International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 294
ISSN 2229-5518
Distribution of trace elements and heavy metals in liver, lung, meat, heart and kidney of cattle, sheep, camel and equine slaughtered in
Casablanca city-Morocco
ChafiK Abdelbasset(1), Eddoha Rabia(2), Bagri Abdallah(1), Nasser Boubker(1) and Essamadi AbdelKhalid(1)
Abstract— The aim of this study was to determine the concentrations of trace elements (copper and zinc) and heavy metals (cadmium and lead) in the different organs of animals slaughtered in municipal slaughterhouse of Casablanca, which is the main source of meat consumption in the study area. Samples of liver, lung, meat, heart and kidney of cattle, sheep, camel and equine were collected. All the samples were digested, mineralized and analyzed for copper, zinc, cadmium and lead using an Inductively Coupled Plasma - Atomic Emission Spectroscopy. The concentrations of trace elements and heavy metals in the liver, lung, meat, heart and kidney of cattle, sheep, camel and equine ranged from 0.78±0.09 to 22±7.07 mg/kg for copper; 0.27±0.27 to 10.88±1.73 mg/kg for zinc; 0±0 to 0.86±0.14 mg/kg for cadmium; and 0.23±0.006 to 1.33±0.29 mg/kg for lead. Concerning concentrations of trace elements, cattle accumulate in their organs the highest concentrations of copper compared to other species. The highest concentrations of copper were observed in liver. The organs of cattle and camel are the richest in zinc in comparison with sheep and equine. However, zinc accumulated mainly in liver and meat. W ith regards to concentrations of heavy metals, camel and equine were deemed contaminated with cadmium compared to cattle and sheep. Cadmium concentrations in kidney and liver were higher than that observed in other organs. Cattle, sheep and camel were deemed the most contaminated by lead with the highest concentrations observed in liver.
Index Terms— Trace elements, heavy metals, organs, animal, consumption, Casablanca.
—————————— ——————————
N recent years, consumption of animal proteins by Moroccan population increased regularly. A great part of nutrients, including the necessary trace elements is provided by slaughtered animals [1], [2], [3], [4]. These, may also accumulate heavy metals which may be a potential health
hazard to humans as consumers [5], [6], [7], [8].
Copper (Cu) and zinc (Zn) remains among the essential trace elements and are essential for the proper functioning of the body [9], [10], [11], [12], [13]. Although they are found only in very low quantities, copper and zinc are implicated in the activity of many enzymes involved in many metabolic processes, and consequently, they ensure the proper functioning of many physiological activities [14], [15], [16], [17], [18]. Copper and zinc deficiency leads to a wide variety of pathological consequences and metabolic defects [19]. The distribution of copper and zinc among the tissues of animals varies with the age, sex, diet composition and physiological status [20].
However, cadmium (Cd) and lead (Pb) are the heavy metals among the most toxic substances present in the environment.
————————————————
• (1) Laboratory of Biochemistry and Neuroscience-Team Biochemistry and Toxicology Applied, University Hassan First / FST Settat, BP 577 Settat, Morocco
• (2) Laboratory of Biochemistry, Nutrition and Value of Natural Resources, University Chouaib Doukkali-Faculty of Sciences El Jadida, Ben Maachou
24000 El Jadida, Morocco
Natural geologic or anthropogenic industrial sources have been largely responsible for environmental pollution with heavy metals [21]. Environmental pollution with heavy metals is considered as one of the most important problems as these metals cannot be degraded and remain permanently in the environment [22]. Finally, heavy metals appear in food chain when they are accumulated in plant and animal tissues [11], [23], [24], [25]. One of the earliest effects is the disruption of trace element metabolism [26], [27], [28]. Farm animals reared freely on pasture might be the indicators of environmental contamination with heavy metals [29], [30], [31], [32]. The heavy metals bio-accumulate in the organs and other tissues of the consuming animals [33]. When these animal tissues are consumed by humans, these metals may bio-accumulate in human tissues and organs [34] [35].
Monitoring levels of trace elements and heavy metals in the different organs of animals destined for human consumption, allows assessing the quality and nutritive value for this purpose.
The main aim of the present study was to investigate the distribution of copper, zinc, cadmium and lead in liver, lung, meat, heart and kidney of cattle, sheep, camel and equine slaughtered in municipal slaughterhouse of Casablanca and destined for consumption by population of this city.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 295
ISSN 2229-5518
During the period of March-April 2013, samples of liver, lung, meat, heart and kidney were collected at the municipal slaughterhouse of Casablanca from 50 cattle, 50 sheep, 30 camels and 40 equines. Cattle, camel and equine that were the subject of this study are adult animals, while that sheep have an age not exceeding one year. For each animal, samples were collected accompanied by a veterinary hygiene inspector who helped in the collection of organs. All samples were packed in labeled plastic bags and immediately transported in a cooler to the laboratory and stored at -27°C until analysis.
For each collected sample a quantity of 0.2 to 0.7 g was digested with sulfuric acid in eppendorf tubes, and mineralized, using the method described by Hill et al., [36]. Thus, the content for each eppendorf was transferred into a test tube into which 3 ml of nitric acid (HNO3 65%) were added. The test tubes were heated on a hot plate, first at low temperature and then at high temperature (until 450°C). The beginning of the mineralization is characterized by the presence of a brown fume. After the total disappearance of this fume, the test tubes were cooled and 2 ml of hydrogen peroxide (H2 O2 35%) were added, then the test tubes were returned onto a hot plate until a limpid solution was obtained. Finally, the test tubes were cooled again and transferred into plastic tubes. The volume for each tube was adjusted to 10 ml with double distilled water and stored to room temperature for mineral analysis.
Trace elements analyses were conducted at the Technological and Scientific Research Support Units (UATRS) of the National Center for Scientific and Technical Research (CNRST) at Rabat, Morocco. The elements were determined by Inductively Coupled Plasma - Atomic Emission Spectroscopy (ICP-AES). The standards used are of commercial type containing the mineral analyzed in a pure state. Each analysis was repeated in triplet.
Statistical differences between the different organs (liver, lung, meat, heart and kidney) were determined by one-way analysis of variance (ANOVA). The limit of significant level was accepted at p<0.05. The significance of correlations between the levels of trace elements and heavy metals in organs in each animal were calculated using Pearson correlation analysis.
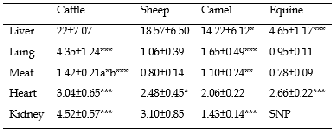
The results found for copper concentrations in liver, lung, meat, heart and kidney of cattle, sheep, camel and equine are presented in Table 1.
The highest concentration of copper in liver was noted in cattle compared to camel (p<0.05) and equine (p<0.001). The lowest concentration was recorded in liver of equine (p<0.001). Copper concentration in liver of sheep is comparable with those found in cattle and camel. Our values
TABLE 1: COPPER CONCENTRATIONS (MG/KG) IN ORGANS OF DIFFERENT ANIMALS (MEAN ± SD)

*p<0.05, **p<0.01, ***p<0.001, a: difference (Cattle Vs Camel), b: difference (Cattle Vs Sheep and Equine); SD: Standard Deviation; SNP: Sampling Not Performed.
for copper concentrations in camel and cattle liver are comparable to those presented by Essamadi [37], showing concentration in liver lower in camel than in cattle. However, the results reported in Saudi Arabia [38], China [39] and Sudan [40] showed that copper concentration in liver was significantly higher for camel than for cattle and sheep. If the normal values of copper in cattle liver range from 35 to 91 mg/kg [37], it is evident that the result of this study is lower and also compared to values found in a previous study [41], [42], [43], [44], [45], but our result is comparable to that reported in Iraq [46] and higher to that reported in Pakistan [47]. Moreover, the present concentration of copper in sheep liver is comparable with those found in Turkey [45] and Iraq [46], and lower comparing to those found in other countries [48], [49]. On the other hand, our value in camel was in the range (10-26 mg/kg) reported in Morocco [37] and comparable to that found in Saudi Arabia [50]. In equine, hepatic copper concentration in this study is lower compared to those observed in other studies [51], [52].
