22. E Rajanarendar ,M Afzal & D Karunakar , Indian J. Chem,
42B,353, 2003.
23. F C Brown , C K Bradsher , E
C Morgan , M Tetenbaum & P Wilder , J. Am. Chem. Soc, 78,
384, 1956 .
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 2093
ISSN 2229-5518
Shalini Jaiswal1 , I. R. Siddiqui2 & Subhadra Rajput3
Department of Chemistry, DIT School of Engineering , Greater Noida, India
Email: shaliniajaiswal@gmail.com
Abstract : An expeditious mineral (Montmorillonite K-10 clay) catalysed cyclo- condensation of chloroacetic acid with arylthiourea followed by Knoevenagel condensation with aromatic aldehyde in presence of sodium acetate under solvent-free microwave irradiation yields 3-aryl-5-benzylideno-2-thiohydantoins 4(a-l). Compounds 4 (a-l) with hydrazine hydrate in glacial acetic acid furnishe the intermediate acetyl hydrazone of 4(a-l) which on cycloisomerisation under reaction conditions give 1,8-diaryl-4,8-dihydro-1-acetylpyrazolo[3,4-c]imidazol-2-thiones 5(a- l). On glycosylation with 1,2,3,5-tetra-O-acetyl-β-D-furanose in presence p-toluene sulfonic acid under microwave irradiation followed by deacetylation with NaOMe / MeOH compounds 5 (a-l) yields, 1,8-diaryl-3(β-D-ribofuranosyl)-4,8-dihydro-1- acetyl[3,4-c]imidazol-2-thione 6(a-l).
Keywords: Pyrazoloimidazol-2-thioneN-nucleoside, montmorillonite K-10 clay supported, Knoevenagel condensation ,solvent-free, microwave-irradiation, glycosylation , Green chemistry ,.
Introduction: Compounds with imidazole scaffolds have recently received attention because of their pharmacological properties.1-3 Most important of these are 2-thioxo- imidazolidinones which exhibit antiviral particularly anti HIV activity.4-5 Most available drugs approved by FDA for the treatment of AIDS include nucleoside analogues and protease inhibitor but no attempt has been made so far to synthesize nucleoside analogues incorporating 2-
thioxoimidazolidinones as nucleobase although they appear to be attractive structural class for exploiting chemical diversity and generating a drug like library to screen for lead candidates. Similarly pyrazole moiety containing compounds gained importance in recent years due to their antiviral, antibacterial and other interesting
biological effects.6 The study on the influence of structure on activity have shown that by fusing one heterocyclic moiety with other, most of the time the pharmacological profile was enhanced many folds than any one of the
heterocyclic moiety.7 The fight against
HIV by developing more efficacious multi-target drugs has been the driving force for fusing pyrazole moiety with and glycosylation of 2- thioxoimidazolidinones.
The application of molecular diversity technique to drug discovery is a multidisciplinary effort ranging from computational chemistry to engineering to organic synthesis to molecular biology. The main objective of the work described here is to provide an account of one aspect of molecular diversity based drug
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 2094
ISSN 2229-5518
discovery viz. the development of general synthetic strategy for generation of nucleoside analogues for screening of lead molecules for novel assays in the archives a chemicals
amassed through organic synthesis.8
With increasing global environmental concerns application of eco-friendly and mineral supported reagents, solvent-free reactions and microwave
irradiation techniques9-12 has increased
dramatically in recent years since by doing so use of expensive and hazardous organic solvents and reagents can be avoided significantly.13-16 Use of mineral supported reagents assisted by microwave irradiation under solvent- free conditions provide
environmentally benign protocol with additional advantages such as enhanced reaction rate, higher yields of pure product, easier workup, better selectivity, improved ease of manipulation, rapid optimization of reactions in parallel which fulfill basic principles of green chemistry.
Encouraged by above reports and as part of our research programme for development of eco-friendly synthetic protocol for biologically active
compounds17-21 as well as in pursuing
of our work on new solvent-free cyclisation process we developed a regioselective, novel, montmorillonite K-10 clay catalysed, microwave activated synthesis of hitherto unknown pyrazoloimidazol-2-thione N-nucleosides (Scheme 1). Interestingly it is the first example of microwave induced synthesis of pyrazoloimidazol-2-thione N- nucleosides. The key element in our approach is the utilization of α- haloacid as a bifunctional building block whose application to the construction of various heterocycles of
chemical and biological interest is well documented.22-23
Results &Discussion: An expeditious mineral (montomorillonite K-10clay) catalysed cyclocondensation of arylthiourea 1 with chloroacetic acid 2 under solvent-free microwave irradiation regioselectively gave 3- aryl-2-thioxoimidazolidin-4-one (Scheme 2) which on Knoevenagel condensation with aromatic aldehyde 3 in presence of CH3 COONa furnished
3-aryl-5-benzylideno-2-thiohydantoins
4a-l (Scheme 1) with 80-88% yields (Table I). Regioselectivity obtained in cyclocondensation of arylyhiourea with chloroacetic acid was due to difference in nucleophilicity of –NH2 and –NHAr groups. Additional delocalisation of electrons on aromatic ring in –NHAr makes it a poor nucleophile and hence –NH2 group by nucleophilic substitution of α-halogen of ClCH2 COOH resulted an intermediate which cyclised to produce
4 a-l (Scheme 2). Structure of 4 a-l was supported by IR and 1H NMR spectral analysis. Absorption bands in the region of 3300-3350 cm-1 for N-H,
3050 cm-1 for aromatic C-H, 1690 cm-1
for C=O, 1620 cm-1 for C=C, 1600,
1500, 1468 cm-1 for aromatic C-C,
1200 cm-1 for C=S and 1180 cm-1 for
C-N stretching in IR spectra and signals at δ 7.2 - 7.4 as multiplet for aromatic protons, δ 4.80 as singlet for vinylic proton of benzylideno group and δ 2.1 - 2.5 as singlet for –NH-
proton in 1H NMR spectra were
indicative of the synthesis of compounds 4a-l.
