Fibronectin is a glycoprotein in the ground substance which binds the cells to the extracellular matrix [8]. Tenascin is present in HERs during odontoblast differentiation and also
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 2268
ISSN 2229-5518
Abstract- Cementum was first described by Denton GB in 1835 as a calcified, avascular mesenchymal tissue that covers the entire root surface. It approximately comprises of 50% of hydroxyappetite and 50% of organic matrix which consist of non collagenous and collagenous proteins. Protein extract of mature cementum promotes cell attachment, migration and stimulates protein synthesis of gingival fibroblast and periodontal ligament cells. One of the main functions of the cementum is to anchor the principle collagen fibers of the periodontal ligament to the root surface. It plays a role in adaptive and reparative function to maintain the occlusal relationship and to predict the integrity of the root surface. Regeneration of cementum on denuded root surface has been the subject of interest in the modern era of periodontal plastic surgery.
Index terms- Cementum, cementum composition, extracellular matrix, growth factors, periodontal regenaration, Hertzwig’s epithelial root sheath, plasma rich platelets
‘Unique’ the word describes a structure being the only one of a particular type. Cementum is described as a unique structure since it is found to be critical in various events responsible in maturation of the periodontal tissues, both in development as well as regeneration.
tissue that covers the entire root surface [1]. One of the main functions of the cementum is to
Cementum was first described by Denton GB in 1835 as a calcified, avascular mesenchymal
anchor the principle collagen fibers of the periodontal ligament to the root surface. It plays a role in adaptive and reparative function to maintain the occlusal relationship and to predict the integrity of the root surface. Understanding the exact molecular and biology of cementum development, is of utmost importance in the regeneration aspect of the periodontium.
Periodontitis is the destruction of the supporting periodontal tissues, including the cementum, periodontal ligament and alveolar bone. Deepening of the periodontal pocket results in degeneration of collageneous remnants of Sharpeys fibres in the cementum creating an environment favorable for penetration in that area. Changes in the cementum during this destruction have been earlier demonstrated at the ultramicroscopic level which includes areas of
increased mineralization in diseased root surface, loss of or reduction in the cross bending of the
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 2269
ISSN 2229-5518
collagen near the cementum surface and a subsurface condensation of organic material which was of exogenous origin [2]. Areas of demineralization here also found due to the proteolysis of embedded remnants of Sharpeys fibres on exposure to oral fluid and bacterial plaque. Failure to restore this alteration can ultimately lead to tooth loss.
Regeneration of periodontal structures includes the reconstruction of all the three tissues which is the cementum, periodontal ligament and alveolar bone. Various attempts have been made to achieve this by the use of bone graft, guided tissue regeneration membrane, growth factors, etc but failure to achieve desired results can be due to the lack of understanding on the possible molecular factors responsible in cementogenesis and tissue engineering. Dental cementum and alveolar bone shares the same qualities. Alveolar bone is avascular but does not undergo continues remodeling like cementum which can grow in thickness throughout life.
mature cementum promotes cell attachment migration and can stimulate protein synthesis of
Cementum expresses specific proteins which contain factors common to bone. Protein extracts of
gingival fibroblast and periodontal ligament cells.
The aim of this review is to highlight the important aspects of cementum in development and regeneration. It also focuses in highlighting the recent advances in tissue engineering aspects, like effects of certain molecular factors which promoted cementum formation.
Cementogenesis begins with deposition of matrix on dentin surface by Hertwigs epithelial root sheath (HERs). Disruption of HERs causes migration and organization of ectomesenchymal cells from the dental papilla to differentiate into cementoblast. According to
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 2270
ISSN 2229-5518
Thomas and Kollar in 1988, cementoblasts can also be of epithelial origin arising from enamel organ which gives rise to Hertwigs epithelial root sheath (HERs) [3]. Diekwish in 2001 demostrated that the disintergration of HERs prior to cementogenesis followed by penetration of dental follicular cells on the root surface depositing cementum [4].