Copper concentration in lung was statistically higher in cattle when compared to other species (p<0.001). Another significant difference in lung was observed in camel compared to sheep and equine (p<0.001). The copper concentrations in lung of sheep and equine were comparable. In this study, copper concentration in cattle lung is comparable with that found in Pakistan [53]. At reverse, our values are lower compared with those reported for sheep [52] and camel [39], [54].
The highest concentration of copper in meat was recorded in cattle when compared to camel (p<0.05), sheep and equine (p<0.001). A significant difference in meat was also observed for camel compared to sheep and equine (p<0.01). Copper concentrations in meat of sheep and equine were comparable. In the present study, copper concentration in meat is comparable to those reported in many studies for cattle [41], [42], [44], [55], [56], [57], [58], but lower to that reported in Pakistan [47]. Moreover, the value found for sheep is comparable to that reported from Poland [48] and Egypt [59]. In camel, the values found in Saudi Arabia [50] and Iran [60] are comparable to those of the present study.
For heart, the copper concentration was statistically higher in cattle when compared to camel (p<0.001). The lowest
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 296
ISSN 2229-5518
concentration was recorded for camel when compared to sheep (p<0.05) and equine (p<0.001). Copper concentrations in heart of cattle, sheep and equine were comparable. The present concentrations of copper in heart are comparable to those reported from other countries for cattle [45], [59] and sheep [59], [61]. In comparison with previous study [60], our value is slightly lower in camel. Similarly, the value found for equine is lower compared to that found in China [52].
The highest concentration of copper in kidney was observed for cattle when compared to other animals (p<0.001). A significant difference in kidney was also observed for camel compared to sheep (p<0.001). The result of this study on the copper concentration in cattle kidney is comparable to the results reported by different literature sources [41], [43], [44], [55], [57], [62]. Moreover, in sheep, our value is comparable with that found in Turkey [45], Iceland [49] and Egypt [59], and slightly lower of the values found in other countries [48], [63], [64]. In camel, the value of the current study is low compared to concentrations reported in other studies [50], [60].
It appears from this study that the copper concentrations in different organs of different animals studied, are generally in agreement with the literature data. Our study also shows that copper concentrations are slightly low and limited to threshold deficiencies especially in cattle [37]. The lower concentrations observed in our study may be attributed to copper deficiency in diet source offered to animals. In camel, it was observed in several studies that this animal regulates its copper contents at levels much lower than other ruminants [37], [65], [66]. In equine, although there are limited data studying the tissue distribution of copper in this species, the values of this study are much lower compared to those reported by other studies [51], [52]. The comparison of copper values in the different organs showed that this element is mainly concentrated in liver. The low concentrations observed in other organs attested the predominant role of this organ in the copper storage. In animals, the results suggest that meat and edible offal of cattle are the richest in copper. However, in spite the age of sheep that does not exceed 12 months, copper concentrations in sheep and camel are comparable. This is due to the fact that camel behaves like a species of small ruminants [66]. While meat and edible offal of equine contain lower concentrations compared to other species. This difference could be explained by the fact that equine destined for slaughtering are the draft animals, and therefore probably they suffer from several deficiency, especially those in trace elements.
The results found for zinc concentrations in liver, lung, meat, heart and kidney of cattle, sheep, camel and equine are presented in Table 2.
The highest concentration of zinc in liver was found in camel compared to cattle (p<0.05), sheep (p<0.01) and equine (p<0.001). However, the lowest concentration was recorded in liver of equine (p<0.001). Zinc concentrations in liver of cattle and sheep were comparable. A study from Morocco [37] reported that zinc concentrations in liver were lower in camel than in cattle. Moreover, some results reported from Sudan
TABLE 2: ZINC CONCENTRATIONS (MG/KG) IN ORGANS OF DIFFERENT ANIMALS (MEAN ± SD)
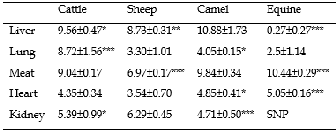
*p<0.05, **p<0.01, ***p<0.001, SNP: Sampling Not Performed; SD: Standard
Deviation.
[40] indicated that the hepatic concentrations of zinc in camel were less concentrated than other ruminants. The results reported from Saudi Arabia [38] showed that differences in liver zinc concentrations between camel, cattle and sheep were not statistically significant. The present results on zinc concentrations in liver for different animals studied are low compared to those reported in different literature sources for cattle [37], [38], [40], [41], [42], [43], [44], [45], [46], [47], [55], [56], [57], [59], [62], [64], [67], sheep [45], [46], [47], [48], [49], [59], [64], camel [37], [40], [50] and equine [52], [68], [69].
In lung, the highest concentration of zinc was observed in cattle when compared to other species (p<0.001). Another significant difference in lung was observed between camel and sheep (p<0.05). However, zinc concentrations in lung of sheep and equine were comparable. Zinc concentrations in lung obtained in this study are low compared to those reported in previous studies for cattle [62], sheep [52], camel [54] and equine [69].
Zinc concentration in meat was statistically higher in equine when compared to other species (p<0.001). The lowest concentration in meat was recorded for sheep (p<0.001). In comparison with previous observations for cattle [41], [42], [44], [47], [55], [56], [57], [58], [59], [64], [67], sheep [48], [59], [64], camel [50] and equine [69], the results of this work on zinc concentrations in meat for different animals studied appeared lower.
The highest concentration of zinc in heart was observed in equine when compared to cattle and sheep (p<0.001). A significant difference in heart was also observed between cattle and camel (p<0.05). However, zinc concentrations in heart of camel and equine were comparable. The results of this study on zinc concentrations in heart are low compared to those found for cattle [47], [59] and camel [54]. But the results reported in Turkey [45] in cattle heart are lower to that found in the present study. However, the concentration found in sheep is comparable with that found in Iran [61], while she is lower as compared to that reported in many studies [45], [47], [59]. In equine, the value found in China [52] is comparable to that of the present study.
The highest concentration of zinc in kidney was recorded in sheep when compared to cattle (p<0.05) and camel (p<0.001). Zinc concentrations in kidney of cattle and camel were comparable. Our results on zinc concentrations in kidney for the different animals studied are lower compared to those
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 297
ISSN 2229-5518
reported from many country for cattle [41], [42], [43], [44], [47], [55], [56], [57], [59], [62], [64], sheep [47], [48], [49], [59], [63], [64] and camel [50], [54]. However, our results are comparable to those reported in Turkey for cattle and sheep [45].