Other mineral supports viz. silica gel, neutral or basic alumina were far less effective resulting in either no reaction (in case of basic alumina) or relatively very low yields (15-30%) of 4 (in case of silica gel and neutral alumina). In order to compare the final temperature
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 2095
ISSN 2229-5518
was measured by immersing a glass thermometer into the reaction mixture immediately after MW irradiation and was found to be <88oC. The reactions
were also carried out using a thermostated oil-bath at the same temperature (88oC) as for the MW activated method but for a longer period of time (Table I). It was found that MW method has improved the yields significantly. MW enhancement of yields and reduction in reaction time
can be rationalized on the basis of the formation of dipolar activated complex I from uncharged reactants complex II from uncharged adduct in these reactions (Scheme 2) and greater stabilization of the more dipolar activated complex by dipole-dipole interaction with electric field of the microwaves as compared to the less polar adduct which may reduce the
activation energy [G#] resulting in the rate enhancement.Compounds 4a-l
with NH2 NH2. H2 O in glacial acetic acid produced acetyl hydrazones which on cycloisomerisation under reaction conditions at room temperature gave
1,8-diaryl-4,8-dihydro-1- acetylpyrazolo[3,4-c]imidazol-2- thiones 5a-l (Scheme 1 and 2) in 78-
86% yields (Table I). Absorption peaks at 3300-3350 cm-1 (N-H), 3050 cm-1 (Aromatic C-H), 1690 cm-1 (C=O), 1640 cm-1 (C=N), 1600, 1500,
1468 cm-1 (Aromatic C-C), 1200 cm-1
(C=S), in IR spectra and doublet at δ
2.0 for –NH- with J= 2.5 Hz, singlet at
δ 2.00-2.08 for –COCH3 , multiplet at δ
3.63 for H-4 and doublet at δ 5.1-5.6
with J= 3.2 Hz for H-8 and multiplet at δ 6.90-7.80 for aromatic protons supported the formation of 5 a-l.
Microwave assisted and p-CH3 - C6 H4 .SO3 H catalysed glycosylation of
5 a-l with 1,2,3,5-tetra-O-acetyl-β-D- ribofuranose followed by deacetylation
with NaOMe / MeOH yielded 1,8- diaryl-3-( β-D-ribofuranosyl)-4,8-
dihydro-1-acetylpyrazolo[3,4- c]imidazol-2-thiones 6 a-l (Scheme 1 and 2) in 75-88% yields (Table I). The structure of 6 a-l were also confirmed by spectral and elemental analysis. Spectra of all the synthesised compounds showed close similarity with spectral pattern of fused pyrazole moiety with 2-thioxoimidazolidine ring as well as β-D-ribofuranose sugar residue.Singlet at δ 2.02 for -COCH3 protons, doublets at δ 3.60-3.65 with J= 3.2 Hz and at δ 5.1-5.6 with J= 3.2
Hz due to H-4 and H-8 respectively
and multiplets at δ 6.46-8.14 due to aromatic protons in 1H NMR spectra of
at δ 3.65-3.80 due to four sugar
protons as well as doublets at δ 4.96
with J= 4.2 Hz due to anomeric proton and broad singlet at δ 2.00 exchangeable with D2 O due to three – OH group were indicative of β-D- ribofuranosyl moiety in 6 a-l.
In 13C NMR spectral analysis in the region δ 108-165 for aromatic carbons and at δ 165-175 for C=S and C=O carbons and at δ 61-178 for sugar carbons supported that all synthesized compound have pyrazoloimidazolidin-
2-thione N-ribofuranosidic skeleton.
420 spectrophotometer. 1H NMR and
13C NMR spectra were recorded at
400oC on a Bruker AVANCE DPX (400 MHz) FT spectrometer in CDCl3
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 2096
ISSN 2229-5518
using TMS as an internal reference (chemical shift in δ, ppm). Mass spectra were recorded on JEOL SX-
303 (FAB) mass spectrophotometer at
70ev. Elemental analyses were carried out using a Coleman automatic C,H,N analyser.
chloroacetic acid 2 aromatic aldehyde
3 (0.01 mol) and CH3 COONa (0.001 mol) in DMF (50 mL) was added montomorillonite K-10 clay (0.50 g) with constant stirring and the reaction mixture was refluxed on thermostated oil-bath at 90 oC for the time specified in Table I. Progress of the reaction was monitored by TLC (Hexane: MeOH; 6:4 v/v). After completion of
the reaction the reaction mixture was cooled and poured into water, filtered and the product was extracted with ethanol (3x50 mL). The extract was evaporated under reduced pressure to obtain the final product which was recrystallised from ethanol to get analytically pure compounds (4a-l).
1 (0.01mol) chloroacetice acid 2 (0.01 mol) aromatic aldehyde 3 (0.01 mol) and CH3 COONa (0.001 mol) in DMF (10ml) with thorough mixing and the solvent was evaporated under reduced pressure. The contents were taken in
20 ml vial and subjected to microwave irradiation at 600 W for 2 min. The reaction mixture was then thoroughly mixed outside the microwave oven for
2 min and again irradiated for another
2 min. This irradiation-mixing cycle
was repeated for the total irradiation time Table I. After completion of the reaction as indicated by TLC (Hexane : MeOH, 6:4 v/v) the product was
extracted with ethanol (3x8 mL). The extract was filtered and filtrate was evaporated under reduced pressure to obtain the product. The final product was recrystallised from ethanol to obtain analytically pure compounds (4a-l).