Gottlieb in 1942 demonstrated that, the expression of amelogenin mRNA’s and enamel proteins on the enamel area of the crown after HERs was removed from the root surface prior to cementum deposition [4]. These results confirmed that, cementum is derived from the dental follicular tissue that forms after HER’s disintegration. Earlier HER’s was believed to disintegrate into small clusters/strands of epithelial cells that survived indefinitely in the PDL. The epithelial cells of HERs undergoes epithelial mesenchymal transformation into fibroblasts and cementoblasts that deposit acellular and cellular cementum [3]. The inner layer of root
during the formation of apical part of the root. The only incontrovertible fact is that many cells of
sheath gets incorporated in cellular cementum or gets trapped between cementum and dentin
HERs retain as epithelial phenotype and survive in PDL as epithelial cell rest of Malassez [5,6]. Studies indicate that PDL may be a source of cementoblast progenitor cells in adult human [7] .
Cementum is composed of 50% organic and 50% inorganic component. Cementum is broadly classified as cellular and acellular. Shroeder and Page has classified cementum into Acellular afibrillar cementum (AAC), Acellular extrinsic fiber cementum (AEFC), Cellular mixed stratified cementum (CMSC), Cellular intrinsic fiber cementum (CIFC) and Intermediate cementum based on its location, morphology and appearance [8]. AEFC is more highly mineralized than other types. Cementum has increased capacity for absorption of fluoride and other element due to the lower crystallizing of mineral component compared to
other hard tissues. It also readily decalcifies in the presence of acidic conditions [9,10].
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 2271
ISSN 2229-5518
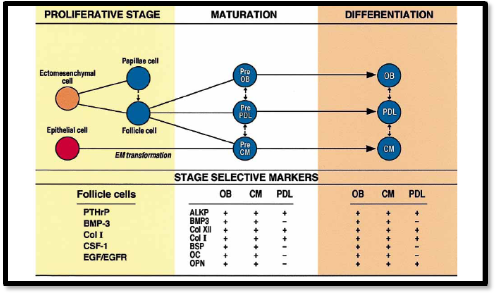
The organic matrix is comprises of 95% of Type I collagen and 5% of Type III collagen [6]. Cementum contains two major noncollagenous proteins, Bone sialoprotein (BSP) and osteopontin (OPN) [11]. They are phosporylated, sulfated glycoprotein that are prominently expressed in AAC and CIFC. They are expressed during early tooth root development by cells along the root surface. They are also seen in periodontal ligament region of the matured teeth. They play a major role in the differentiation of cementoblast progenitor cells to cementoblast [12]. The phospoglycoproteins contains well recognized adhesion domain, arginnine – glycine aspartic acid (RGD) which targets specific intergrin receptors, as well as other adhesion regions that promotes migration and cell adhesion [13,14]. Molecular factors present in the cementum have been proved to be important for development, maintenance and regeneration of cementum. Figure 1 shows diagrammatic illustration of factors responsible for proliferation and differentiation of new cementum.
Fibronectin is a glycoprotein in the ground substance which binds the cells to the extracellular matrix [8]. Tenascin is present in HERs during odontoblast differentiation and also
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 2272
ISSN 2229-5518
found in PDL [15]. Other matrix component includes osteonectin, osteocalcin and laminin, found responsible for the formation and mineralization. Proteins like proteoglycans, chondroitin sulfate, dermatan sulfate and hyaluronic acid are also found. All this can contribute in binding the cell to the extracellular matrix. Several polypeptide growth factor like Bone morphogenic protein (BMP) 2, 3 and 4, Platelet derived growth factor (PDGF), alpha and beta fibroblast derived growth factor( FGF), transforming growth factor (TGF) beta and parathyroid hormone( PTH ) are found in cementum matrix plays a role in cell differentiation and mineralization [12,16,17]. Some unique molecular factors includes, cementum growth factors (CGF),Insulin like growth factor (IGF) 1 Isoform [18] and cementum attachment protein (CAP) are found to promote adhesion and spreading of mesenchymal cells. They also play a role in adhesion of mineralized tissue forming cells [19,20].
which helps in proliferation and differentiation of some periodontal cell types [15]. These
Cementum contain molecules which promotes chemokines, integrins, adherins,
molecules are not detected in other periodontal structures. Adherin molecule present in the cementum have capability to exclude the unwanted cells [19].