In general, the results of this study on zinc concentrations in different organs of the different animals studied are low compared to the results reported by many studies. These lower concentrations observed in our study may be attributed to the probable zinc deficiency in the diet offered to the sampled animals. On the other hand, it seems that camel regulates its zinc contents at the lower levels than those of other ruminants [37], [65], [66]. For equine, low zinc concentrations obtained in liver and lung compared to other species, could only be explained by the fact that equine destined for slaughtering are the draft animals, and therefore probably they suffer more deficiency, especially for trace elements. It appears that the highest concentrations of zinc were observed in liver and meat. Cattle and camel accumulate higher concentrations of zinc in liver, lung and kidney compared to equine. In contrast, equine present higher concentrations in meat and heart as compared to other species. While sheep that have been used in this study did not exceed
12 months of age, their concentrations of zinc were comparable to those of cattle and camel.
TABLE 3: CADMIUM CONCENTRATIONS (MG/KG) IN ORGANS OF DIFFERENT ANIMALS (MEAN ± SD)

**p<0.01, ***p<0.001, SNP: Sampling Not Performed; SD: Standard Deviation.
The results found for cadmium concentrations in liver, lung, meat, heart and kidney for cattle, sheep, camel and equine are presented in Table 3.
The highest concentration of cadmium in liver was observed in equine and camel when compared to cattle and sheep (p<0.001). Our results on the cadmium concentrations in liver of cattle (0.10 mg/kg) and sheep (0.04 mg/kg) are below the level recommended by the European Commission (0.5 mg/kg) [70]. Concentrations reported from other countries for cattle are similar [44], [55], [59], higher [47], [56], [64], [71] and lower [41], [42], [43], [45], [57], [62], [72], [73], [74] compared to our value. In sheep, the concentration of this study is lower compared to those reported in many studies [45], [47], [52], [59], [64], [75], [76], [77], while it is higher compared to that reported from Italy [72] and comparable to that reported from Iceland [49]. Cadmium concentrations reported for this study in camel liver (0.25 mg/kg) were lower than the limit of
European Commission in cattle liver (0.5 mg/kg) [70]. Moreover, in equine, comparing our value (0.86 mg/kg) with that recommended by the European Commission (0.5 mg/kg) [70], it is clear that the cadmium concentration obtained in this species is higher. However, our value in equine remains lower compared to those reported in other studies [52], [68], [69], [78].
Cadmium concentration in lung was statistically higher in equine and camel when compared to cattle and sheep (p<0.001). The cadmium concentrations in lung of cattle and sheep were comparable. Cadmium concentrations found in lung of different animals studied are lower compared to those reported from many country for cattle [62], [72], sheep [52], [72] and equine [69].
In meat, the highest concentration of cadmium was observed in camel when compared to other species (p<0.001). However, no accumulation of cadmium was observed in meat of cattle, sheep and equine. In spite of our results that suggested the absence of cadmium in meat of cattle, sheep and equine, the levels of this element in meat should not exceed the limits proposed by the European Commission for cattle and sheep (0.05 mg/kg), on one hand, and for equine (0.2 mg/kg), on the other hand [70]. In camel, cadmium concentrations found in meat (0.12 mg/kg) appeared widely higher than the tolerable values in cattle meat (0.05 mg/kg) as proposed by the European Commission [70]. Contrary to previous observations, the values of this study for the different animals studied are lower compared to those reported for cattle [41], [42], [44], [47], [55], [56], [57], [58], [59], [64], [71], [73], [74], sheep [52], [59], [64], [76], [77] and equine [69], [78].
The highest concentration of cadmium in heart was
reported for camel compared to other species (p<0.001).
Cadmium concentrations in heart for cattle, sheep and equine
were comparable. While the results of this study showed the
absence of cadmium in heart of cattle and equine, the previous
studies have reported high values in cattle [45], [47], [59] and
equine [52]. In sheep, the concentration of this study is lower
compared to those reported from many studies [45], [47], [52],
[59], [76], while it is comparable to that reported in Italy [72].
Cadmium concentration in kidney was statistically higher
in camel when compared to other species (p<0.001). Another
significant difference in kidney was observed between cattle and sheep (p<0.01). The present concentrations of cadmium in kidney for cattle (0.21 mg/kg) and sheep (0.085 mg/kg), do not exceed the limits as recommended by the European
Commission (1 mg/kg) [70]. By comparing our values to those found in other studies, higher values in cattle have been reported by different literature sources [41], [42], [44], [47], [55], [56], [59], [64], [71], while lower values have been reported from other countries [43], [45], [57], [62], [72], [73], [74]. Also, the higher values have been reported for sheep [45], [47], [52], [59], [64], [75], [76], while lower values have been reported from Italy [72] and Iran [77]. Cadmium concentrations reported for this study in camel’s kidney (0.69 mg/kg) were lower than the limit of European Commission for cattle’s kidney (1 mg/kg) [70].
Cadmium concentrations found in liver, kidney and meat of cattle, sheep and equine are below the acceptable levels
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 298
ISSN 2229-5518
recommended by the European Commission, with the exception of equine’s liver and camel’s meat. Also, our results are lower compared to those reported in many studies. Indeed, it was difficult to state if cadmium contaminations of camel organs were important or not, as to our knowledge, no reference was available for this species in the literature. On the other hand, our data show that cadmium concentrations vary in different organs for different animals studied. The differences in animals may be attributed to the pasture on which these animals grazed and the source of water from which these animals drank. Camel and equine were deemed the species contaminated by cadmium than cattle and sheep. Concerning the differences in organs, it appears that liver and kidney are the organs that accumulate the highest concentrations of cadmium compared to other organs. The highest concentrations of cadmium detected in kidney followed by liver attested the preferential accumulation of cadmium in those organs linked to their functional role in the detoxification process.
TABLE 4: LEAD CONCENTRATIONS (MG/KG) IN ORGANS OF DIFFERENT ANIMALS (MEAN ± SD)
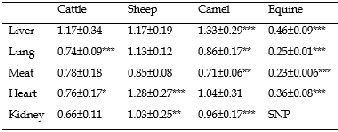
*p<0.05, **p<0.01, ***p<0.001, SNP: Sampling Not Performed; SD: Standard
Deviation.
presented in Table 4.
In liver, the highest concentration of lead was observed in
camel (p<0.001), while the lowest concentration was recorded
for equine (p<0.001). Lead concentrations in liver of cattle,
sheep and camel were comparable. The present results on lead
concentrations in cattle (1.17 mg/kg) and sheep (1.17 mg/kg)
liver are higher than the permissible limit recommended by
the European Commission (0.5 mg/kg) [70]. Similarly, our
values are higher compared to those found in other studies for
cattle [41], [42], [43], [44], [45], [55], [57], [59], [62], [71], [72], [73], [79], [80] and sheep [45], [52], [59], [72], [79]. At reverse, the values reported from Pakistan [47], [64] are higher than those found in this study for cattle and sheep. Lead
concentration found in camel’s liver (1.33 mg/kg) is higher than the tolerable values for cadmium in this organ (0.5 mg/kg) as proposed by the European Commission [70]. In equine, our value is lower compared to those reported from Kazakhstan [69] and China [52], while it is higher compared to that reported from the United States [78].
The highest concentration of lead was present in sheep’s lung compared to camel (p<0.01), cattle and equine (p<0.001).
The lowest concentration of lead was recorded in lung of equine (p<0.001). Lead concentrations in lung of cattle and camel were comparable. The present lead concentrations in lung of cattle (0.74 mg/kg) and sheep (1.13 mg/kg) exceeded the acceptable limits proposed by the European Commission (0.5 mg/kg) [70]. Thus, our values are higher compared to those reported in other studies for cattle [62], [72] and sheep [72]. At reverse, our values are lower to those reported in previous studies for sheep [52] and equine [69]. In camel, our value (0.86 mg/kg) is higher than the tolerable one for cadmium in cattle’s lung (0.5 mg/kg) as proposed by the European Commission [70].