(0.001 mol), p-TsOH (0.03 g) and compound 5a-l (0.001 mol) were
mixed thoroughly and taken in 20 mL
vial. The reaction mixture was
subjected to microwave irradiation for
2 min and again irradiated for another
2 min. This irradiation-mixing cycle was repeated for the total irradiation time (Table I). After completion of reaction as indicated by TLC, the resulting oil was dissolved in absolute MeOH (1.5 mL) and allowed to stand for 1 h at room temperature to get the crude product. It was filtered, recrystallised from absolute MeOH and dried in vacuum to get the crude product (acylated N-nucleoside).
The acylated N-nucleosides (0.0016
mol) in dry MeOH (20 mL) and 1 mL solution of MeONa (prepared by adding 0.1 g Na in 20 mL of dry MeOH) were taken in 100 mL stoppered flask and the reaction mixture was allowed to stand for 3-4 h with occasional shaking. The resulting solution on neutralization with dil. HCl
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 2097
ISSN 2229-5518
yielded nucleosides 6a-l which were filtered and recrystallised from ethanol to get analytically pure samples.
1. P Franchetti, S Marchetti, L Capellacci,J A Yalowitz, H N
Jayaram, B M Goldstein & Grifantini M Grifantini, Bioorg
2. A M Agh-Atabay, B Dulger &
F Gucin, Eur. J. Med. Chem,
38,875, 2003.
3. E D Chrysina, M N Kosmopoulou, C Tiraidis, R Kardakaris, N Bischler, D D Leonidas, Z Hadady, L Somsak, T Docsa, P Gergely & N G Oikonomakos, Proteinsci,
14, 873, 2005.
4. A Rosario, J I Andres, M T
Lopez, F G Heras, R Herranz, B Alarcon & L Carrasco, J. Med. Chem, 28,834, 1985.1
5. D P C Mc Gee, J C Martin, D F Smee & J P H Verheyden, Nucleosides and Nucleotides,
9,815, 1990.
6. F Pancic, B A Stenberg, G D
Diana, P M Carabates, W G Gorman & P E Came, Antimicrob Agents Chemother,
19 (3),470, 1981.
7. M Pierrot, B Garrigus, J Fifani, Em Eessasi & B Djerrari, Indian J. Chem, 42 B,2820,
8. M Nuss John, Manoj C Manoj Desai, N Zuckermann Ronald, R Singh, S Subramanian , Pure
& Appl. Chem, 69 (3),447,
9. J Ugi & A Domling,
Endeavour, 18,115, 1994.
10. G A Krous & J O Nagy,
Tetrahedron, 41,3537, 1985.
11. I J Uji, Prakt. Chem, 339, 499,
12. A Loupy , A Petit ,J Hamelin ,
F Texier-Bouller ,P Jacquatt & D Mathe , Synthesis, 1213
13. R S Varma , Green Chem, I,
43, 1999.
14. M Balogh & P Laszlo,
Organic Chemistry Using
Clays; Springer; Berlin, 1993.
15. P Lidstrom ,J Tierney,B Wathey & J Westman, Tetrahedron, 57,9225, 2001.
16. H M Meshram , K C Shekharm
,Y S S Ganesh & J S Yadav ,
Synlett, 1273, 2000.
17. I RSiddiqui , P K Singh ,J Singh &.J Singh , J. Agri. And Food Chem, 51, 7062, 2003.
18. I R Siddiqui , P K Singh , J
Singh &.J Singh , J. Chem, Res, 8, 554, 2004.
19. JI R Siddiqui ,P K Singh ,J Singh &.J Singh , Indian J.
Chem, 44B,2102, 2005.
20. I R Siddiqui ,P K Singh , V
Srivastava & J Singh , Indian J. Chem, 44B,2178, 2005.
21. I R Siddiqui ,P K Singh ,J Singh & J. Singh , Indian J.
Heterocyclic Chem, 15,
375,2003.
22. E Rajanarendar ,M Afzal & D Karunakar , Indian J. Chem,
42B,353, 2003.
23. F C Brown , C K Bradsher , E
C Morgan , M Tetenbaum & P Wilder , J. Am. Chem. Soc, 78,
384, 1956 .![]()
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 2098
ISSN 2229-5518
![]()
Ar
![]()
S NHAr HO O
![]()
![]() + + Ar'
+ + Ar'
O

N
Montomorillonite K-10 clay S
CH3COONa
O NH2NH2.H2O
![]()
glacial CH COOH
NH2
X
MW, 5-8 min HN
Ar' 3
![]()

Ar AcO
OAc
![]()
O Ar

N
![]()
S N
![]()
HN N
COCH3
OAc OAc
TsOH
MW, 4-8 min
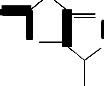
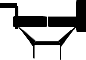
N S
AcO N O
N
![]()
N COCH3
Ar'
OAc OAc
![]()
Ar'
NaOMe / MeOH (Dry)
![]()
Ar


N S
HO N O
N
![]()
N COCH3
OH OH