After the periodontal therapy, major changes occur only in the epithelium and connective tissue but not in the cementum. Various phases of wound healing in the periodontal therapy include blood clot formation, re-epithelisation at the wound margin, angiogenesis and collagen synthesis and remodeling phase [15].
Regeneration of periodontium requires the reestablishment alveolar bone height to
the original position at the cementoenamel junction and the formation of acellular extrinsic fiber
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 2273
ISSN 2229-5518
cementum (AEFC) on the previously exposed root surface [21]. Grzesik at al proposed that the local environment for regeneration should be conductive for the recruitment and functioning of cells forming cementum. The wound matrix that is formed, favours repair rather than regeneration [15]. New therapeutic approaches includes guided tissue regeneration, application of growth factors and nanomatrix proteins to the root surface. However the formation of cementum remains unpredictable [15].
According to Melcher, only PDL cells have progenitor cells or stem cells have the capability to differentiate into cementoblast, osteoblast and fibroblast. They are found to be depleted or absent during the process of tissue destruction. The molecular factors responsible for recruiting and differentiating these cells like chemotactic factors, adhesion molecules, growth factors and matrix constituents which are found only in the cementum are also depleted. Hence
proliferation and differentiation during periodontal regeneration. Alteration of this
microenvironment of cementum contains all the components are necessary for cell recruitment,
microenvironment happens during periodontal destruction [22].
Many treatments has been tried to restore the integrity of the cementum like root conditioning, application of growth factors, barrier membranes and bone grafting materials [15]. Several biologically active mediators have been reviewed by various investigators which prove to facilitate in periodontal regeneration [15]. The existing periodontal ligament is the only tissue comprising of precursor cells/ progenitor cells. The local environment provides the instructions and the stimulating signals for these cells to differentiate. The extracellular matrix and the growth factors present in ECM and substances present in circulation play a pivotal role in providing stimulus and directional signals for the cells to migrate.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 2274
ISSN 2229-5518
Extracellular matrix is a 3 dimensional matrix and has collagen, fibronectin, elastin, other non collagenous proteins and proteoglycan as their contents. They act as :-
1. Substrate for cell adhesion [23]
2. Promotes spreading of cells and organization of cytoskeleton [23]
3. Determines the development of 3 dimensional structures [24]
4. Regulate gene expression of growth factor and its receptors [25]
5. Determines the outcome of cell response to growth factors [25]
6. It can induce signaling pathway which controls the biological function [25]
Binding the cells to ECM requires the help of intergrins and this binding can
1. Thyrosine phosphorylation of focal adhesion kinase (FAK)
initiate a cascade of signaling process. Signaling reactions which are activated includes [26,27]:-
2. Activation of mitogen activated protein (MAP) kinase cascade
3. Expression of c-fos
4. Elevation of cycline levels
Expressions of intergrins and growth factors induced signals which are important for the expression of G₁ Cyclins and cell cycle progression from G₀/G₁ to S phase [28]. ECM induces a sustained increase in activity of intergrin and growth factor induced signaling pathways by formation of MAP kinases as well as activation of extracellular signal regulated kinases 1 and 2 (ERK1/2) resulting in increased cyclin D₁ expression [29]. The ECM also up
regulates cyclin dependent kinase (cdk) inhibitors [30].
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 2275
ISSN 2229-5518
ECM is responsible for the recruitment of specific cell type during wound healing. Fibronectin promotes cell adhesion of most of the cell types whereas laminin and type IV collagen are helpful in the adhesion of selective cells. Tenacin has an anti adhesive property. Hence ECM can determine the cells to be elicited during wound healing depending on whether the healing is by regeneration or repair [15].