Lead concentration in meat was statistically higher for sheep when compared to camel (p<0.01) and equine (p<0.001). The lowest concentration of lead was found in meat of equine (p<0.001). Lead concentrations in meat of cattle, sheep and camel were comparable. Our results for lead concentrations in meat of cattle (0.78 mg/kg) and sheep (0.85 mg/kg) exceeded the maximum acceptable levels for this element as proposed by the European Commission (0.1 mg/kg) [70]. Similarly, the values reported in previous studies remain lower compared to our values for cattle [41], [42], [44], [55], [57], [58], [59], [71], [73], [74], [79] and sheep [59], [79]. However, the values found in other studies are higher than those of the present work for cattle [47], [64] and sheep [52], [64]. Lead concentration in camel’s meat (0.71 mg/kg) appeared widely higher than the tolerable values for meat (0.1 mg/kg) of cattle as proposed by the European Commission [70]. For equine, the concentration obtained in the present study is lower than that reported in Kazakhstan [69], while it is higher than that reported from the United States [78].
In heart, lead concentration was statistically higher for
sheep when compared to cattle and equine (p<0.001). The
lowest concentration of lead was found in heart of equine
(p<0.001). A significant difference was also observed between
cattle and camel (p<0.05). However, lead concentrations in
heart of camel and sheep were comparable. Our results on
lead concentrations in heart of cattle (0.76 mg/kg) and sheep
(1.28 mg/kg) are higher than the permissible limit as
recommended by the European Commission (0.5 mg/kg) [70].
Thus, the values of the current study are higher than those
reported in many studies for cattle [45], [59], [79] and sheep
[45], [59], [72], [79]. In contrast, a previous study [52] reported higher values compared to those of the present work for sheep and equine. For camel, our value (1.04 mg/kg) is higher than the tolerable values for cadmium in cattle’s heart (0.5 mg/kg)
as proposed by the European Commission [70].
The highest concentration of lead in kidney was observed
for sheep compared to cattle (p<0.01). Another significant
difference in kidney was observed between cattle and camel
(p<0.001). Lead concentrations in kidney of camel and sheep
were comparable. Lead concentrations found in kidney of
cattle (0.66 mg/kg) and sheep (1.03 mg/kg) exceeded the
permissible levels recommended by the European
Commission (0.5 mg/kg) [70]. Lower values compared to those of the current study have been reported in various literature sources for cattle [41], [42], [43], [44], [45], [55], [57], [59], [62], [71], [72], [73], [74], [79], [80] and sheep [45], [52],
[59], [72], [79]. However, the values reported from Pakistan
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 299
ISSN 2229-5518
[47] and Egypt [64] for cattle and sheep are higher than those found in the present work. Lead concentration in camel’s kidney (0.96 mg/kg) appeared widely higher than the tolerable values for this organ (0.5 mg/kg) as proposed by the European Commission [70].
The results of this study showed that meat and edible offal of cattle, sheep and camel contained slightly higher lead levels than permissible ones recommended by the European Commission, as well as the results obtained in many studies. Similarly, for equine, our values are higher compared to those reported in a previous study [78]. Because, data on lead concentrations are unavailable for camel organs, it was difficult to attest if lead contaminations of this species organs were important or not. The higher concentrations obtained in this study may suggest an environmental pollution by this metal. The sources of lead may be traced to the pasture on which these animals grazed and the source of water from which they drank. Cattle, sheep and camel were deemed the most contaminated by lead. Lead accumulates mainly in liver.
A correlation analysis among copper, zinc, cadmium and lead concentrations was carried out for liver, lung, meat, heart and kidney of cattle, sheep, camel and equine (Table 5).
In the present study, copper for cattle showed significant negative correlations with zinc in meat, cadmium in liver and kidney and with lead in liver, meat and kidney. Positive correlations were detected between copper and lead in lung, zinc and lead in meat and also between cadmium and lead in liver and kidney. A recent study showed that significant negative correlations were observed in cattle meat between zinc-copper and zinc-lead, but a significant positive correlation was observed between copper and lead [81]. There was also a very high positive correlative dependence between lead and cadmium concentrations in the liver and muscle of cattle in Poland [82]. However, another study has reported
positive correlations between copper and zinc in liver, meat and kidney; zinc and cadmium in kidney; zinc and lead in liver, meat and kidney; cadmium and lead in liver, meat and kidney; But at reverse negative correlations were observed between copper and cadmium in meat and kidney [83]. Also, copper in liver is negatively correlated to lead and positively correlated to zinc and cadmium [84]. A previous study showed that significant positive correlations were observed between copper and lead in liver, and between copper and zinc in kidney [43]. Some results found from Jamaica showed that zinc is positively correlated to copper, cadmium and lead in liver, and to copper and cadmium in kidney [85]. Significant positive correlations were observed in kidney between copper, zinc, cadmium and lead, and between zinc, cadmium and lead in liver; But significant negative correlations were observed between copper, cadmium and lead in liver [44]. Although another study has also reported that copper was significantly correlated with zinc in liver, cadmium was positively correlated with copper and zinc in kidney, and with zinc in liver, lead was significantly correlated with copper in liver [42].
For sheep, strong significant positive correlations were observed between copper and zinc in meat, heart and kidney. Significant positive correlations were also observed between copper, cadmium and lead in liver and kidney. At reverse, significant negative correlations were observed between copper and zinc in liver, copper and cadmium in heart and kidney, zinc and cadmium in liver and heart, zinc and lead in liver and lung, cadmium and lead in lung, heart and kidney. At the opposite of our results, a recent study reported a significant negative correlation between lead and copper levels in liver [86]. However, another study has reported positive correlations between cadmium and lead in liver and meat [87]. These associations are similar to those found in our study.
For camel, copper and zinc were highly and positively
TABLE 5: PEARSON CORRELATIONS AMONG MINERALS’ LEVELS IN ORGANS OF ANIMALS
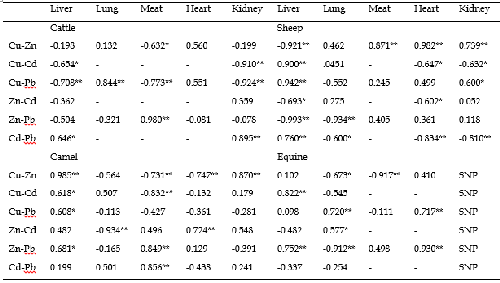
**Correlation is significant at the 0.01 level, *Correlation is significant at the 0.05 level, SNP: Sampling Not Performed.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 300
ISSN 2229-5518
correlated in liver and kidney. Positive correlations were also observed between copper, cadmium and lead in liver, zinc and cadmium in heart, zinc and lead in liver and meat, cadmium and lead in meat. Negative correlations were observed between copper and cadmium in meat and lung. But at reverse, strong negative correlations were observed in lung between zinc and cadmium, in meat between copper, zinc and cadmium, and in heart between copper and zinc. A recent study reported a negative correlation between copper and zinc in meat, but in liver and kidney the correlations were not significant [50]. On the other hand, as it was observed in case of a global pollution in other substrates like camel milk [88], cadmium and lead were highly correlated.
In equine, significant positive correlations were observed between copper and cadmium in liver, copper and lead in lung and heart, zinc and cadmium in lung, zinc and lead in liver and heart. At the opposite, copper and zinc are negatively correlated in lung and meat. A negative correlation was also observed between zinc and lead in lung.