Ar'
Compd | Ar | Ar' | Compd | Ar | Ar' |
4a | C6 H5 | C6 H5 | 5g | 4-Me- C6 H4 | C6 H5 |
4b | C6 H5 | 4-Cl-C6 H4 | 5h | 4-Me- C6 H4 | 4-Cl-C6 H4 |
4c | C6 H5 | 4-HO-C6 H4 | 5i | 4-Me- C6 H4 | 4-HO- C6 H4 |
4d | C6 H5 | 4-MeO- C6 H4 | 5j | 4-Me- C6 H4 | 4-MeO- C6 H4 |
4e | C6 H5 | 2-NO2 - C6 H4 | 5k | 4-Me- C6 H4 | 2-NO2 - C6 H4 |
4f | C6 H5 | 3-MeO, 4-HO- C6 H3 | 5l | 4-Me- C6 H4 | 3-MeO, 4-HO-C6 H3 |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 2099
ISSN 2229-5518
4g | 4-Me- C6 H4 | C6 H5 | 6a | C6 H5 | C6 H5 |
4h | 4-Me- C6 H4 | 4-Cl-C6 H4 | 6b | C6 H5 | 4-Cl-C6 H4 |
4i | 4-Me- C6 H4 | 4-HO- C6 H4 | 6c | C6 H5 | 4-HO- C6 H4 |
4j | 4-Me- C6 H4 | 4-MeO- C6 H4 | 6d | C6 H5 | 4-MeO- C6 H4 |
4k | 4-Me- C6 H4 | 2-NO2 - C6 H4 | 6e | C6 H5 | 2-NO2 - C6 H4 |
4l | 4-Me- C6 H4 | 3-MeO, 4-HO- C6 H3 | 6f | C6 H5 | 3-MeO, 4-HO-C6 H3 |
5a | C6 H5 | C6 H5 | 6g | 4-Me- C6 H4 | C6 H5 |
5b | C6 H5 | 4-Cl-C6 H4 | 6h | 4-Me- C6 H4 | 4-Cl-C6 H4 |
5c | C6 H5 | 4-HO- C6 H4 | 6i | 4-Me- C6 H4 | 4-HO- C6 H4 |
5d | C6 H5 | 4-MeO- C6 H4 | 6j | 4-Me- C6 H4 | 4-MeO- C6 H4 |
5e | C6 H5 | 2-NO2 - C6 H4 | 6k | 4-Me- C6 H4 | 2-NO2 - C6 H4 |
5f | C6 H5 | 3-MeO, 4-HO- C6 H3 | 6l | 4-Me- C6 H4 | 3-MeO, 4-HO-C6 H3 |
Table 1: Physical and spectral data of compounds 4 a-l, 5 a-l and 6 a-l
Cmp d | Mol.Formulaa (Mol. wt) | Time | Yield b(%) | m.p . (oC) | IR (KBr, υ cm-1) | 1H NMR (CDCl 3, δ, ppm) | MS (EI, m/z (M+) | |||
MW (min) | Thermal (h) | M W | Thermal | |||||||
4a | C16 H 12N 2 OS (280) | 6 | 4 | 82 | 32 | 225 | 3320, 3050, 1690, 1620, 1600,1500, 1468, 1200, 1180 cm- 1 | δ 2.1 (s, 1H, -NH-), 4.8 (s, 1H, -C=CHAr), 7.2- 7.4 (m, 10 H, Ar-H) | 280 (M+) | |
4b | C16 H 11NOSCl (314.5) | 7 | 5 | 81 | 32 | 240 | 3330, 3052, 1695, 1620, 1600, 1500, 1460, 1210, 1190 cm- 1 | δ 2.4 (s, 1H, -NH-), 5.2 (s, 1H, -C=CHAr), 7.21-7.51 (m, 9H, Ar- H) | 314.5(M+) | |
4c | C16 H 12N 2 O 2S (296) | 5 | 3 | 84 | 34 | 130 | 3320, 3050, 2530, 1685, 1618, 1600, 1502, 1469, 1205, 1185 cm-1 | δ 2.2 (s, 1H, -NH-), 4.4 (s, 1H, -OH exchangeable with D2 O), 4.78 (s, 1H, -C=CHAr), 6.9-7.4 (m, 9H, Ar-H) | 296.12(M+) | |
4d | C17 H 14N 2 O 2S (310) | 5 | 3 | 88 | 35 | 210 | 3320, 3050, 3000, 1690, 1620, 1600, 1500, 1468, 1200, 1180, 1120 cm-1 | δ 2.3 (s, 1H, -NH-), 3.3 (s, 3H, -OCH3), 4.82 (s, 1H, -C=CHAr), 7.07-7.76 (m, 9H, Ar-H) | 310(M+). | |
4e | C16 H 11N 3 O 3S (325) | 8 | 6 | 80 | 30 | 245 | 3330, 3045, 1690, 1625, 1600, 1504, 1470, 1210, 1190 cm- 1 | δ 2.5 (s, 1H, -NH-), 4. 2 (s, 1H, -C=CHAr), 6.92-7.40 (m, 9H, Ar- H) | 325.13(M+) | |
4f | C17 H 14N 2 O 3S (326) | 5 | 3 | 87 | 34 | 195 | 3320, 3050, 3000, 2520, 1690, 1620, | δ 2.1 (s, 1H, -NH-), 3.3 (s,3H, OCH3), 4.4 (brs, | 326.10(M+) |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 2100
ISSN 2229-5518
4g | C17 H 14N 2 OS | 6 | 4 | 82 | 31 | 230 | 3320, | 3050, | 2960, 1H NMR (CDCl 3): δ 294.16(M+) | |
(294) | 1690, | 1620, | 1600, 1.32 (s, 3H, -CH3), 2.1 | |||||||
1500, | 1468, | 1200, (s, 1H, -NH-), 4. 8 (s, | ||||||||
1180 cm-1 | 1H, | |||||||||
-C=CHAr), 7.2-7.4 (m, | ||||||||||
4h | C17 H 13N 2 OSC | 7 | 6 | 83 | 33 | 248 | 3330, 3052, | 2960, | 9H, Ar-H) δ 1.31 (s, 3H, -CH3), | 328.10(M+) |
l | 1695, 1620, | 1600, | 2.4 (s, 1H, -NH-), 5.2 |
(328.5)
1500, 1460, 1210,
1190 cm-1
(s, 1H, -C=CH Ar),
7.21-7.51 (m, 8H, Ar-H
4i | C17 H 14N 2 O 2S | 6 | 4 | 86 | 33 | 180 | 3320, | 3050, | 2950, | δ 1.32 (s, 3H, -CH3), | 310.14(M+) |
(310) | 2540, | 1685, | 1618, | 2.2 (s, 1H, -NH-), 4.4 | |||||||
1600, | 1502, | 1469, | (s, 1H, -OH | ||||||||
1205, 1 | 185 cm | -1 | exchangeable with | ||||||||
D2 O), 4. 78 (s, 1H, | |||||||||||
-C=CHAr), 6.9-7.4 (m, | |||||||||||