ECM can modify the binding of growth factors to cell surface receptors at multiple sites. During periodontal destruction, soluble factors like growth factors, cytokines and chemokines are secreted by inflammatory cells. Their activity will be targeted towards cells which take part in the wound healing. The activity of the component in the ECF will be limited
to the cell type needed for tissue homeostasis and some will be tissue specific in their
functionally in
Ithe prJocess of
Swound healingE. Flow chartRshows the function of ECM
distribution. Hence the composition of ECM and GF regulates the receptors and biochemical
signals induced [31]. The components of ECM can also determine how the cell respond
components at various phases of wound healing.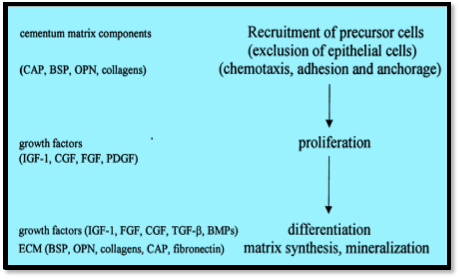
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 2276
ISSN 2229-5518
Structural integrity and unique biochemical composition of the ECM is essential for their tissue homeostasis in healthy condition as well as regeneration after injury. Composition of ECM varies with progression of wound healing. Table 1 shows some of the growth factors which can be derived from cementum as well as blood.
Table 1 : Growth factors responsible in wound healing [15]![]()

• Transforming growth
• Fibroblast growth factor
• Platlet-derived growth
• Epidermal growth
• Heparin-binding EGF
(FGF) 1 and 2
• Vascular endothelial
• TGF-beta
factor (PDGF)
• TGF-beta
• Connective tissue
growth factor (VEGF)
Formation of granulation tissue is constituted by Type III, IV,V and VI collagen, fibronectin and vitronectin. This is replaced by Type I collagen in later stages [32]. This change of composition in ECM does not mimic the local environment of the healthy tissue. Failure in
regeneration after injury can be due to the difficulty in reconstituting the local environment as it
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 2277
ISSN 2229-5518
is found in the healthy stage. Hence the provisional matrix offered by granulation tissue can be conductive more to repair than regeneration [15].
Success of periodontal healing relies in reconstruction of destroyed components of periodontium ie, the alveolar bone, cementum and the PDL. The tissue engineering concept of regeneration stresses in the three component of the triad: ie, the cells, GF and the scaffold.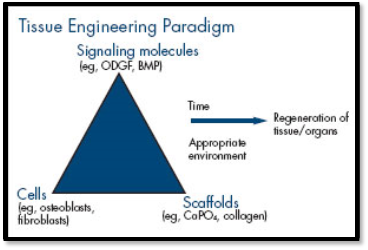
Figure 2 : Three basic elements in tissue engineering concept
IJSER
Several attempts have been made to allow the PDL cells to come into the wound space to facilitate the cells which are expected to populate in that particular area. Variety of chemotactic factors, adhersin and ECM constitutes participate in the recruitement of progenitor cells, their expansion and differentiation. Most of these molecules are pleotropic and are not cell specific. For achievement of cell specificity, various in vivo experiments evaluated the effect of growth factors targeting specific cell types, unique ECM composition and conditions permissive
for needed cells but refractory to other cells. The concentrations of chemical mediators were
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 2278
ISSN 2229-5518
relatively higher in alveolar bone and cementum which was found to be altered in pathological condition.
Different therapies have been experimented to restore the integrity of cementum. Application of root conditioner can only expose molecules like Type I collagens which has poor cell specificity [28]. Growth factor application did not provide a complete repair of the needed molecules, whereas their concentration and type can change during the healing process. Wang et al in 1993 demonstrated the effect of root conditioning agent like citric acid and tetracycline on the wound healing with or without GTR and found that there was no definite advantage [15].
Clinical trials using guided tissue regeneration (GTR) membrane can facilitate the
periodontal ligament cells to populate into wound area but they are not likely to provide the local
to wound contamIinationJ[33].