In general, the relationships between elements could not be
well explained, especially when those relationships change
from one organ to another. Scarce information is available on
interactions between elements in camel, sheep and equine. The
highest number of significant correlations between copper,
zinc, cadmium and lead in this study were found especially in
liver and kidney. This may reflect a main role of both organs,
especially the kidney, in trace elements metabolism [89], [90].
The interactions between copper, zinc and cadmium probably
indicate that mineral balance in the body is regulated by
important homeostatic mechanisms, especially in the kidney,
in which toxic elements compete with the essential metals [42]. These interactions are consequences of the shared ability of these metals to induce metallothionein synthesis and thus compete for binding sites of metallothionein [42], [83], [89],
[91], [92], [93], [94], [95], [96], [97], [98], [99].
The status of copper and zinc concentrations in organs of animals is obviously linked to diet composition. But other parameters, such as physiological stage, health conditions and form of elements, could explain the observed variations. However, the animals of our study could have been exposed to cadmium and lead either through their feed (pasture) or drinking water. This is an indication that the environment, where these animals were reared, was potentially contaminated with cadmium and lead. Finally, the status of trace elements and heavy metals in animals in the different regions of Morocco needs to be studied. Studies should be done to document the status of trace elements and heavy metals in animals to be slaughtered for human consumption in order to monitor the possible risk of deficiency or poisoning.
The authors thank the President of urban municipalities of Casablanca, the Director and the responsible for veterinary service of Casablanca slaughterhouse. Our thanks to Dr. Chriyaa Abdelouahid for the English lecture.
[1] C.F. Mills, “Trace Elements in Animals,” Philos. Trans. R. Soc. Lond. B Biol. Sci., vol. 288, no. 1026, pp. 51-63, Dec 1979.
[2] M.H. Golden, “Trace elements in human nutrition,” Hum. Nutr. Clin.
Nutr., vol. 36, no. 3, pp. 185-202, 1982.
[3] J.T. Yen, “Impact of trace element nutrition of animals on the food chain,” Trace Elements in Man and Animals – 9, Proceedings of the Ninth International Symposium on Trace Elements in Man and Animals. Edited by P.W.F. Fischer, M.R. L'Abbe, K.A. Cockell, and R.S. Gibson, NRC Research Press, Ottawa, Canada, pp. 294-297, 1997.
[4] K.O. Soetan, C.O. Olaiya and O.E. Oyewole, “The importance of mineral elements for humans, domestic animals and plants: A review,” Afr. J. Food Sci., vol. 4, no. 5, pp. 200-222, May 2010.
[5] L. Järup, “Hazards of heavy metal contamination,” Br. Med. Bull., vol.
68, no. 1, pp. 167-82, 2003.
[6] R.K. Sharma and M. Agrawal, “Biological effects of heavy metals: an overview,” J. Environ. Biol., vol. 26, no. 2 Suppl, pp. 301-313, Jun 2005.
[7] W. de Vries, P.F. Römkens and G. Schütze, “Critical soil concentrations of cadmium, lead, and mercury in view of health effects on humans and animals,” Rev. Environ. Contam. Toxicol., vol.
191, pp. 91-130, 2007.
[8] O. Kaplan, N.C. Yildirim, N. Yildirim and M. Cimen, “Toxic Elements in Animal Products and Environmental Health,” Asian J. Anim. Vet. Adv., vol. 6, no. 3, pp. 228-232, Mar 2011.
[9] H. Tapiero and K.D. Tew, “Trace elements in human physiology and
pathology: zinc and metallothioneins,” Biomed. Pharmacother., vol. 57, no. 9, pp. 399-411, Nov 2003.
[10] H. Scherz and E. Kirchhoff, “Trace elements in foods: Zinc contents of raw foods—A comparison of data originating from different geographical regions of the world,” J. Food Comp. Anal., vol. 19, no. 5, pp. 420-433, Aug 2006.
[11] L.S.L.S. Reis, P.E. Pardo, A.S. Camargos and E. Oba, “Mineral element and heavy metal poisoning in animals,” J. Med. Med. Sci., vol. 1, no. 12, pp. 560-579, Dec 2010.
[12] M. Angelova, S. Asenova, V. Nedkova and R. Koleva-Kolarova, “Copper in the human organism,” Trakia J. Sci., vol. 9, no. 1, pp. 88-
98, 2011.
[13] M. Šoch, P. Vydrová, J. Brouček, K. Suchý, L. Smutný, Š. Smutná, B.
Čermák, L. Zábranský, A. Šimková, K. Švejdová and Š. Václav, “Relationship between Copper and Zinc Content in the Soil and Plants and their Consequent Content in Blood and Excrements of Cattle and Sheep under Various Forms of Breeding,” Scientific Papers: Animal Science and Biotechnologies, vol. 46, no. 1, pp. 316-320, Jan 2013.
[14] M.A. Oliver, “Soil and human health: a review,” Eur. J. Soil Sci., vol.
48, no. 4, pp. 573-592, Dec 1997.
[15] L. Minate and J.C. Carfagnini, “Evaluation of the diagnostic value of plasma copper levels in cattle,” Prev. Vet. Med., vol. 53, no. 1-2, pp. 1-
5, Feb 2002.
[16] C. Binet, “ Les oligo-éléments,” Oligo-éléments et oligothérapie : Matière médicale, propriétés et indications thérapeutiques. Argile et compléments alimentaires diététiques, Dangles Editions., France, pp. 9-18, 2007.
[17] J. Healy and K. Tipton, “Ceruloplasmin and what it might do,” J.
Neural Transm., vol. 114, no. 6, pp. 777-781, Apr 2007.
[18] S.I. Liochev and I. Fridovich, “Mechanism of the peroxidase activity of Cu, Zn superoxide dismutase,” Free Radic. Biol. Med., vol. 48, no.
12, pp. 1565-1569, Mar 2010.
[19] A. Deen, A. Bhati and M. Sahani, “Trace mineral profiles of camels blood and sera,” J. Camel Pract. Res., vol. 11, no. 2, pp. 135-136, 2004.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 301
ISSN 2229-5518
[20] J.J. Doyle, “Genetic and nongenetic factors affecting the elemental composition of human and other animal tissues-a review,” J. Anim. Sci., vol. 50, no. 6, pp. 1173-1183, Jun 1980.
[21] G.B. Van der Voet, J.A. Centeno, F.G. Mullick and P.B. Tchounwou, “Metal-Induced Toxicologic Pathology: Human Exposure and Risk Assessment,” Encyclopedia of Environmental Health - ISBN: 978-0-444-
52272-6 (5,016 pages). J.O. Nriagu, S. Kacew, T. Kawamoto, J.A. Patz, D.M. Rennie, G.A.S. Ansari, T. Bachmann, M. Blaxter, N. Dainiak, K.L. Ebi, M. Ezzati, Y. Fukuda, J.E. Kenigsberg, B.M. Lee, P. Lercher, K. McAuley, J. Meliker, O.A. Ogunseitan, O.E. Orisakwe, L. Ritter, A.D. Roux, D. Roy, M. Slotnick, B. Sonawane, H-J. Su, P.B. Tchounwou, C-Y. Yang and J.M. Zielinski, (Eds), Elsevier B.V. Oxford, pp. 713-721, 2011.
[22] B.D. Baykov, M.P. Stoyanov and M.L. Gugova, “Cadmium and lead bioaccumulation in male chickens for high food concentrations,” Toxicol. Environ. Chem., vol. 54, no. 1-4, pp. 155-159, 1996.