4j | C18 H 16N 2 O 2S | 6 | 4 | 87 | 34 | 220 | 3320, | 3050, | 3000, | 8H, Ar-H) δ 1.31 (s, 3H, -CH3), | 324.18(M+) |
(324) | 2950, | 1690, | 1620, | 2.3 (s, 1H, -NH-), 3.3 | |||||||
1600, | 1500, | 1468, | (s, 3H,-OCH3 ), 4.82 |
1200, 1180, 1120 cm-
1
(s, 1H,
-C=CHAr), 7.07-7.76 (m, 8H, Ar-H
1504, 1470, 1210,
1190 cm-1
(s, 1H, -C=CHAr),
6.92-7.40 (m, 8H, Ar-H
4l | C18 H 16N 2 O 3S | 5 | 4 | 88 | 35 | 200 | 3320, | 3050, | 3000, | δ 1.30(s, 3H, -CH3), | 340.15(M+) |
(340) | 2960, | 2520, | 1690, | 2.1(s, 1H, -NH-), 3.3 | |||||||
1620, | 1600, | 1500, | (s, 3H, -OCH3 ), 4.4 (s, | ||||||||
1468, 1200, 1180, 1H, -OH exchangeable 1120 cm-1 with D2O), 4. 8 (s, 1H,- |
C=CHAr),7.1-7.9(m,
7H, Ar-H)
Compd | Ar' | Mol.Formulaa (Mol. wt) | Yield b (%) | m.p. (oC) | IR (KBr, υ cm-1) | 1H NMR (400 MHz,CDCl 3, δ, ppm) | MS (EI, m/z (M+) |
MW | |||||||
5a | C6 H 5 | C18 H 16N 4 OS (336) | 78 | 112 | 3320, 3050, 1690, 1640, 1600,1500, 1468, 1200, 1180 cm- 1 | δ 2.0 (d, 1H, J= 2.5 Hz, - NH-), 2.02 (s, 3H, - COCH3 ), 3.6 (m, 1H, H-4), 5.1 (d, 1H, J= 3.2 Hz, H-8), 6.46-7.27 (m, 10 H, Ar-H) | 336.10(M+) |
5b | 4-Cl- C6 H 4 | C18 H 15ClN4 OS (370.5) | 76 | 122 | 3330, 3050, 1690, 1640, 1600,1500, 1468, 1200, 1180 cm- 1 | δ 2.0 (d, 1H, J= 2.5 Hz, - NH-), 2.02 (s, 3H, - COCH3 ), 3.6 (m, 1H, H-4), 5.1 (d, 1H, J= 3.2 Hz, H-8), 6.46-7.22 (m, 9 H, Ar-H) | 370.5(M+) |
5c | 4-HO- C6 H 4 | C18 H 16 N 4 O 2S (352) | 80 | 124 | 3320, 3050, 2900, 2560, 1690, 1640, 1600,1500, 1468, 1200, 1180 cm-1 | δ 2.0 (d, 1H, J= 2.5 Hz, - NH-), 2.02 (s, 3H, - COCH3 ), 3.6 (m, 1H, H-4), 5.0 (brs, 1H, ArOH exchangeable with D2O), 5.1 (d, 1H, J= 3.2 Hz, H-8), 6.46-7.01 (m, 9 H, Ar-H) | 352.14(M+) |
5d | 4-MeO- | C19 H 18 N 4 O 2S | 82 | 119 | 3320, 3050, 2930, 1690, 1640, | δ 2.0 (d, 1H, J= 2.5 Hz, - | 366.16(M+) |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 2101
ISSN 2229-5518
C6 H 4 (366) 1600,1500, 1468, 1200, 1180,
1120 cm-1
NH-), 2.02 (s, 3H, - COCH3 ), 3.6 (m, 1H, H-4),
3.73 (s, 3H, -OCH3),5.1 (d,
1H, J= 3.2 Hz, H-8), 6.46-
7.01 (m, 9 H, Ar-H)
5e 2-NO2 - C6 H 4
5f 3-MeO,
4-HO- C6 H 3
C18 H 15 N 5 O 3S (381)
C19 H 18 N 4 O 3S (381)
73 115 3320, 3050, 1690, 1640,
1600,1500, 1468, 1200, 1180, cm-1
86 130 3320, 3050, 2930, 2560, 1690,
1640, 1600,1500, 1468, 1200,
1180, 1120 cm-1
δ 2.0 (d, 1H, J= 2.5 Hz, - NH-), 2.02 (s, 3H, - COCH3 ), 3.6 (m, 1H, H-4),
5.1 (d, 1H, J= 3.2 Hz, H-8),
6.46-8.14 (m, 9 H, Ar-H)
δ 2.0 (d, 1H, J= 2.5 Hz, - NH-), 2.02 (s, 3H, - COCH3 ), 3.6 (m, 1H, H-4),
3.73 (s, 3H, -OCH3 ), 5.0 (brs, 1H, ArOH exchangeable with D2O),
5.1 (d, 1H, J= 3.2 Hz, H-8),
6.46-7.01 (m, 8H, Ar-H)
381(M+)
381.15(M+)
5g C6 H 5 C19 H 18 N 4 OS (350)
80 110 3320, 3050, 2930, 1690, 1640,
1600,1500, 1468, 1200, 1180, cm-1
δ 2.0 (d, 1H, J= 2.5 Hz, - NH-), 2.02 (s, 3H, - COCH3 ), 2.35 (s, 3H, - CH3 ),3.6 (m, 1H, H-4), 5.1 (d, 1H, J= 3.2 Hz, H-8),
6.46-7.21 (m, 9H, Ar-H)
350.10(M+)
5h 4-Cl- C6 H 4
5i 4-HO- C6 H 4
5j 4-MeO- C6 H 4
5k 2-NO2 - C6 H 4
5l 3-MeO,
4-HO- C6 H 3
C19 H 17ClN4 OS (384)
C19 H 18N 2 O 2S (366)
C20 H 20N 4 O 2S (380)
C19 H 17N 5 O 3S (395)
C20 H 20N 4 O 3S (395)
85 118 3330, 3050, 2940, 1690, 1640,
1600,1500, 1468, 1200, 1180, cm-1
86 123 3320, 3050, 2940, 2900, 2560,
1690, 1640, 1600,1500, 1468,
1200, 1180, cm-1
87 126 3320, 3050, 2930, 2900, 1690,
1640, 1600,1500, 1468, 1200,
1180, 1120 cm-1
83 128 3320, 3050, 2930, 1690, 1640,
1500, 1468, 1200, 1180, cm-1
88 132 3320, 3050, 2930, 2900, 2560,
1690, 1640, 1600, 1500, 1468,
1200, 1180, 1120 cm-1
δ 2.0 (d, 1H, J= 2.5 Hz, - NH-), 2.02 (s, 3H, - COCH3 ), 2.35 (s, 3H, - CH3 ),3.6 (m, 1H, H-4), 5.1 (d, 1H, J= 3.2 Hz, H-8),
6.46-7.22(m, 8H, Ar-H).