SER
environment for their further differentiation [15]. Reis EC et al in 2013 demonstrated the
efficacy of rigid absorbable membrane and found that the effect of regeneration was limited due
Enamel matrix derivates are found to be effective on cells during the differentiation process and hence found to be favourable for regeneration [34]. Lindhe et al in
1997 demonstrated that enamel protein was expressed in early cementogenesis but did not provide the environment for recruitment for cementoblasts progenitor cells in adults and their differentiation [35].
Protein and peptide based therapeutics provides a unique strategy for controlling highly specific and complex biological aspects. They are found to accelerate repair and regenerate when compared with other treatment. Miron RJ et al in 2013 has suggested that
emdogain (EMD) has the ability to enhance the speed of new bone formation when combined
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 2279
ISSN 2229-5518
with natural bone mineral (NBM) particles in rat osseous defects. Therefore it was found that the use of enamel matrix derivative (EMD) in combination with a natural bone mineral (NBM) was able to stimulate periodontal ligament cell and osteoblast proliferation and differentiation [34].
Growth factor released by the inflammatory cells can initiate the healing after periodontal surgery. The provisional matrix formed by blood clot and granulation tissue has a different composition from that of healthy cementum. Absence of progenitors in the pathological condition can be compensated by allowing stem cells to populate that area, but their differentiation requires those events that take place during cementogenesis. Signaling molecules for differentiation of stem cells are absent in wound healing environment. Hence a right combination of ECM and GF plays a pivotal role in cementum formation.
Stem cells derived from the exfoliated decidous teeth, periodontal ligament and
effect of allogenic stem cell from the exfoliated deciduos tooth could successfully regenerate the
dental follicle, have been successfully used for regeneration. Fu X et al in 2013 demonstrated the
periodontium [36].
Plasma rich in growth factors exerts positive effects on periodontal ligament fibroblasts. Suaid FF et al in 2012 evaluated that platelet rich plasma could enhance the cementum formation in class II furcation defects [37]. It was found that, they could enhance cementum formation. Eduardo Anitua et al in 2013 have proved that, autologous platelet-rich plasma stimulates periodontal ligament regeneration [38].
In early and moderate periodontitis, coronal half of the root which is affected includes acellular cementum whereas in advanced cases, damage extends to cellular cementum.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 2280
ISSN 2229-5518
However, whether the amount of cellular cementum is sufficient for successful regeneration remains unclear.
The concentration of growth factors and the adhesion molecules present in the cementum released during the inflammatory process are likely to be lesser than those available in blood and inflammatory cells [39,40]. Hence the contribution by molecules of cementum during regeneration can be marginal.
Many studies were conducted about gene therapy approaches to bone and periodontal tissue engineering as it is being widely explored. Flores MG et al in 2008
demonstrated a sItudy wJith periodoSntal ligament Ecell sheets whiRch was cultured with osteogenic
differentiation medium on periodontal fenestration defect of immunodeficient rat and found that
it could induce periodontal regeneration containing a cemental layer and Sharpey’s fibers [41]. Another study done by Rodrigues TL et al in 2011 suggested that reduced local levels of phosphate or pyrophosphate could promote increase cementum regeneration [42]. Seranno et al in 2013 conducted a study on the effect of cementum protein 1 (CEMP-1) and was demonstrated that treatment with CEMP-1 on defects in rat cranium stimulated bone formation and regeneration [43].
Noble cementoblasts and PDL near the cementum plays a critical role in the regeneration of periodontium, when avulsed tooth was re-implanted along with viable cells [44]. In vitro study done by D’Errico et al has demonstrated that isolation and propagation of cells exhibited an apparent cementoblastic phenotype. When transplanted into immunodeficient mice,
they formed a histologically proven cementum like tissue [45]. Hence, novel in vitro/in vivo
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 2281
ISSN 2229-5518
models offer promise towards developing relatively simple means of recruitment of cells with cementoblastic potential and differentiation as well as excluding unwanted osteoblastic precursors. This can allow the investigators for direct in vivo experimentation using human cells.
