aqueous Solution Using Trametes Versicolor Polyporus Fungi, Vol.5,No.3,499-510,E-Journal of chemistry,2006.
[18] Yarong Li, Narayan K. Pradhan, Roy Foley, Gary K.C. Low, Selective determination of airborne hexavalent chromium using inductively coupled plasma mass spectrometry, Vol.57 1143–
1153,Talanta,2002.
IJSER © 2011 http://www.ijser.org