δ 2.0 (d, 1H, J= 2.5 Hz, - NH-), 2.02 (s, 3H, - COCH3 ), 2.35 (s, 3H, - CH3 ),3.6 (m, 1H, H-4), 5.0 (brs, 1H, ArOH exchangeable with D2O),5.1 (d, 1H, J= 3.2 Hz, H-8),
6.46-7.01(m, 8H, Ar-H).
δ 2.0 (d, 1H, J= 2.5 Hz, - NH-), 2.02 (s, 3H, - COCH3 ), 2.35 (s, 3H, - CH3 ),3.6 (m, 1H, H-4), 3.73 (s, 3H, -OCH3), 5.1 (d, 1H, J= 3.2 Hz, H-8), 6.46-
7.01(m, 8H, Ar-H).
δ 2.0 (d, 1H, J= 2.5 Hz, -
NH-), 2.02 (s, 3H, - COCH3 ), 2.35 (s, 3H, - CH3 ),3.6 (m, 1H, H-4), 3.73 (s, 3H,
-OCH 3), 5.1 (d, 1H, J= 3.2
Hz, H-8), 6.46-8.14(m, 8H,
Ar-H).
δ 2.0 (d, 1H, J= 2.5 Hz, -
NH-), 2.02 (s, 3H, - COCH3 ), 2.35 (s, 3H, - CH3 ), 3.6 (m, 1H, H-4),
3.73 (s, 3H, -OCH3 ), 5.0 (brs, 1H, ArOH exchangeable with D2O),
5.1 (d, 1H, J= 3.2 Hz, H-8),
6.46-7.01(m, 7H, Ar-H).
384.5(M+)
366.14(M+)
380.16(M+)
395(M+)
395.15(M+)
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 2102
ISSN 2229-5518
Compd | Ar | Ar' | Mol.Formulaa (Mol. wt) | Time | Yield b (%) | m.p. (oC) | 1H NMR (400 MHz,CDCl 3, δ, ppm) | 13C NMR (100 MHz,CDCl 3, δ, ppm) | MS (EI, m/z (M+) |
MW (min) | MW | ||||||||
6a | C6 H 5 | C6 H 5 | C23 H 24N 4 O 5S (468) | 4 | 78 | 110 | δ 2.0(brs, 3H, 3 x OH exchangeable with D2O), 2.02 (s, 3H, -COCH3 ), 3.6 (d, 1H, J= 3.2 Hz, H-4), 3.65-3.88 (m, 4H, H-2', H- 3'', 2 x H-5'), 3.92 (m, 1H, H-4'), 4.96 (d, 1H, J= 4.2 Hz, H-1'), 5.1 (d, 1H, J= 3.2 Hz, H-8), 6.46-7.12 (m, 10 H, Ar-H) | δ 125.3-142.4 (Aromatic carbons), 171(C=O), 155 (C=N-), 180.9 (C=S), 85.2 (C- 1'), 76.6 (C-3'), 76.3 (C-4'), 76.0 (C-2'), 61.9 (C-5'), 49.6 (C-4), 49.4 (C-8), 18.1 (-COCH3 ). | 468.15(M+) |
6b | C6 H 5 | 4-Cl- C6 H 4 | C23 H 23ClN4 O 5S (502) | 5 | 77 | 120 | δ 2.0(brs, 3H, 3 x OH exchangeable with D2O), 2.02 (s, 3H, -COCH3), 3.6 (d, 1H, J= 3.2 Hz, H-4), 3.65-3.88 (m, 4H, H-2', H- 3'', 2 x H-5'), 3.92 (m, 1H, H-4'), 4.96 (d, 1H, J= 4.2 Hz, H-1'), 5.1 (d, 1H, J= 3.2 Hz, H-8), 6.46-7.22 (m, 9 H, Ar-H) | δ 180.9 (C=S), 171(C=O), 155 (C=N- ),125.3-140.5(Aromatic carbons), 85.2 (C-1'), 76.6 (C-3'), 76.3 (C-4'), 76.0 (C-2'), 61.9 (C-5'), 49.6 (C-4), 49.4 (C-8), 18.1 (-COCH3 ). | 502.11(M+) |
6c | C6 H 5 | 4-OH- C6 H 4 | C23 H 24 N 4 O 6S (484) | 5 | 80 | 116 | δ 2.0(brs, 3H, 3 x OH exchangeable with D2O), 2.02 (s, 3H, -COCH3), 3.6 (d, 1H, J= 3.2 Hz, H-4), 3.65-3.88 (m, 4H, H-2', H- 3'', 2 x H-5'), 3.92 (m, 1H, H-4'), 4.96 (d, 1H, J= 4.2 Hz, H-1'), 5.0 (brs, 1s, Ar- OH exchangeable with D2 O),5.2 (d, 1H, J= 3.2 Hz, H-8), 6.46-7.01 (m, 9 H, Ar-H) | δ 180.9 (C=S), 171(C=O), 155 (C=N- ),125.3-155.3 (Aromatic carbons), 85.2 (C-1'), 76.6 (C-3'), 76.3 (C-4'), 76.0 (C-2'), 61.9 (C-5'), 49.6 (C-4), 49.4 (C-8), 18.1 (- COCH3 ). | 484.14(M+) |