Once the molecule critical for cementum regeneration is identified, a simple delivery system is required for applying it in specific location. Further, it should remain bioavailable and bioactive for long period of time [15]. The anatomical position of cementum implies that the successful delivery system should reconcile the connecting mineralized dentin as well as no mineralized periodontal ligament. The regenerated cementum should confine only to tooth root surface. This can be made possible by targeting and binding bioactive molecule to the mineralized surface of the tooth root. Lack of stability of bioactive molecules to mineralized tooth surface can lead to unpredictable effect[46,47].
acidic motifs like polyaspartate and polyglumate are found in these molecules. Eg Cementum
Proteins which have the binding capability have been studied and found that
attached protein (CAP) contain bioactive part which helps in cementoblastic differentiation as well as mineral binding domain for efficient targeting to the tooth surface.
Integrin matrix interaction occurs in healing/ regeneration. Several integrin and their ligands like collagen, BSP, OPN, CAP are expressed by cementoblastic cells which could regulate cementogenesis [48]. Hence targeting the integrin receptors are important for cemental regeneration. Experiments have established that, those synthetic peptides contain RGD sequence and polyglutamate domain derived from BSP when bonded to hydoxyappitite phosphate (HAP)/ tricalcium phosphate (TCP) induced into immnunodificient mice. Cementum formation was
more efficient and abundant to untreated ceramics. Hence increase in number of active integrin
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 2282
ISSN 2229-5518
ligands on mineralized surface enhance formation of cementum by committed cementogenic cells [15]. Further research needs to be done to demonstrate on directing the bioactive peptides to naturally occurring mineral matrices of bone and dentin.
Knowledge of structure-function relationships of cemental matrix interphases is fundamental in order to develop more predictable regenerative therapy aimed at substituting pathologically altered mineralized tissues. The major biological problems are physical and chemical alteration of the root surface, these changes are incompatible with mesenchymal cell matrix interaction and impair matrix-matrix interaction. Hence a better understanding of molecular biology gives us a better insight to what lies beneath and in improvising the
therapeutic outcoImes oJf regeneratiSon. ER
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 2283
ISSN 2229-5518
[1] Jung-Chul Park, Yoo-Jung Um, Ui-Won Jung, Chang-Sung Kim, Seong-Ho Choi, Chong- Kwan Kim.
Histological characteristics of newly formed cementum in surgically created one wall intra bony defects in a
canine model. J Periodontal Implant Sci. 2010 February; 40(1): 3–10.
[2] Page RC, Baab DA. A new look at the etiology and pathogenesis of early onset periodontitis. Cementopathia
Revisited. J Periodontol. 1985 Dec; 56(12): 748-51.
[3] Patricia Furtado Gonçalves, Enilson Antonio Sallum, Antonio Wilson Sallum, Márcio Zaffalon Casati, Sérgio de Toledo, Francisco Humberto Nociti Junior. Dental cementum reviewed: development, structure, composition,regeneration and potential functions. Brazilian Journal of Oral Sciences. 2005 Jan/Mar; 4(12):
651-658.
[4] Diekwisch TIG. TheJdevelopmentSal biology of cemeEntum. Int J Dev BRiol. 2001 Sep;45(5-6):695-706.
[5] J. C. Rincon, W. G. Young, P. M. Bartold. The Epithelial Cell Rests of Malassez: A Role in Periodontal
Regeneration? Journal of Periodontal Research. 2006; 41 (4): 245-252.
[6] Bosshardt DD, Selvig KA. Dental cementum: the dynamic tissue covering of the root. J Periodontol. 1997;
13:41–75.
[7] Roguljic H, Matthews BG, Yang W, Cvija H, Mina M, Kalajzic I. In
vivo identification of periodontal progenitor cells. J Dent Res. 2013 Aug;92(8):709-15.