[23] D. Demirezen and A. Aksoy, “Accumulation of heavy metals in Typha angustifolia (L.) and Potamogeton pectinatus (L.) living in Sultan Marsh (kayseri, Turkey),” Chemosphere, vol. 56, no. 7, pp. 685-
696, Aug 2004.
[24] Q. Cai, M.L. Long, M. Zhu, Q.Z. Zhou, L. Zhang and J. Liu, “Food chain transfer of cadmium and lead to cattle in a lead-zinc smelter in Guizhou, China,” Environ. Pollut., vol. 157, no. 11, pp. 3078-3082, Nov
2009.
[25] X. Liu, Q. Song, Y. Tang, W. Li, J. Xu, J. Wu, F. Wang and P.C.
Brookes, “Human health risk assessment of heavy metals in soil- vegetable system: a multi-medium analysis,” Sci. Total Environ., vol.
463-464, pp. 530-540, Oct 2013.
[26] R.A. Goyer, “Toxic and essential metal interactions,” Annu. Rev.
Nutr., vol. 17, no. 1, pp. 37-50, 1997.
[27] M.M. Brzóska and J. Moniuszko-Jakoniuk, “Interactions between cadmium and zinc in the organism,” Food Chem. Toxicol., vol. 39, no.
10, pp. 967-980, Oct 2001.
[28] M. Lopez-Alonso, J.L. Benedito, M. Miranda, C. Castillo, J.
Hernandez and R.F. Shore, “Cattle as biomonitors of soil arsenic, copper and zinc concentrations in Galicia (NW Spain),” Arch. Environ. Contam. Toxicol., vol. 43, no. 1, pp. 103-108, Jul 2002a.
[29] M. Debackere, “Environmental Pollution: The animal as a Source, Indicator and Transmitter,” Veterinary pharmacology and toxicology,
2nd Edition., Westport, CT, AVI Publishing Co., pp. 595-608, 1983. [30] M.O. Akinola and T.O. Ekiyoyo, “Accumulation of Lead, Cadmium
and Chromium in some plants cultivated along the bank of River Ribila at Odo-nla Area of Ikorodu, Lagos State, Nigeria,” J. Environ. Biol., vol. 27, no. 3, pp. 3-4, Jul 2006.
[31] D.O. Nwude, P.A.C. Okoye and J.O. Babayemi, “Heavy metal level in animal muscle tissue. A case study of Nigeria raised cattle,” Res. J. Appl. Sci., vol. 5, no. 2, pp. 146-150, Jan 2010.
[32] A. Roegner, F. Giannitti, L.W. Woods, A. Mete and B. Puschner, “Public health implications of lead poisoning in backyard chickens and cattle: four cases,” Vet. Med. Res. Rep., vol. 4, pp. 11-20, Apr 2013.
[33] C. Makridis, C. Svarnas, N. Rigas, N. Gougoulias, L. Roka and S.
Leontopoulos, “Transfer of Heavy Metal Contaminants from Animal Feed to Animal Products,” J. Agr. Sci. Tech., vol. 2, no. A, pp. 149-154, Feb 2012.
[34] M. Miranda, J.L. Benedito, I. Blanco-Penedo,C. Lopez-Lamas, A.
Merino and M. Lopez-Alonso, “Metal accumulation in cattle raised in a Serpentine-soil area: Relationship between metal concentrations in Soil, Forage, and Animal tissues,” J. Trace Elem. Med Biol., vol. 23, no.
3, pp. 231-238, May 2009.
[35] S.A. Saïdi, M.S. Azaza, P. Windmolders, J. van Pelt and A. El-Feki, “Cytotoxicity evaluation and antioxidant enzyme expression related to heavy metals found in tuna by-products meal: An in vitro study in human and rat liver cell lines,” Exp. Toxicol. Pathol., vol. 65, no. 7-8, pp. 1025-1033, Apr 2013.
[36] A.D. Hill, K.Y. Patterson, C. Veillon and E.R. Morris, “Digestion of biological materials for mineral analyses using a combination of wet and dry ashing,” Anal. Chem., vol. 58, no. 11, pp. 2340-2342, Sept
1986.
[37] A.K. Essamadi, “Etude du metabolism des oligo-éléments (cuivre, zinc et selenium) chez le dromadiare-étude comparative avec les bovins,” PhD dissertation, Université Chouaib Eddoukali, Faculté des Sciences, El-Jadida, 2000.
[38] K.A. Al-Busadah, “Trace-element status in camels, cattle and sheep in Saudi Arabia,” Pakistan J. Biol. Sci., vol. 6, no. 21, pp. 1856-1859,
2003.
[39] L. Zongping, “Studies on the haematology and trace element status of adult Bactrian camels (Camelus bactrianus) in China,” Vet. Res. Commun., vol. 27, no. 5, pp. 397-405, Jul 2003a.
[40] A.O. Bakhiet, A.A. Mohammed, E.S.M. Siham and M.A.S. El-Badwi,
“Some trace-elements profile in the liver of camels, cattle, sheep and goats,” Int. J. Trop. Med., vol. 2, no. 1, pp. 1-2, 2007.
[41] M. Lopez-Alonso, J.L. Benedito, M. Miranda, C. Castillo, J.
Hernández and R.F. Shore, “Arsenic, cadmium, lead, copper and zinc in cattle from Galicia, NW Spain,” Sci. Total Environ., vol. 246, no. 2-3, pp. 237-248, Feb 2000a.
[42] M. Lopez-Alonso, F.P. Montaña, M. Miranda, C. Castillo, J.
Hernández and J.L. Benedito, “Interactions between toxic (As, Cd, Hg and Pb) and nutritional essential (Ca, Co, Cr, Cu, Fe, Mn, Mo, Ni, Se, Zn) elements in the tissues of cattle from NW Spain,” Biometals., vol. 17, no. 4, pp. 389-397, Aug 2004.
[43] I. Blanco-Penedo, J.M. Cruz, M. Lopez-Alonso, M. Miranda, C.
Castillo, J. Hernández and J.L. Benedito, “Influence of copper status on the accumulation of toxic and essential metals in cattle,” Environ. Int., vol. 32, no. 7, pp. 901-906, Sep 2006.
[44] N. Waegeneers, J.C. Pizzolon, M. Hoenig and L. De Temmerman, “Accumulation of trace elements in cattle from rural and industrial areas in Belgium,” Food Addit. Contam. Part A, Chem. Anal. Control Expo. Risk. Assess., vol. 26, no. 3, pp. 326-332, Mar 2009.
[45] D. Mendil and M. Tuzen, “Assessment of trace elements in animal
tissues from Turkey,” Environ. Monit. Assess., vol. 182, no. (1-4), pp.
423-430, Nov 2011.
[46] L.S.Z. Al-omran, “Determination of some essentiel trace elements in cattle liver and sheep liver in Basrah,” Basra studies journal, vol. 8, no.
8, pp. 1-11, 2009.
[47] M.A. Nasser, S. Ahmad, A. Basir, A.K. Rais, A. Bibi, R. Ullah, A.A.
Shad, Z. Muhammad and I. Hussain, “Distribution of Heavy Metals in the Liver, Kidney, Heart, Pancreas and Meat of Cow, buffalo, Goat, Sheep and Chicken from Kohat market Pakistan,” Life Sci. J., vol. 10, no. 7s, pp. 937-940, 2013.