6d | C6 H 5 | 4-OMe- C6 H 4 | C24 H 26 N 4 O 6S (498) | 5 | 86 | 115 | δ 2.0(brs, 3H, 3 x OH exchangeable with D2O), 2.02 (s, 3H, -COCH3 ), 3.6 (d, 1H, J= 3.2 Hz, H-4), 3.65-3.88 (m, 4H, H-2', H- 3', 2 x H-5'),3.72 (s, 3H, - OCH3 ), 3.92 (m, 1H, H- 4'), 4.96 (d, 1H, J= 4.2 Hz, H-1'), 5.1 (d, 1H, J= 3.2 Hz, H-8), 6.46-7.01 (m, 9 H, Ar-H) | δ 180.9 (C=S), 171(C=O), 155 (C=N- ),125.3-160.0 (Aromatic carbons), 85.2 (C-1'), 76.6 (C-3'), 76.3 (C-4'), 76.0 (C-2'), 61.9 (C-5'), 56.0 (- OCH3 ), 49.6 (C-4), 49.4 (C-8), 18.1(- COCH3 ). | 498.5(M+) |
6e | C6 H 5 | 2-NO2 - C6 H 4 | C23 H 23 N 5 O 7S (513) | 8 | 75 | 118 | δ 2.0(brs, 3H, 3 x OH exchangeable with D2O), 2.02 (s, 3H, -COCH3 ), 3.6 (d, 1H, J = 3.2 Hz, H-4), 3.65-3.88 (m, 4H, H-2', H- 3', 2 x H-5'), 3.91 (m, 1H, H-4'), 4.96 (d, 1H, J = 4.2 Hz, H-1'), 5.1 (d, 1H, J = 3.2 Hz, H-8), 6.46-8.14 (m, 9 H, Ar-H). | δ 180.9 (C=S), 171(C=O), 155 (C=N- ),125.3-156.2 (Aromatic carbons), 85.3 (C-1'), 76.6 (C-3'), 76.3 (C-4'), 76.0 (C-2'), 61.9 (C-5'), 49.6 (C-4), 49.4 (C-8), 18.1(- COCH3 ). | 513.13(M+) |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013 2103
ISSN 2229-5518
Hz, H-1'), 5.0 (brs, 1s, Ar- OH exchangeable with | 49.6 (C-4), COCH3 ). | 18.1(- | ||||||||
D2 O), 5.1 (d, 1H, J = 3.2 | ||||||||||
Hz, H-8), 6.46-7.07 (m, 8 | ||||||||||
6g | 4- | C6 H 5 | C24 H 26 N 4 O 5S | 4 | 79 | 114 | H, Ar-H). δ 2.0(brs, 3H, 3 x OH | δ 180.9 | (C=S), | 482.16(M+) |
Me- | (482) | exchangeable with D2O), | 171(C=O), 15 | 5 (C=N- | ||||||
C6 H 4 | 2.02 (s, 3H, -COCH3), | ),125.2-142.4 | ||||||||
2.35 (s, 3H, -CH3), 3.6 (d, | (Aromatic | carbons), | ||||||||
1H, J = 3.2 Hz, H-4), 3.65- | 85.2 (C-1'), 76.6 (C-3'), | |||||||||
3.88 (m, 4H, H-2', H-3', 2 | 76.3 (C-4'), 76.0 (C-2'), | |||||||||
x H-5'), 3.91 (m, 1H, H- | 61.9 (C-5'), 49.6 (C-4), | |||||||||
4'), 4.96 (d, 1H, J = 4.2 | 49.4(C-8), 20.9 (-CH3), | |||||||||
Hz, H-1'), 5.1 (d, 1H, J = | 18.1(-COCH3). | |||||||||
3.2 Hz, H-8), 6.34-7.21 | ||||||||||
6h | 4- | 4-Cl- | C24 H 25ClN4 O 5S | 5 | 77 | 124 | (m, 9 H, Ar-H). δ 2.0 (brs, 3H, 3 x OH | δ 180.9 (C=S), | 516.11(M+) | |
Me- | C6 H 4 | (516) | exchangeable with D2O), | 171(C=O), 155 (C=N- | ||||||
C6 H 4 | 2.02 (s, 3H, -COCH3), | ),125.2-140.5 | ||||||||
2.35 (s, 3H, -CH3), 3.6 (d, | (Aromatic carbons), | |||||||||
1H, J = 3.2 Hz, H-4), 3.65- | 85.2 (C-1'), 76.6 (C-3'), | |||||||||
3.88 (m, 4H, H-2', H-3', 2 | 76.3 (C-4'), 76.0 (C-2'), | |||||||||
x H-5'), 3.91 (m, 1H, H- | 61.9 (C-5'), 49.6 (C-4), | |||||||||
4'), 4.96 (d, 1H, J = 4.2 | 49.4(C-8), 20.9 (-CH3), | |||||||||
Hz, H-1'), 5.1 (d, 1H, J = | 18.1(-COCH3). | |||||||||
3.2 Hz, H-8), 6.46-7.22 | ||||||||||
6i | 4- | 4-OH- | C24 H 26N 4 O 6S | 4 | 84 | 122 | (m, 8 H, Ar-H). δ 2.0(brs, 3H, 3 x OH | δ 180.9 (C=S), | 498.14(M+) | |
Me- | C6 H 4 | (498) | exchangeable with D2O), | 171(C=O), 155 (C=N- | ||||||