[8] Newman MG, Takei HH, Klokkevold PR. Caranza’s Clincal Periodontology. 10th ed. Los Angeles , California: Elsevier Inc Inc; 2006.
[9] Nakagaki H, Koyama Y, Sakakibara Y, Weatherell JA, Robinson C. Distribution of fluoride across human dental enamel, dentine and cementum. Arch Oral Biol. 1987;32(9):651-4.
[10] T. Murakami, H. Nakagaki, Y. Sakakibara, J.A. Weatherell, C. Robinson. The distribution pattern of fluoride concentrations in human cementum. Arch Oral Biol. 1987; 32(8): 567-571.
[11] Bosshardt DD, Zalzal S, McKee MD, Nanci A. Developmental appearance and distribution of bone sialoprotein and osteopontin in human and rat cementum. Anat Rec. 1998 Jan;250(1):13-33.
[12] Saygin NE, Giannobile WV, Somerman MJ. Molecular and cell biology of cementum. Periodontol. 2000;
24:73–98.
[13] Ganss B, Kim RH, Sodek J (1999). Bone sialoprotein. Crit Rev Oral Biol Med10:79–98. [14] Sodek J, Ganss B, McKee MD (2000). Osteopontin. Crit Rev Oral Biol Med11:279–303.
[15] Grzesik WJ, Narayanan AS. Cementum and periodontal wound healing and regeneration. Crit Rev Oral Biol
Med. 2002;13(6):474-84.
[16] Cochran DL, Wozney JM. Biological mediators for periodontal regeneration.Periodontol. 2000; 19:40–58.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 2284
ISSN 2229-5518
[17] MacNeil RL, Somerman MJ. Development and regeneration of the periodontium: parallels and contrasts. Periodontol. 2000; 19:8–20
[18] Ikezawa K, Hart CE, Williams DC, Narayanan AS. Characterization of a cementum derived growth factor as an insulin-like growth factor-I like molecule.Connect Tissue Res. 1997; 36:309–319.
[19] Olson S, Arzate H, Narayanan AS, Page RC (1991). Cell attachment activity of cementum proteins and mechanism of endotoxin inhibition. J Dent Res. 1991; 70:1272–1277.
[20] Pitaru S, Narayanan SA, Olson S, Savion N, Hekmati H, Alt I, et al. Specific cementum attachment protein enhances selectively the attachment and migration of periodontal cells to tooth root surfaces. J Periodontal Res. 1995; 30:360–368.
[21] http://www.homesteadschools.com/dental/courses/GrowthFactorsPeriodontalRegeneration/Chapter05.html
[22] Melcher AH. Cells of periodontium:Their role in the healing of wounds. Ann R Coll Surg Engl 1985: 67:
130—131
[23] Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–349.
[24] Huang S, Ingber DE. Shape-dependent control of cell growth, differentiation and apoptosis: switching between attractors in cell regulatory networks. Exp Cell Res. 2000; 261:91–103
[25] Adams JC, Watt FM . Regulation of development and differentiation by the extracellular matrix. Development. 1993; 117:1183–1198
[26] Juliano RL, Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993; 120:577–585. [27] Lewis TS, Shapiro PS, Ahn NG. gnal transduction through MAP kinase cascades. Adv Cancer Res.
1998; 74:49–139
[28] Polson AM. The root surface and regeneration; present therapeutic limitations and future biologic potentials. J Clin Periodontol. 1986; 13:995–999.
[29] Zhu X, Assoian RK. Integrin-dependent activation of MAP kinase: a link to shape-dependent cell proliferation. Mol Biol Cel. 1995;6:273–282.
[30] Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996; 87:1069–1078
[31] Saito M, Narayanan AS. Signaling reactions induced in human fibroblasts during adhesion to cementum- derived attachment protein. J Bone Miner Res. 1999; 14:65–72.
[32] Eckes B, Zigrino P, Kessler D, Holtkötter O, Shephard P, Mauch C, et al. Fibroblast-matrix interactions in wound healing and fibrosis. Matrix Biol.2000; 19:325–332.