[48] J. Falandysz, W. Kotecka and K. Kannan, “Mercury, lead, cadmium, manganese, copper, iron and zinc concentrations in poultry, rabbit and sheep from the northern part of Poland,” Sci. Total Environ., vol.
141, no. (1-3), pp. 51-57, Jan 1994.
[49] O. Reykdal and A. Thorlacius, “Cadmium, mercury, iron, copper, manganese and zinc in the liver and kidney of the Icelandic lamb,” Food Addit. Contam., vol. 18, no. 11, pp. 960-969, Nov 2001.
[50] M.A. Mutassim, S.R. Aljumaah and M. Ayadi, “Variation of Copper,
Zinc, Manganese and Magnesium in Blood Serum and Tissues of
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 302
ISSN 2229-5518
Two Breeds of Dromedary Camels in Saudi Arabia,” Asian. J. Anim. Vet. Adv., vol. 8, no. 1, pp. 91-99, Jan 2013.
[51] D.A. Egan and M.P. Murrin, “Copper concentration and distribution in the livers of equine fetuses, neonates and foals,” Res. Vet. Sci., vol.
15, no. 1, pp. 147-148, Jul 1973.
[52] L. Zongping, “Lead poisoning combined cadmium in sheep and horses in the vicinity of non-ferrous metal smelters,” Sci. Total Environ., vol. 309, no. 1-3, pp. 117-126, Jun 2003b.
[53] M. Ayub, M. Zuber, M. Yousaf, A.F. Zahoor, Z.I. Khan, K. Ahmad and M. Mansha, “Assessment of copper intensity in selected tissues of two different classes of ruminants in Punjab, Pakistan,” Afr. J. Pharm. Pharacol., vol. 7, no. 21, pp. 1396-1403, Jun 2013.
[54] L. Zongping, “Studies on rickets and osteomalacia in Bactrian camels
(Camelus bactrianus),” Vet. J., vol. 169, no. 3, pp. 444-453, May 2005. [55] M. Lopez-Alonso, J.L. Benedito, M. Miranda, C. Castillo, J.
Hernandez and R.F. Shore, “Toxic and trace elements in liver, kidney and meat from cattle slaughtered in Galicia (NW Spain),” Food Addit. Contam., vol. 17, no. 6, pp. 447-457, Jun 2000b.
[56] A. Sedki, N. Lekouch, S. Gamon and Pineau A., “Toxic and essential trace metals in muscle, liver and Kidney of bovies from a polluted area of Morocco,” Sci. Total Environ., vol. 317, no. 1-3, pp. 201-205,
2003.
[57] M. Miranda, M. Lopez-Alonso, C. Castillo, J. Hernandez and J.L.
Benedito, “Effects of oderate pollution on toxic and trace metal levels in calves from a polluted area of Northern Spain,” Environ. Int., vol.
31, no. 4, pp. 543-548, May 2005.
[58] T.V. Petukhova, “Content of heavy metals in the muscle tissue of cattle,” E3S Web of Conferences, vol. 1, pp. 1-3, 2013.
[59] A.A.K. Abou-Arab, “Heavy metal contents in Egyptian meat and the role of detergent washing on their levels,” Food Chem. Toxicol., vol. 39, no. 6, pp. 593-599, Jun 2001.
[60] K. Badiei, K. Mostaghni, M. Pourjafar and A. Parchami, “Serum and
tissue trace elements in Iranian camels (Camelus dromedarius),”
Comp. Clin. Path., vol. 15, no. 6, pp. 58-61, Jun 2006.
[61] G.A. Kojouri, E. Aghajani, S. Jahanabadi and A. Kojour, “Mineral status of myocardial Sarcocystosis,” Iranian J. Parasitol., vol. 6, no. 2, pp. 17-22, Jun 2011.
[62] C. Jukna, V. Jukna and J. Siugzdaite, “Determination of heavy metals in viscera and muscles of catlle,” Bulg. J. Vet. Med., vol. 9, no. 1, pp.
35-41, 2006.
[63] C.J. Botha, A.S. Shakespeare, R. Gehring and D. Van der Merwe, “Evaluation of a commercially available molybdate formulation and zinc oxide boluses in preventing hepatic copper accumulation and thus enzootic icterus in sheep,” J. S. Afr. vet. Assoc., vol. 72, no. 4, pp.
183-188, Dec 2001.
[64] M. Irfana, S. Iqbal and S.A. Nagra, “Distribution of some trace and macrominerals in beef, mutton and poultry,” Int. J. Agri. Biol., vol. 6, no. 5, pp. 816-820, 2004.
[65] B. Faye, C. Grillet and A. Tessema, “Teneur en oligo-éléments dans les fourrages et le plasma des ruminants domestiques en Ethiopie,” Rev. Elev. Med. Vet. Pays. Trop., vol. 39, no. 2, pp. 227-237, 1986.
[66] B. Faye, M. Kamil and M. Labonne, “Teneur en oligo-éléments dans les fourrages et le plasma des ruminants domestiques en République de Djibouti,” Rev. Elev. Med. Vet. Pays. Trop., vol. 43, no. 3, pp. 365-
373, 1990.
[67] B. Koréneková, M. Skalická and P. Naï, “Concentration of some heavy metals in cattle reared in the vicinity of a metallurgic industry,” Vet. Arhiv., vol. 72, no. 5, pp. 259-267, 2002.
[68] C.D. Salisbury, W. Chan and P.W. Saschenbrecker, “Multielement
concentrations in liver and kidney tissues from five species of Canadian slaughter animals,” J. Assoc. Off. Anal. Chem., vol. 74, no. 4, pp. 587-591, Jul-Aug 1991.
[69] A.A. Farmer and A.M. Farmer, “Concentrations of cadmium, lead and zinc in livestock feed and organs around a metal production centre in eastern Kazakhstan,” Sci. Total Environ., vol. 257, no. 1, pp.
53-60, Jul 2000.
[70] Anonymous, “Règlement (CE) No 1881/2006 de la commission, du
19 décembre 2006, portant fixation de teneurs maximales pour certains contaminants dans les denrées alimentaires,” Journal officiel de l’Union européenne (Publié le : 20/12/2006). Accessed 08
Septembre 2013 on the site: http://eur- lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:00
24:FR:PDF.
[71] J.N. Ihedioha and C.O. Okoye, “Cadmium and lead levels in muscle and edible offal of cow reared in Nigeria,” Bull. Environ. Contam. Toxicol., vol. 88, no. 3, pp. 422-427, Mar 2012.
[72] G. Forte and B. Beatrice, “Quantification of cadmium and lead in offal by SF-ICP-MS: Method development and uncertainty estimate,” Food Chem., vol. 105, no. 4, pp. 1591-1598, Mar 2007.
[73] F.A. Khalafalla, F.H. Ali, F. Schwagele and M.A. Abd-El-Wahab, “Heavy metal residues in beef carcasses in Beni-Suef abattoir, Egypt,” Vet. Ital., vol. 47, no. 3, pp. 351-361, Jul-Sep 2011.
[74] S.H. Al-naemi, “Estimation of lead and cadmium levels in muscles, livers and kidneys of slaughtered cattle in Mosul city,” Mesopotamia J. of Agric., vol. 39, no. 3, pp. 8-15, 2011.
[75] M. Kramarova, P. Massanyi, A. Jancova, R. Toman, J. Slamecka, F.