C6 H 4 | 2.02 (s, 3H, -COCH3), | ),125.5-155.5 | ||||||||
2.35 (s, 3H, -CH3), 3.6 (d, | (Aromatic carbons), | |||||||||
1H, J = 3.2 Hz, H-4), 3.65- | 85.2 (C-1'), 76.6 (C-3'), | |||||||||
3.88 (m, 4H, H-2', H-3', 2 | 76.3 (C-4'), 76.0 (C-2'), | |||||||||
x H-5'), 3.91 (m, 1H, H- | 61.9 (C-5'), 49.6 (C-4), | |||||||||
4'), 4.96 (d, 1H, J = 4.2 | 49.4(C-8), 20.9 (-CH3), | |||||||||
Hz, H-1'), 5.0 (brs, 1s, Ar- | 18.1(-COCH3). | |||||||||
OH exchangeable with | ||||||||||
D2 O), 5.2 (d, 1H, J = 3.2 | ||||||||||
Hz, H-8), 6.46-6.95 (m, 8 | ||||||||||
6j | 4- | 4-OMe- | C25 H 28N 4 O 6S | 4 | 86 | 124 | H, Ar-H). δ 2.0(brs, 3H, 3 x OH | δ 180.9 (C=S), | 512.17(M+) | |
Me- | C6 H 4 | (512) | exchangeable with D2O), | 171(C=O), 155 (C=N- | ||||||
C6 H 4 | 2.02 (s, 3H, -COCH3), | ),113.0-160.0 | ||||||||
2.35 (s, 3H, -CH3), 3.6 (d, | (Aromatic carbons), | |||||||||
1H, J = 3.2 Hz, H-4), 3.65- | 85.2 (C-1'), 76.6 (C-3'), | |||||||||
3.88 (m, 4H, H-2', H-3', 2 | 76.3 (C-4'), 76.0 (C-2'), | |||||||||
x H-5'), 3.73 (s, 3H, - | 61.9 (C-5'), 56.0 (- | |||||||||
OCH3 ), 3.91 (m, 1H, H- | OCH3 ), 20.9 (-CH3), | |||||||||
4'), 4.96 (d, 1H, J = 4.2 | 18.1(-COCH3). | |||||||||
Hz, H-1'), 5.1 (d, 1H, J = | ||||||||||
3.2 Hz, H-8), 6.34-7.07 | ||||||||||
6k | 4- | 2-NO2 - | C24 H 25N 5 O 7S | 8 | 80 | 130 | (m, 8 H, Ar-H). δ 2.0(brs, 3H, 3 x OH | δ 180.9 (C=S), | 528.16(M+) | |
Me- | C6 H 4 | (528) | exchangeable with D2O), | 171(C=O), 155 (C=N- | ||||||
C6 H 4 | 2.02 (s, 3H, -COCH3), | ),125.2-142.0 | ||||||||
2.35 (s, 3H, -CH3), 3.6 (d, | (Aromatic carbons), | |||||||||
1H, J = 3.2 Hz, H-4), 3.65- | 85.2 (C-1'), 76.6 (C-3'), | |||||||||
3.88 (m, 4H, H-2', H-3', 2 | 76.3 (C-4'), 76.0 (C-2'), | |||||||||
x H-5'), 3.91 (m, 1H, H- | 61.9 (C-5'), 49.6 (C-4), | |||||||||
4'), 4.96 (d, 1H, J = 4.2 | 49.4(C-8), 20.9 (-CH3), | |||||||||
Hz, H-1'), 5.1 (d, 1H, J = | 18.1(-COCH3). | |||||||||
3.2 Hz, H-8), 6.34-7.60 | ||||||||||
6l | 4- | 3-OMe, | C25 H 28N 4 O 7S | 4 | 88 | 131 | (m, 8 H, Ar-H). δ 2.0(brs, 3H, 3 x OH | δ 180.9 (C=S), | 528.17(M+) | |
Me- | 4-OH- | (528) | exchangeable with D2O), | 171(C=O), 155 (C=N- | ||||||
C6 H 4 | C6 H 3 | 2.02 (s, 3H, -COCH3), | ),116.5-140.9 | |||||||
2.35 (s, 3H, -CH3), 3.6 (d, | (Aromatic carbons), |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 9, September-2013
ISSN 2229-5518
1H, J = 3.2 Hz, H-4), 3.65-
3.88 (m, 4H, H-2', H-3', 2 x H-5 , 3. 73 (s, 3H, - OCH 3),3.91 (m, 1H, H-4'),
4.96 (d, 1H, J = 4.2 Hz, H-
1 ' 5.0 (brs, 1s, Ar-OH
exchangeable with D2 0),
5.2 (d, 1H, J = 3.2 Hz, H-
8), 6.34-6.81 (m, 7H, Ar
H).
2104
85.2 (C-1'), 76.6 (C-3'),
76.3 (C-4'), 76.0 (C-2'),
61.9 (C-5'), 56.0 ( OCH3), 49.7 (H-8),
49.6 (C-4), 20.9 (
CH3), 18.1(-COCH3).
IJSER 2013