[33] Reis EC, Borges AP, del Carlo RJ, Oliveira PM, Sepúlveda RV, Fernandes NA, Martins LM, Carvalho TB.
Guided tissue regeneration using rigid absorbable membranes in the dog model of chronic furcation defect.
Acta Odontol Scand. 2013 May-Jul;71(3-4):372-80
[34] Miron RJ, Wei L, Bosshardt DD, Buser D, Sculean A, Zhang Y. Effects of enamel matrix proteins in combination with a bovine-derived natural bone mineral for the repair of bone defects. Clin Oral Investig. 2013 May 8. [Epub ahead of print]
[35] Lindhe J.A biological approach to periodontal regeneration. J Clin Periodontol.1997; 24:657–714.
[36] Fu X, Jin L, Ma P, Fan Z, Wang S. Allogeneic Stem Cells From Deciduous Teeth Mediated Treatment for
Periodontitis in Miniature Swine. J Periodontol. 2013 Sep 3. [Epub ahead of print]
[37] Suaid FF, Carvalho MD, Ambrosano GM, Nociti FH Jr, Casati MZ, Sallum EA. Platelet-rich plasma in the treatment of Class II furcation defects: a histometrical study in dogs. J Appl Oral Sci. 2012 Mar- Apr;20(2):162-9.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 12, December-2013 2285
ISSN 2229-5518
[38] Anitua E, Troya M, Orive G. An autologous platelet-rich plasma stimulates periodontal ligament regeneration. J Periodontol. 2013 Nov;84(11):1556-66.
[39] Narayanan AS, Bartold PM. Biochemistry of periodontal connective tissues and their regeneration: a current perspective. Connect Tissue Res.1996; 34:191–201.
[40] Bartold PM, McCulloch CAG, Narayanan AS, Pitaru S. Tissue engineering: a new paradigm for periodontal regeneration based on molecular and cell biology.J Periodontol.2000; 24:253–269.
[41] Flores MG, Yashiro R, Washio K, Yamato M, Okano T, Ishikawa I. Periodontal ligament cell sheet promotes periodontal regeneration in athymic rats. J Clin Periodontol. 2008 Dec;35(12):1066-72
[42]. Rodrigues TL, Foster BL, Silverio KG, Martins L, Casati MZ, Sallum EA, Somerman MJ, Nociti FH Jr.Correction of hypophosphatasia-associated mineralization deficiencies in vitro by phosphate/pyrophosphate modulation in periodontal ligament cells. J Periodontol. 2012 May;83(5):653-63
[43] Serrano J, Romo E, Bermúdez M, Narayanan AS, Zeichner-David M, Santos L, Arzate H. Bone Regeneration in Rat Cranium Critical-Size Defects Induced by Cementum Protein 1 (CEMP1). PLoS One. 2013 Nov 12;8(11)
[44] Boyd DH, Kinirons MJ, Gregg TA. A prospective study of factors affecting survival of replanted permanent incisors in children. Int J Paediatr Dent.2000; 10:200–205.
[45] D’Errico JA, MacNeil RL, Takata T, Berry J, Strayhorn C, Somerman MJ . Expression of bone associated
markers by tIooth rooJt lining cells,Sin situ and in vivoE.1997 R
[46] Caffesse RG, Nasjleti CE, Anderson GB, Lopatin DE, Smith BA, Morrison EC. Periodontal healing
following guided tissue regeneration with citric acid and fibronectin application. J Periodontol.
1991; 62:21–29
[47] Choi SY, Nilvéus RE, Minutello RD, Zimmerman GJ, Wikesjö UME. Effect of a collagen matrix on healing in periodontal fenestration defects in dogs. J Periodontol. 1993; 64:878–882.
[48] Steffensen B, Duong AH, Milam SB, Potempa CL, Winborn WB, Magnuson VL,et al. Immunohistological localization of cell adhesion proteins and integrins in the periodontium. J Periodontol. 1992; 63:584–592.
IJSER © 2013 http://www.ijser.org