Tataruch, J. Kovacik, J. Gasparik, P. Nad, M. Skalicka, B. Korenekova, R. Jurcik, J. Cubon and P. Hascik, “Concentration of cadmium in the liver and kidneys of some wild and farm animals,” Bull. Vet. Inst. Pulawy, vol. 49, no. 4, pp. 465-469, 2005.
[76] A.R. Katarzyna, J. Monkiewicz and S. Kaszyca, “Correlations in
cadmium concentrations in the body of the sheep poisoned subacutely and nourished with or without a supplement of detoxicating preparation,” Bull. Vet. Inst. Pulawy, vol. 52, pp. 135-140,
2008.
[77] H.R. Kazemeini, E. Rahimi, A.A. Kharrattaherdel, N. Nozarpour and A.G. Ebadi, “Cadmium concentration in muscle, liver and kidney of sheep slaughtered in Falavarjan abattoir, Iran,” Toxicol. Ind. Health, vol. 26, no. 5, pp. 259-263, Jun 2010.
[78] L. Penumarthy, F.W. Oehme and R.H. Hayes, “Lead, cadmium, and
mercury tissue residues in healthy swine, cattle, dogs, and horses from the midwestern United States,” Arch. Environ. Contam. Toxicol., vol. 9, no. 2, pp. 193-206, 1980.
[79] M.A. Abou-Donia, “Lead concentrations in different animals muscles and consumable organs at specific localities in Cairo,” Global Veterinaria, vol. 2, no. 5, pp. 280-284, 2008.
[80] A. Bala, A.U. Junaidu, M.D. Salihu, K.I. Onifade, A.A. Magaji, O.O.
Faleke, M.A. Saulawa, A.I. Musawa, M. Mohammed, L.U. Muhammad, S.B. Pewan, S.A. Anzaku and P. Emenna, “Survey of lead (Pb) residue in kidney and liver of slaughtered cattle in Sokoto Central Abattoir, Sokoto State, Nigeria,” J. Vet. Adv., vol. 2, no. 3, pp.
132-138, 2012.
[81] M. García-Vaquero, M. Miranda, J.L. Benedito, I. Blanco-Penedo and M. Lopez-Alonso, “Effect of type of muscle and Cu supplementation on trace element concentrations in cattle meat,” Food Chem. Toxicol., vol. 49, no. 6, pp. 1443-1449, Jun 2011a.
[82] M. Rudy, “Correlation of lead, cadmium and mercury levels in tissue
and liver samples with age in cattle,” Food Addit. Contam. Part A,
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 303
ISSN 2229-5518
Chem. Anal. Control Expo. Risk. Assess., vol. 26, no. 6, pp. 847-853, Jun
2009a.
[83] M. Lopez-Alonso, J.L. Benedito, M. Miranda, C. Castillo, J.
Hernández and R.F. Shore, “Interactions between toxic and essential trace metals in cattle from a region with low levels of pollution,” Arch. Environ. Contam. Toxicol., vol. 42, no. 2, pp. 165-172, Feb 2002b.
[84] M. García-Vaquero, M. Lopez-Alonso, J.L. Benedito, J. Hernández, B.
Gutiérrez and M. Miranda, “Influence of Cu supplementation on toxic and essential trace element status in intensive reared beef cattle,” Food Chem. Toxicol., vol. 49, no. 12, pp. 3358-3366, Dec 2011b.
[85] J. Nriagu, M. Boughanen, A. Linder, A. Howe, C. Grant, R. Rattray, M. Vutchkov and G. Lalor, “Levels of As, Cd, Pb, Cu, Se and Zn in bovine kidneys and livers in Jamaica,” Ecotoxicol. Environ. Saf., vol.
72, no. 2, pp. 564-571, Feb 2009.
[86] I. Jankovská, J. Száková, D. Lukešová, I. Langrová, P. Válek, J.
Vadlejch, Z. Cadková and M. Petrtyl, “Effect of lead in water on the absorption of copper, iron, manganese and zinc by sheep (Ovis aries) infected with sheep tapeworm (Moniezia expansa),” Exp. Parasitol., Vol. 131, no. 1, pp. 52-56, May 2012.
[87] M. Rudy, “The analysis of correlations between the age and the level
of bioaccumulation of heavy metals in tissues and the chemical composition of sheep meat from the region in SE Poland,” Food Chem. Toxicol., vol. 47, no. 6, pp. 1117-1122, Jun 2009b.
[88] G. Konuspayeva, B. Faye, G. Loiseau, E. Diacono and S.
Akhmetsadykova, “Pollution of camel milk by heavy metals in
Kazakhstan,” Open Environ. Pollut. Toxicol. J., vol. 1, pp. 112-118, 2009. [89] A. Taylor, “Detection and monitoring of disorders of essential trace
elements,” Ann. Clin. Biochem., vol. 33, no. 6, pp. 486-510, Nov 1996. [90] R. Rahil-Khazen, B.J. Bolann and R.J. Ulvik, “Correlations of trace
element levels within and between different normal autopsy tissues analysed by Inductively coupled plasma atomic emission spectrometry (ICP-AES),” Biometals, vol. 15, no. 1, pp. 87-98, Mar
2002.
[91] M. Webb, “The chemistry, biochemistry and biology of cadmium,”
Topics in environmental health, Amsterdam: Elsevier, 1979.
[92] I. Bremner and J.H. Beattie, “Copper and zinc metabolism in health and disease: speciation and interactions,” Proc. Nutr. Soc., vol. 54, no.
2, pp. 489-499, Jul 1995.
[93] F. Bebe and M. Panemangalore, “Modulation of tissue trace metal concentrations in weanling rats fed different levels of zinc and exposed to oral lead and cadmium,” Nutr. Res., vol. 16, no. 8, pp.
1369-1380, 1996.
[94] M. Bengoumi, K. Essamadi, J.C. Tressol and B. Faye, “Comparative study of copper and zinc metabolism in cattle and camel,” Biol. Trace Elem. Res., vol. 63, no. 2, pp. 81-94, Aug 1998.
[95] A. Frank, R. Danielsson and B. Jones, “The 'mysterious' disease in Swedish moose. Concentrations of trace elements in liver and kidneys and clinical chemistry. Comparison with experimental molybdenosis and copper deficiency in the goat,” Sci. Total Environ., vol. 249, no. 1-3, pp. 107-122, Apr 2000.
[96] G.J. Komarnicki, “Tissue, sex and age specific accumulation of heavy metals (Zn, Cu, Pb, Cd) by populations of the mole (Talpa europaea L.) in a central urban area,” Chemosphere, vol. 41, no. 10, pp. 1593-
1602, Nov 2000.
[97] P.G. Reeves and R.L. “Chaney, Marginal nutritional status of zinc, iron, and calcium increases cadmium retention in the duodenum and other organs of rats fed rice-based diets,” Environ. Res., vol. 96, no. 3, pp. 311-322, Nov 2004.
[98] C.J. Phillips, P.C. Chiy and E. Zachou, “Effects of cadmium in
herbage on the apparent absorption of elements by sheep in comparison with inorganic cadmium added to their diet,” Environ. Res., vol. 99, no. 2, pp. 224-234, Oct 2005.
[99] C. Coudray, C. Feillet-Coudray, M. Rambeau, J.C. Tressol, E. Gueux, A. Mazur and Y. Rayssiguier, “The effect of aging on intestinal absorption and status of calcium, magnesium, zinc, and copper in rats: a stable isotope study,” J. Trace Elem. Med. Biol., vol. 20, no. 2, pp. 73-81, 2006.
IJSER © 2014 http://www.ijser.org